Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
miR145 Regulates the Proliferation and Apoptosis of Rat Vascular Endothelial Cells under Hyperglycemia by Targeting the ANGPT2 Gene and Involving the NFκB Signaling Pathway
Authors Zhang W , Tang XH, Zhang JJ, He Q
Received 22 July 2020
Accepted for publication 14 October 2020
Published 18 November 2020 Volume 2020:13 Pages 4435—4446
DOI https://doi.org/10.2147/DMSO.S273451
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Wen Zhang,1 Xin-Hua Tang,2 Jin-Juan Zhang,3 Quan He4
1Clinical Medical Research Center and Yunnan Provincial Key Laboratory of Clinical Virology (2018DG010), First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, Yunnan 650032, People’s Republic of China; 2Center of Genetic Diagnosis, First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, Yunnan 650032, People’s Republic of China; 3Kunming Institute of Zoology, Chinese Academy of Science (CAS), Kunming, Yunnan, 650223, People’s Republic of China; 4Emergency Department, First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, Kunming, Yunnan, 650032, People’s Republic of China
Correspondence: Quan He
Emergency Department, First People’s Hospital of Yunnan Province, Affiliated Hospital of Kunming University of Science and Technology, 157 Jinbi Road, Xishan District, Kunming 650032, Yunnan Province, People’s Republic of China
Tel +86 871 6363 9921
Fax +86 871 6362 7731
Email [email protected]
Purpose: A majority of diabetes mellitus patients with disturbances of glucose metabolism present with vascular complications. This study aimed to explore regulatory mechanisms of miR145 and its potential target gene ANGPT2 on diabetic vasculopathy under hyperglycemia.
Methods: Based on the fact that miR145 is detected in rat aortic endothelial cells (RAECs) under hyperglycemia, RAECs were transfected with miR145 mimics/inhibitor for further confirmation. RAEC proliferation was detected with CCK8 assays, and cell apoptosis and CD34+-cell population with annexinV–PI staining and anti-CD34FITC on flow cytometry, respectively. Then, qPCR and Western blot were applied to detect mRNA and protein expression of ANGPT2 and involved pathway factor NFκB p65. Subsequently, dual luciferase–reporter gene analysis was utilized to verify whether miR145 acted directly upon the 3ʹUTR of ANGPT2 mRNA.
Results: The ANGPT2 gene was confirmed to be a direct target of miR145. miR145 mimics markedly downregulated the expression of ANGPT2 and NFκB p65, boosted the percentage of the CD34+ phenotype, and promoted proliferation and suppressed apoptosis of RAECs under hyperglycemia.
Conclusion: miR145 might regulate the viability of RAECs via targeting ANGPT2 and involving NFκB signaling to exert a protective effect on diabetic vasculature.
Keywords: diabetic vasculopathy, miR145, angiopoietin 2, ANGPT2, rat aortic endothelial cells, RAECs, hyperglycemia
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterized by the disturbance of glucose metabolism.1 There are >300 million individuals suffering from DM worldwide, and approximately 60%–80% of DM patients present with vascular complications.2 Diabetic vascular complications are characterized by endothelial cell dysfunction and the formation of atherosclerotic plaques, due to micro- and macrovascular lesions.3 DM and its complications have become epidemic, and pose a serious challenge to worldwide health-care systems.4
According to the current consensus, DM is a kind of chronic low-level inflammatory disease. As an autocrine regulator of inflammatory reactions, ANGPT2 expression in vascular endothelial cells may act as a potential target in DM and its vascular complications.5 Studies have showed that ANGPT2 participates in inducing inflammatory reactions, promoting the apoptosis of diabetic vascular endothelial cells,6 and causing diabetic vasculopathy.7
miRNA has been found to be a posttranscriptional gene-expression regulator with specific sequences.8 Studies have showed that miRNA might play a major role in the pathogenesis of DM and microvascular DM complications,9 as well as in diabetic vascular endothelial dysfunction.10 Located at chromosome 5q32, miR145 has been reported to be a potent regulatory miRNA that plays important roles in vascular smooth-muscle cells. The high expression of miR145 in the vascular wall is probably essential for vascular function.11 Studies have also shown that miR145 participates in regulating the progression of metabolic inflammation in DM and atherosclerosis. With potent anti-inflammatory effects, miR145 can relieve the inflammatory status of DM hyperglycemia and delay the progression of DM vasculopathies.12
As such, it seems that miR145 has anti-inflammatory and ANGPT2 proinflammatory effects in DM. Since miRNA always acts upon mRNA to downregulate protein levels posttranscriptionally, we can hypothesize that miR145 might exert a potential protective effect on DM vasculopathy. However, whether miR145 definitely interacts with ANGPT2 and what the effect of their interaction is on vasculopathy remains unknown. Therefore, this study intended to verify interactions between ANGPT2 and miR145 so as to explore their effects on vascular cells under a certain DM condition — hyperglycemia.
Methods
Target Prediction and miRNA Acquisition
miRNA analysis for mature seed sequences and target prediction were searched on miRBase (http://www.mirbase.org) and achieved via miRDB (http://mirdb.org/miRDB/index.html) and TargetScan (http://www.targetscan.org/vert_71). Then, miRNA mimics of miR145, including miR145 mimic, miR145-mimic control, miR145 inhibitor, and miR145-inhibitor control were synthesized.
Cell Culture and Transfection
The rat aortic endothelial cell (RAEC) line was purchased from the Cell Bank of the Shanghai Institute of Cellular Biology, Chinese Academy of Sciences. FBS, RPMI 1640 culture medium and PBS were purchased from HyClone. RAECs were cultured in RPMI 1640 medium with 10% FBS at 37°C in 5% CO2. RAECs were then plated at a density of 106 cells per well into a six-well plate. When cellular confluence had reached approximately 50%–70%, 250 pmol of mimics (miR145 mimic, miR145-mimic negative control [NC], miR145 inhibitor, and miR145-inhibitor NC) mixed with 100 μL Lipofectamine 2000 (Invitrogen) transfection reagent were added to each well within 1 mL FBS-free medium. The final concentration of the mimics was adjusted to 10 pmol/mL. The complete medium was changed 5 hours after transfection.
Experimental Groups
For detection of miR145 expression under different glucose concentrations, when the cellular confluence of plated RAECs had reached approximately 80%, the culture medium was changed to FBS-free medium to starve cells for synchronization for about 12 hours. Then, RAECs were cultured with different glucose-concentration media and grouped: normal glucose (NG) concentration (5.6 mmol/L D-glucose as control), high glucose (HG) concentration (divided gradually into 12.5 mmol/L, 25 mmol/L, 50 mmol/L, and 75 mmol/L D-glucose as hyperglycemia groups), and 25 mmol/L, 100 mmol/L, and 300 mmol/L D-mannitol as hyperosmotic control groups. The incubation time for detection of each group was 30 minutes and 1, 2, 4, 6, and 8 hours, respectively. D-glucose and D-mannitol were obtained from the Kunming Institute of Zoology of the Chinese Academy of Sciences.
After transfection with miRNA mimics, RAECs were divided into six groups: one NG control group (5.6 mmol/L D-glucose in medium) and five HG groups with 25 mmol/L of D-glucose in medium, of which four with miRNA transfection were defined: HG+miR145 mimics, HG+NC mimics, HG+miR145 inhibitor, and HG+NC inhibitor.
Quantitative RT-PCR
qRT-PCR primers were synthesized by Shanghai Huada Gene, and primer sequences were: ANGPT2 — forward 5ʹ-GACCACGAGACTTGAACTTCAG-3ʹ, reverse 5ʹ-GGATGATGTGCTTGTCTTCCATAG-3ʹ; NFκB p65 — forward 5ʹ-TGATGTGCATCGGCAAGTG-3ʹ, reverse 5ʹ-AGAAGTTGAGTTTCGGGTAG-3ʹ; hsa-miR145 — forward 5ʹ-TGCTGAGAACGAGCAGTAA-3ʹ, reverse 5ʹ-CGAGGTCCGAAGACTCATTT-3ʹ; and β-actin — forward 5ʹ-CCTCAAGACATCAGCAATGCCTCCT-3ʹ, reverse 5ʹ-GGTCATGAGTCCTTCCACGATACCAA-3ʹ. When cellular confluence had reached approximately 80% after transfection for about 24–36 hours, total RNA of RAECs from each group was isolated (Bio-Rad) and potential genomic DNA contamination removed with an On-Column DNase I digestion kit (R&D Systems). Then, total RNA (0.5–1.0 μg) was reverse-transcribed with and iScript cDNA-synthesis kit (Bio-Rad), and 2×SYBR Green 1 Mix (Applied Biological Materials) applied to perform qRT-PCR assays to quantify the mRNA expression of miR145, ANGPT2, and NFκB p65 according to the manufacturer’s instructions. β-actin was used as an internal control.
Western Blot
Western blot was employed to detect protein levels of ANGPT2 and NFκB p65 from RAECs harvested at 48 hours posttransfection. β-actin was used as an internal reference. SDS-PAGE was routinely performed, as described in Sun et al.13 ImageJ software was used to analyze the gray scales of the bands.
Proliferation-Activity Assays
RAECs were seeded at a density of 5,000 cells per well in a 96-well plate. Then 25 pmol mimics (miR145 mimic, miR145-mimic NC, miR145 inhibitor, and miR145-inhibitor NC) and 10 μL Lipofectamine 2000 mixed within 100 μL FBS-free medium were added to each well. After 4 hours’ transfection, the culture medium was replaced and CCK8 proliferation assays (KeyGen Tech, Nanjing, China) employed to detect the proliferation activity of transfected RAEC cells. From this time point on, 100 μL culture medium with 10 μL CCK8 detection reagent was added to each well from day 1 to day 6 posttransfection. Two hours after the CCK8 reagents had been added, the optical density (OD) level of each well was detected with an enzyme reader to generate a cellular proliferation-activity curve for these days. Each group was assessed four times. For calculation purposes, relative proliferation activity = OD of the assay group/OD of the normal control group.
Flow Cytometry
RAECs were harvested from each group at 24 hours posttransfection. Then, a PI–annexin V apoptosis detection–reagent kit (KeyGen Tech) was applied to detect cell apoptosis following the manufacturer’s instructions. Briefly, after cell collection and rinsing, 500 μL binding buffer was used to resuspend cells at a concentration of 106cells/mL. Subsequently, 5 mL annexin V and 10 μL PI (20 g/L) were added to the suspension. After incubation in the dark for 5 minutes at room temperature, cells were loaded into a flow cytometer to detect the apoptosis rate in each cell group. Live-cell FITC–/PI–, apoptotic-cell FITC+/PI–, and necrotic-cell FITC+/PI+ were applied to calculate the ratio of apoptotic cells. Similarly, RAECs were harvested at 72 hours posttransfection and labeled with anti-CD34FITC fluorescent antibody (BD Biosciences). All detections were performed with flow cytometry FACSCalibur (BD Biosciences), and Flowjo version 7.6 was used for analysis.
Dual Luciferase–Reporter Gene Assay
Amplification of Full-Length 3ʹUTR of ANGPT2
Bioinformatics was applied to predict the target-conjugation point between miR145 and ANGPT2, and point mutation was performed. Primer sequences of hsa-miR145-5p and ANGPT2 3ʹUTR were synthesized with equipment from Beijing Sunbiotech. The full-length 3ʹUTR of ANGPT2 gene was amplified with primers: forward 5ʹ-ACCCCACTGTTGCTAAAGATTCAAGAGATCTTTAGCAACAGTGGGGTTTTTTT-3ʹ, reverse 5ʹ-AATTAAAAAAACCCCACTGTTGCTAAGATCTCTTGAATCTTTAGCAACAGTGGGGTGGCC-3ʹ.
Cloning of 3ʹUTR Constructs
The pMIR-Report luciferase-reporter plasmid was constructed by inserting a firefly luciferase–reporter gene into the pMIR-Report empty vector (Origene) between EcoRI and Sgf I enzyme-cut sites. Then, ANGPT2 3ʹUTR wild-type (WT) and mutant (Mut) segments (murine ANGPT2) were inserted into the pMIR-Report luciferase vector between HindIII and MluI sites to construct the pMIR-Report ANGPT2 3ʹUTR plasmid. Restriction endonucleases HindIII, MluI HpaI, EcoRI, and SgfI and T4 DNA ligase were purchased from Takara (Dalian, China). A plasmid miniprep reagent kit, endotoxin-free plasmid large-prep reagent kit, and DNA gel-recycling reagent kit were used for extraction and purification of constructed reporter plasmids (Tiangen). After construction, pMIR-Report ANGPT2 3ʹUTR plasmids, including WT and Mut, were sent for sequencing to confirm their validity.
Luciferase-Reporter Gene Assay
RAEC cells were plated in 12-well plates at 3×105cells/well. The miR145 mimic and its NC, together with miR145 inhibitor and its NC and the ANGPT2 reporters (pMIR-Report ANGPT2 3ʹUTR WT and pMIR-Report ANGPT2 3ʹUTR Mut) were cotransfected into the RAECs. At 48 hours posttransfection, whole-cell lysate was collected for luciferase-reporter assays. A pRL-TK vector expressing Renilla luciferase was utilized as an internal reference to calibrate differences in cell numbers and transfection efficiency. Dual-luciferase activity was measured in triplicate using the Bright-Glo luciferase–assay system (Promega) according to the instructions provided.11
Statistical Analysis
SPSS version 13.0 was used, and data are expressed as means ± SD. Univariate ANOVA was employed to compare means among multiple groups. P<0.05 was considered statistically significant. The data sheet was prepared using GraphPad Prism 5.0 software.
Results
Sustained Hyperglycemia Decreased miR145 Expression and Increased Expression of ANGPT2 and NFκB p65 in RAECs
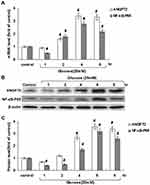
In order to explore the effect of hyperglycemia on miR145 expression, different concentrations of glucose were added to the culture medium for 30 minutes. As shown in Figure 1A, compared with the NG group of 5.6 mmol/L D-glucose, miR145 significantly increased with the addition of D-glucose of 12.5–75 mmol/L, showing a maximal effect at 25 mmol/L (Figure 1A, left). Therefore, 25 mmol/L glucose was used for subsequent experiments. In order to exclude the osmotic effect of hyperglycemia on miR145 expression, triple concentrations of mannitol were added in parallel, which failed to affect the expression of miR145 (Figure 1A, right).
Based on results with different concentrations at one time point (Figure 1A), incubation was prolonged to 1, 2, 4, and 6 hours to determine the most effective concentration at different time points, as shown in Figure 1B. Taking the average expression of normal group with 5.6 mmol/L glucose along time as control normalized as “1”, an interesting reversed effect emerged. Though 25 mmol/L of glucose maximally increased the expression of miR145 above level 3 at 30 minutes (Figure 1A), this effect gradually decreased below level 2 when detected at 1, 2, 4, and 6 hours, and was significantly less than the control level after 4–6 hours (Figure 1B). These results indicated that miR145 might participate in some related biological process of diabetic vasculopathy under hyperglycemia, with somehow temporal and spatial attributes.
MiRDB and TargetScan were utilized to search and analyze the target genes of miRNAs, and ANGPT2 was pinpointed as a potential target of miR145. The effect of hyperglycemia on ANGPT2 expression in RAECs was then examined. Initially, hyperglycemia with 25 mmol/L glucose did not have an effect on ANGPT2 (Figure 2A). However, ANGPT2 expression began to increase after 2 hours of hyperglycemic stimulation, and both mRNA-expression and protein levels of ANGPT2 became significantly elevated when prolonged till 4–8 hours (Figure 2A–C). Similarly, in order to investigate ANGPT2 signal pathways involved, both mRNA-expression and protein levels of NFκB p65 were detected under sustained hyperglycemia, which turned out to have the same expression trend as ANGPT2 (Figure 2). This made us believe that the delayed effect was closely related to the initial temporary upregulation of miR145.
miR145 Promoted Proliferation of Vascular Endothelial Cells
Based on the transient robust expression of miR145 in RAECs under hyperglycemia with 25 mmol/L glucose, the mimics and inhibitor of miR145 and their NCs were transiently transfected into RAECs to explore the effect of miR145 overexpression or miR145 inhibition on vascular endothelial cells. The six groups were NG (control, 5.6 mmol/L D-glucose), HG (25 mmol/L D-glucose), further divided into HG only, HG+miR145 mimic, HG+NC mimic, HG+miR145 inhibitor, and HG+NC inhibitor.
After transfection in the 96-well plate, CCK8 assays were applied to detect the effect of miR145 on the proliferation of RAECs under hyperglycemia. The growth curve by CCK8 OD value from day 1 to day 6 displayed that cellular proliferation following transfection with miR145 mimic became faster than the HG and HG+NC mimic groups (P<0.05, Figure 3), but quite similar to normal cells in the NG group (P>0.05), while proliferation slowed remarkably after transfection with the miR145 inhibitor (P<0.01, Figure 3), indicating that miR145 was necessary for cell proliferation under hyperglycemia. The curve also showed that changes in cell-proliferation activity became strongly pronounced at 3–4 days posttransfection (Figure 3), which indicated that miR145 promote the proliferation of RAECs under hyperglycemia, making it similar to normal cell status.
miR145 Suppressed Cellular Apoptosis of Vascular Endothelial Cells
Annexin V–FITC/PI assays were employed to determine whether miR145 played a regulatory role in apoptosis of RAECs among these six groups. When compared with HG (5.2%+11.5%) and HG+miR145 mimics (11.0%+16.6%), the apoptotic rate of RAECs decreased markedly in the latter (6.8%+7.9%, P<0.05), similar to the NG group (5.0%+7.6%), whereas it increased markedly in both the HG+miR145 inhibitor (18.70%+10.8%) and HG+miR145 inhibitor–control groups (11.3%+18.8%, P<0.05; Figure 4A). This indicated that miR145 overexpression markedly suppressed apoptosis of RAECs, while miR145 silencing promoted apoptosis under hyperglycemia (P<0.01, Figure 4B). Regardless of early or late apoptosis, RAECs with overexpression of miR145 with HG displayed similar normal status as NG.
miR145 Boosted the Ratio of CD34+ Vascular Endothelial Cells
CD34 is considered a marker of early undifferentiated vascular endothelial cells. Labeled with anti-CD34 fluorescent antibody and detected by flow cytometry, CD34+ vascular endothelial cell population to total RAEC counts was higher in HG+miR145 mimics than in the HG+miR145 mimic–control group (13.50%±2.10% vs 6.82%±1.31%, P<0.05). As for the inhibitor groups, the CD34+ cell population reduced in both the HG+miR145-inhibitor and HG+miR145 inhibitor–control groups (6.28%±1.37% vs 4.64%±0.92%, P>0.05). When comparing the miR145-mimic group with the miR145-inhibitor group, a remarkable increase was also found (P<0.01, Figure 5). These results indicated that miR145 significantly boosted the ratio of CD34+ cells in rat vascular endothelial cells, playing a possible protective role in maintaining the characteristics of progenitor vascular endothelial cells under hyperglycemia.
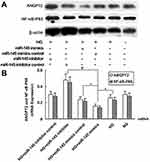
miR145 Regulated Expression of ANGPT2 and NFκB p65 in Rat Vascular Endothelial Cells
Based on these results, hyperglycemia with 25 mmol/L glucose in culture medium maximally elevated miR145 expression at 30 minutes, but declined over time (Figure 1). Conversely, 25 mmol/L HG began to markedly elevate the expression of ANGPT2 and NFκB p65 from hour 4 onward (Figure 2). In order to explore whether there were a direct association between transient high expression of miR145 and delayed high expression of ANGPT2, as well as NFκB p65 in RAECs under hyperglycemia, Western blot analysis was performed to detect protein levels of ANGPT2 and NFκB p65 with 25 mmol/L HG mediated by overexpression or silencing miR145, as well as RT-PCR assays for mRNA expression. Both protein level and mRNA expression of ANGPT2 and NFκB p65 were markedly downregulated in the HG+miR145 mimics group (P<0.05) and markedly upregulated in the HG+miR145-inhibitor group (P<0.01, Figure 6). These results indicated that transient high expression of miR145 downregulated ANGPT2 and pathway-related inflammatory factor NFκB p65, via which the role of miR145 in participating in inflammatory signaling was confirmed in vitro.
miR145 Directly Regulated Its Target ANGPT2
TargetScan predicted ANGPT2 as a potential target gene of miR145 (Figure 7, upper). The acting foci of miR145 and ANGPT2 were found, and turned out to be conserved among many species when comparing a human motif with other species like monkey, dog, mouse, and rat (Figure 7, lower). In order to confirm the targeting effect of miR145 upon ANGPT2 mRNA, the dual luciferase–reporter system was used. Firstly, synthesized miR145 mimics and constructed luciferase-recombinant plasmids, defined as pMIR-Report ANGPT2 3ʹUTR, were co-transfected into RAECs. Based on the plasmids, WT 3ʹUTR and Mut 3ʹUTR of ANGPT2 mRNA were constructed, pMIR-Report ANGPT2 3ʹUTR WT and pMIR-Report ANGPT2 3ʹUTR Mut, marked as ANGPT2 WT and ANGPT2 Mut in the figure. To verify plasmid construction, sequencing was performed to display the original motif of WT 3ʹUTR and Mut 3ʹUTR motifs of ANGPT2 mRNA, AACTGGAA in WT in Figure 7, and CCAGTTCA in Mut (Figure 8A). The luciferase-reporter assay showed that luciferase activity declined approximately 50% in the ANGPT2 3ʹUTR WT group (P<0.001), while there was no obvious change in the ANGPT2 3ʹUTR Mut group. Due to a direct binding effect, no statistically significant change in luciferase activity was observed in the miR145 mimic-control group or the miR145-inhibitor groups (Figure 8B). These results indicated that miR145 directly targeted the 3ʹUTR region of ANGPT2 mRNA and thus downregulated expression of ANGPT2.
 |
Figure 7 Relationship of base-pair complementarity between miR145 and ANGPT2. |
Discussion
DM and its complications are a serious challenge to worldwide health care. One of the major features of diabetic vascular complications lies in endothelial cellular dysfunction. On one hand, many previous studies have indicated that a hyperglycemic environment accelerates the apoptosis of endothelial cells and disrupts the endothelial barrier. These two factors are vital in the occurrence and progression of atherosclerosis in DM.14 On the other hand, the pathological essence of type 2 DM is a kind of subacute or chronic inflammatory lesion, and its occurrence and progression are accompanied by inflammatory reactions.15 To a certain extent, this is correlated with the release of various inflammatory mediators and free radicals as a result of the lesion-induced activation of endothelial cells and macrophages.16
In light of the close relationship between inflammation and vascular diseases, such as atherosclerosis (diabetic macrovasculopathy is an atherosclerotic process), miRNA, as a recently discovered regulatory factor for posttranscriptional gene expression, is taken into consideration. It has been indicated that miRNA exhibits sequence specificity and plays vital roles in the pathogenesis of diabetic vascular endothelial dysfunction.17 Located at chromosome 5q32, miR145 has the highest expression level in the vascular wall, which implies that it probably plays an important role in vascular function. It has also been suggested that miR145 possibly has equal importance in the regulation of metabolic inflammations in DM and atherosclerosis. As demonstrated by both in vivo and in vitro studies, overexpression of miR145 can promote the apoptosis of macrophages, thereby alleviating the infiltration of macrophages and suppressing the production of inflammatory factors by macrophages.18 That is to say, miR145 has potential anti-inflammatory effects.
According to the predictions by MiRDB and TargetScan and Lu et al,19 the ANGPT2 gene is a potential target for miR145. This makes sense, because ANGPT2 specifically expresses in endothelial cells and acts as an independent inflammation-triggering factor. Endothelial cells not only convey harmful stimuli to cause inflammation but also initiate the overall inflammatory cascade under appropriate conditions. Therefore, ANGPT2 has been widely recognized as a therapeutic goal for eliminating the initial proinflammatory focus.20 Moreover, the researchers of this study have previously demonstrated that ANGPT2 inhibited the proliferation and enhanced the apoptosis in vitro of human vascular endothelial cells.21 It could be surmised that the possible mechanism on cell proliferation/apoptosis of vascular endothelial cells lies in the negative regulation of miR145 via its target gene ANGPT2.
High-concentration glucose has toxic effects on vascular endothelial cells, and it may be one major mechanism for DM, leading to injury to endothelial cells. However, the exact molecular pathway remains poorly defined. In light of the miRNA investigations, our study intended to address the core questions on the miR145-mediated regulation of target ANGPT2 and determine whether altered expression of ANGPT2 participates in injury to vascular endothelial cells in glucose-disordered DM. In the meantime, related inflammatory factors/pathways were taken into detection. Expression of miR145 was initially enhanced, but subsequently suppressed in RAECs under hyperglycemia for several hours. The miRNA mimics and inhibitor of miR145 were transfected into RAECs under hyperglycemia to create a temporary miR145-overexpression effect under the most effective glucose concentration — 25 mmol/L. Compared with the silencing effect of the miR145 inhibitor, miR145 mimics promoted cell proliferation and suppressed cell apoptosis of RAECs and downregulated protein level and mRNA expression of ANGPT2 under hyperglycemia. Dual luciferase–reporter gene assays verified that miR145 directly and specifically targeted the 3ʹUTR region of ANGPT2 mRNA to inhibit ANGPT2 expression posttranscriptionally.
During this study, two other factors were detected further verification. CD34 is a surface-specific antigen of progenitor endothelial cells in peripheral blood, which are precursor immature forms of vascular endothelial cells. It was demonstrated that after induced differentiation, the expression of CD34 gradually declined, while other markers remained positive. Due to its atypical expression in most endothelial cells, CD34 has been extensively applied as one of the most specific vascular endothelial markers. With the highest expression specificity in vascular endothelial cells, CD34 has been termed a pan–endothelial cell marker and is superior to other markers of endothelial cells.22 Therefore, CD34+ endothelial cells were used for detection in miR145 mimic/inhibitor–transfected RAECs to verify the proliferation of specific endothelial cell populations. Detection turned out to verified successfully, because miR145 mimics boosted the expression of CD34+ endothelial cells in RAECs.
Since miR145 silenced its target gene, ANGPT2, which acts as an inflammation-triggering factor, the inflammation-associated signal was also taken into account in this study. As an important type of proinflammatory factor that participates in the pathophysiological processes of inflammation and apoptosis,7 the NFκB signal–related factor NFκB p65 was detected as well. The results confirmed that miR145 mimics suppressed the expression of inflammatory factor NFκB p65, thus possibly inhibiting inflammation. What is more, the NFκB family and signaling-transduction pathways also exerted a biregulatory effect in cell apoptosis. Chen et al23 noted that when subunit p65 (RelA) was overexpressed, cell apoptosis was suppressed. However, in the present study miR145 mimics suppressed the expression of NFκB p65, but continued to suppress the apoptosis of RAECs. In this context, it was hypothesized that ANGPT2 gene might be in the upstream region of NFκB p65. The exact relationship between ANGPT2 and NFκB p65 and the mechanism whereby modulation of the NFκB p65–signaling pathway suppresses ANGPT2-induced activation of diabetic vascular endothelial cells need to be further elucidated.
Conclusion
In our study of RAECs, we found that the miR145 expression gradually increased with increased glucose concentrations within a certain range. Under the negative regulation of miR145, ANGPT2 and the involved signaling factor NFκB p65 were downregulated. Through this pathway, miR145 promoted the proliferation and suppressed the apoptosis of RAECs, which might alleviate the dysfunction of vascular endothelial cells under an HG environment. What is more, throughout all the assays, even in hyperglycemia with 25 mmol/L glucose, as long as miR145 was stimulated to be overexpressed, it had a protective effect on vascular endothelial cells, the status of which was similar to cells within NG conditions. As such, we can speculate that miR145 might prevent hyperglycemia-induced vascular endothelial cell injury by targeting ANGPT2 and involving the NFκB pathway and thereby exerting a protective effect on diabetic vasculature. Once confirmed by functional verification in vivo, both miR145 and ANGPT2 could serve as inflammatory markers, carrying important clinical implications for DM-related vascular complications.
Acknowledgments
This work was funded by projects and grants from the Scientific Research Program of Yunnan Provincial Education Bureau (2014CO12Y), the Applied Basic Research Program of the Science and Technology Hall of Yunnan Province and Kunming Medical University (2015FB091 and 2017FE468 [−104]), and by Yunnan financial social security departments (2017-116). Jin-Juan Zhang works at the Kunming Institute of Zoology of the Chinese Academy of Sciences, while the rest of the authors work at the First People’s Hospital of Yunnan Province. All were engaged in the experimental and writing work of this study. Both funding and efforts are appreciated.
Disclosure
The authors declare that there are no conflicts of interest for this work. We have no financial or personal relationships with other people or organizations that could inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service, and/or company that could be construed as influencing the position presented in the manuscript.
References
1. Gong J, Fang K, Dong H, Wang D, Hu M, Lu F. Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: a meta-analysis. J Ethnopharmacol. 2016;194:260–268. doi:10.1016/j.jep.2016.08.003
2. Liu J, Jiang C, Ma X, Feng L, Wang J. Notoginsenoside Fc accelerates reendothelialization following vascular injury in diabetic rats by promoting endothelial cell autophagy. J Diabetes Res. 2019;2019:9696521. doi:10.1155/2019/9696521
3. Lee C, Huxley R, Lam T, et al. Prevalence of diabetes mellitus and population attributable fractions for coronary heart disease and stroke mortality in the WHO South-East Asia and Western Pacific regions. Asia Pac J Clin Nutr. 2007;16(1):187–192.
4. Chakraborty R, Sen S. EDITORIAL (thematic issue: treatment and diagnosis of diabetes mellitus and its complication: advanced approaches). Mini Rev Med Chem. 2015;15(14):1132–1133. doi:10.2174/138955751514151006154616
5. Lim H, Blann A, Chong A, Freestone B, Lip G. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. J Diabetes Care. 2004;27(12):2918–2924. doi:10.2337/diacare.27.12.2918
6. Li L, Yu Z, Qian L, et al. Interleukin-19 and angiopoietin-2 can enhance angiogenesis of diabetic complications. J Diabetes Complications. 2016;30(2):386–387. doi:10.1016/j.jdiacomp.2015.11.002
7. Tabata M, Kadomatsu T, Fukuhara S, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. J Cell Metab. 2009;10(3):178–188. doi:10.1016/j.cmet.2009.08.003
8. Kong L, Zhu J, Han W, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48(1):61–69. doi:10.1007/s00592-010-0226-0
9. Zampetaki A, Kiechl S, Drozdov I, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. J Circ Res. 2010;107(6):810–817. doi:10.1161/circresaha.110.226357
10. Karolina D, Armugam A, Tavintharan S, et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PLoS One. 2011;6(8):e22839. doi:10.1371/journal.pone.0022839
11. Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. J Circ Res. 2009;105(2):158–166. doi:10.1161/circresaha.109.197517
12. Lovren F, Pan Y, Quan A, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. J Circ. 2012;126(11_suppl_1):S81–S90. doi:10.1161/circulationaha.111.084186
13. Sun Y, Bai Y, Zhang F, Wang Y, Guo Y, Guo L. miR-126 inhibits non-small cell lung cancer cells proliferation by targeting EGFL7. Biochem Biophys Res Commun. 2010;391(3):1483–1489. doi:10.1016/j.bbrc.2009.12.098
14. Kirmaz C, Ozbilgin K, Yuksel H, et al. Increased expression of angiogenic markers in patients with seasonal allergic rhinitis. J Eur cytokine Netw. 2004;15(4):317–322.
15. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72(1):219–246. doi:10.1146/annurev-physiol-021909-135846
16. Chan H, Yiu K, Wong C, Li S, Tam S, Tse H. Increased dietary fruit intake was associated with lower burden of carotid atherosclerosis in Chinese patients with type 2 diabetes mellitus. Diabet Med. 2013;30(1):100–108. doi:10.1111/j.1464-5491.2012.03764.x
17. Park S, Moon S, Lee K, Park I, Lee D, Nam S. miR2Diabetes: a literature-curated database of microRNA expression patterns, in diabetic microvascular complications. Genes. 2019;10(10):10. doi:10.3390/genes10100784
18. Odegaard J, Chawla A. Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab. 2008;4(11):619–626. doi:10.1038/ncpendmet0976
19. Lu R, Ji Z, Li X, et al. miR-145 functions as tumor suppressor and targets two oncogenes, ANGPT2 and NEDD9, in renal cell carcinoma. J Cancer Res Clin Oncol. 2014;140(3):387–397. doi:10.1007/s00432-013-1577-z
20. Kim H, Koh G. Ang2, the instigator of inflammation. Blood. 2011;118(18):4767–4768. doi:10.1182/blood-2011-09-377333
21. Quan HE. Construction of shRNA eukaryotic expression vectors targeting angiopoietin-2 gene and effects of angiopoietin-2 gene knockdown on apoptosis in endoplasmic reticulum stress-induced human umbilical vein endothelial cell. Chin J Diabetes. 2015;2(23):158–164.
22. Dore F, Domingues C, Ahmadi N, et al. The synergistic effects of saxagliptin and metformin on CD34+ endothelial progenitor cells in early type 2 diabetes patients: a randomized clinical trial. J Cardiovasc Diabetol. 2018;17(1):65. doi:10.1186/s12933-018-0709-9
23. Chen X, Kandasamy K, Srivastava R. Differential roles of RelA (p65) and c-Rel subunits of nuclear factor kappa B in tumor necrosis factor-related apoptosis-inducing ligand signaling. J Cancer Res. 2003;63(5):1059–1066.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.