Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
miR-623 Targets Metalloproteinase-1 and Attenuates Extravasation of Brain Metastatic Triple-Negative Breast Cancer Cells
Authors Hammash D , Mahfood M, Khoder G, Ahmed M, Tlili A, Hamoudi R , Harati R
Received 24 April 2022
Accepted for publication 21 July 2022
Published 1 August 2022 Volume 2022:14 Pages 187—198
DOI https://doi.org/10.2147/BCTT.S372083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Dua Hammash,1,2 Mona Mahfood,3 Ghalia Khoder,4 Munazza Ahmed,1,2 Abdelaziz Tlili,3 Rifat Hamoudi,2,5,6 Rania Harati1,2
1Department of Pharmacy Practice and Pharmacotherapeutics, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates; 2Sharjah Institute for Medical Research, University of Sharjah, Sharjah, United Arab Emirates; 3Department of Applied Biology, College of Sciences, University of Sharjah, Sharjah, United Arab Emirates; 4Department of Pharmaceutics and Pharmaceutical Technologies, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates; 5Clinical Sciences Department, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates; 6Division of Surgery and Interventional Science, University College London, London, UK
Correspondence: Rania Harati, Department of Pharmacy Practice and Pharmacotherapeutics, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates, Tel +971 6 505 7438, Fax +971 6 558 5812, Email [email protected]
Background: Most breast cancer-related deaths result from metastasis. Understanding the molecular basis of metastasis is needed for the development of effective targeted and preventive strategies. Matrix metalloproteinase-1 (MMP1) plays an important role in brain metastasis (BM) of triple-negative breast cancer (TNBC) by promoting extravasation of cancer cells across the brain endothelium (BE). MMP1 expression is controlled by endogenous microRNAs. Preliminary bioinformatics analysis has revealed that miR-623, known to target the 3ʹUTR of MMP1, is significantly downregulated in brain metastatic tumors compared to primary BC tumors. However, the involvement of miR-623 in MMP1 upregulation in breast cancer brain metastatic cells (BCBMC) remains unexplored. Here, we investigated the role of miR-623 in MMP1 regulation and its impact on the extravasation of TNBC cells through the BE in vitro.
Materials and Methods: A loss-and-gain of function method was employed to address the effect of miR-623 modulation on MMP1 expression. MMP1 regulation by miR-623 was investigated by real-time PCR, western blot, luciferase and transwell migration assays using an in vitro human BE model.
Results: Our results confirmed that brain metastatic TNBC cells express lower levels of miR-623 compared with cells having low propensity to spread toward the brain. miR-623 binds to the 3′-untranslated region of MMP1 transcript and downregulates its expression. Restoring miR-623 expression significantly decreased MMP1 expression, preserved the endothelial barrier integrity, and attenuated transmigration of BCBMC through the BE.
Conclusion: Our study elucidates, for the first time, the crucial role of miR-623 as MMP1 direct regulator in BCBMC and sheds light on miR-623 as a novel therapeutic target that can be exploited to predict and prevent brain metastasis in TNBC. Importantly, the presents study helps in unraveling a brain metastasis-specific microRNA signature in TNBC that can be used as a guide to personalized metastasis prediction and preventive approach with better therapeutic outcome.
Keywords: brain metastasis, triple-negative breast cancer, brain endothelium, brain endothelial junctions, matrix metalloproteinase-1, microRNA-623, personalized preventive therapy, targeted prevention, metastasis prediction, precision medicine, primary and secondary care
Introduction
Breast Cancer is the Leading Malignancy Worldwide
Breast cancer (BC) is one of the leading causes of cancer-related deaths worldwide and the most frequently diagnosed malignancy in women as of 2020.1 Metastasis of cancer cells to distant organs in BC patients is considered one of the major causes of morbidity and mortality.2,3 Approximately 10% to 16% of patients with metastatic disease develop brain metastases, with the highest frequency occurring in patients with triple-negative tumors, where more than 50% of TNBC patients die within the first 6 months of the metastatic disease.4,5
Paradigm Shift from Reactive Medicine to Predictive and Preventive Approach is Essential to Advance Management of Metastatic Breast Cancer
The rapidly increasing incidence of BM in the TNBC sub-type prompts the development of novel personalized predictive and preventive approaches adapted to the individualized patient’s profile as an advanced strategy toward improved management of metastatic breast cancer. In this framework, a better understanding of the molecular mechanisms and markers that regulate BM of TNBC is clinically relevant to enable the development of novel targeted and personalized predictive and preventive therapeutic options that improves individual outcomes and could be applicable to the secondary care to protect against potential development of metastatic disease.5–7
Molecular Marker-Based Targeted Therapy as Potential Strategy for Prediction and Prevention of BM in TNBC
Recent evidence demonstrated that targeted therapies targeting molecular markers that regulate cancer pathogenesis have clinical potential for the prediction and prognosis of various cancers.8,9 Matrix metalloproteinase-1 (MMP1) has been shown to be a potential molecular target as it plays a pivotal role in promoting extravasation of cancer cells through the brain endothelium (BE), a key step in the brain metastatic cascade. During extravasation, BC cells adhere to the BE then transmigrate between neighboring brain endothelial cells to reach the brain.10 In this process, MMP1 plays an essential role by promoting the degradation of inter-endothelial junctions such as claudin-5 which allows the transmigration of BC cells across the BE. Knockdown of MMP1 expression was shown to suppress brain metastasis in vivo by preserving the endothelial barrier integrity which reduces extravasation of metastatic breast cancer cells through the BE.11–15 In patients, high levels of MMP1 were detected in brain metastatic TNBC tumors compared to primary tumors, and MMP1 was the only metalloproteinase to be significantly correlated with triple-negative breast cancer brain metastasis.11 Despite its important role in brain metastasis, the molecular mechanisms involved in the upregulation of MMP1 in TNBC cells is not fully understood.
In the last years, several evidence has suggested microRNAs (miRNAs) as interesting biomarkers in cancer because of their prominent role in the molecular pathogenesis of cancer development and metastasis.16 Interestingly, these small non-coding RNAs have been identified as critical post-transcriptional regulators of MMP1 gene expression.13,17–22
Working Hypothesis
In a previous study, we analyzed, using the GEO2R tool, the microRNA array cohort data (GSE37407) which contain global miRNA expression profiles of 47 tumor samples from 14 patients with paired samples from primary breast tumors of different sub-types and corresponding distant metastases.23–25 We identified three potential microRNAs (miR-202-3p, miR-623, and miR-145) as potential critical regulators of MMP1. Indeed, our analysis showed that these microRNAs, previously found to target the 3ʹUTR of MMP1 in scleroderma fibrosis and pancreatic cancer, were significantly expressed at lower levels in BM tumors than in primary tumors suggesting a potential role in upregulating MMP1 in BM cells.13,20,23,26–28 In a previous study, we examined the role of miR-202 in MMP1 regulation in triple-negative breast cancer brain metastatic cells (BCBMC) and showed that downregulation of miR-202-3p increases MMP1 expression in TNBC cells and promotes the invasive cancer phenotype.13 However, the role of miR-623 in the brain metastatic cascade remains to be determined, In the current study, we investigated the role of miR-623 in the regulation of MMP1 expression and examined its impact on the extravasation of TNBC cells using an in vitro model of human BE. The final aim is to help identify a brain metastasis-specific microRNA signature in TNBC that can be used as a guide to a personalized preventive therapy and metastasis prediction.
Materials and Methods
Cell Culture
Two human TNBC cell lines with different BM propensities were used. The BM cell line MDA-MB-231-BrM2 abbreviated as MDA231-BrM2 and the parental cell MDA-MB-231-TGL abbreviated as MDA231 were obtained from Dr. Joan Massagué Laboratory (Memorial Sloan-Kettering Cancer Center, New York, USA).29 The MDA-MB-231 human TNBC cell line was obtained from American Type Culture Collection (Rockville, MD, USA). All TNBC cells were cultured and maintained in Dulbecco′s Modified Eagle′s Medium (DMEM, Sigma-Aldrich, Germany, cat# D5671) supplemented with 1%
To establish the in vitro model of the human BE,13–15 5×104 hCMEC/D3 cells were seeded on the apical side of a thin collagen I (cat# 08–115, EMD Millipore) and fibronectin (cat# F1141, Sigma-Aldrich) pre-coated transwell membrane (growth area 1.12 cm2) until a tight confluent monolayer of hCMEC/D3 cells formed.30,31
Brain Endothelial Barrier Integrity
We evaluated the trans-endothelial electrical resistance (TEER) of the hCMEC/D3 monolayer using an Endohm 12 chamber and an Endohmeter EVOMX (World Precision Instruments) as previously described.13,32,33 The TEER values were subtracted from the electrical resistance of the control inserts and expressed as Ω.cm2 (surface area of the transwell inserts).
Transient Cell Transfection
Synthetic miR-623 inhibitor (Anti-hsa-miR-623 miScript miRNA Inhibitor), miR-623 mimic (Syn-hsa-miR-623 miScript miRNA Mimic, MIMAT0003292: 5’-AUCCCUUGCAGGGGCUGUUGGGU-3’), negative control (miScript Inhibitor Negative Control, cat# 1027271), and scrambled control (AllStars Negative Control, cat# SI03650318) were purchased from Qiagen. To introduce miR-623, the Lipofectamine™ RNAiMAX reagent (Invitrogen, cat# 13778075) was used to transfect MDA231-BrM2 cells at 70% confluence with 5 nM of miR-623 mimic or its scrambled control for 48 hours according to the manufacturer’s instructions. MDA231 cells were transfected with 30 nM of miR-623 inhibitor (a chemically modified antisense single-stranded RNA) or a negative control for 48 hours to inhibit endogenous miR-623 expression according to the manufacturer’s instructions. To inhibit MMP1 expression, MDA231 cells were transfected for 48 hours with 30 nM siRNA (MMP1) (Santa Cruz, cat# sc-41552), or co-transfected with 30 nM miR-623 inhibitor and 30 nM MMP1 siRNA, according to the transfection reagent instructions. Real-time PCR was used to measure transfection efficiency.
For the transfections, cells were seeded till 70% confluence in a 6-well plate. For each well, lipofectamine RNAiMAX Reagent (7.5 μL) was diluted in reduced serum Opti-MEM™ Medium (125 μL) according to the manufacturer’s instructions. Separately, microRNAs mimic (5 nM), inhibitor (30 nM), siRNA (MMP1) (30 nM), and negative or scrambled controls (5 nM) were diluted in reduced serum Opti-MEM™ Medium (125 μL) according to the manufacturer’s instructions. The diluted mimic, inhibitor and/or siRNA were then mixed with the diluted Lipofectamine® RNAiMAX Reagent (1:1 ratio) by vortexing and incubated for 5 minutes at room temperature to allow formation of transfection complexes. The media were then removed from cells and replaced with reduced serum Opti-MEM™ Medium and the transfection complexed added onto cells drop-wise. After 4 hours, the transfection media were removed from cells and replaced with complete media.
Real-Time PCR
Total RNA from MDA231 and MDA231-BrM2 cells was extracted using the miRNeasy Micro Kit (cat# 217084, Qiagen, Germany). A NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) was used to evaluate RNA purity and concentration using two absorbance ratios (A260/A280 and A260/A230). For single-stranded cDNA synthesis, the RT2 First Strand Kit (cat#, 330401, Qiagen) or the miScript II RT Kit (cat#, 218161, Qiagen) was used as per the manufacturer’s protocols. Real-time quantitative PCR was performed on the “Applied Biosystems® StepOne™ Real-Time PCR System” with the RT2 SYBR Green ROX qPCR Mastermix (cat# 330522, Qiagen) for mRNA expression and the miScript SYBR Green PCR Kit (Cat# 218075, Qiagen) for miRNA expression using the “Applied Biosystems® StepOne™ Real-Time PCR System”.13 hsa-miR-623 and MMP1 relative expressions were normalized to the housekeeping gene U6 small nuclear RNA and GAPDH (glyceraldehyde-3-phosphate dehydrogenase), respectively, and the data were analyzed using the 2–ΔCt and 2–ΔΔCt methods.34 The primers used were obtained from Qiagen: Hs_miR-623_1 miScript Primer Assay (Qiagen), Hs_RNU6-2_11 miScript Primer Assay (cat# MS00033740), RT2 qPCR Primer Assay for Human MMP1 (cat# PPH00120B-200), and RT2 qPCR Primer Assay for Human GAPDH (Cat# PPH72843A).
Transwell Migration Assay
Transmigration of transfected or control MDA231 and MDA231-BrM2 cells was assessed as previously described.13,15 Briefly, pre-transfected MDA231 and MDA231-BrM2 cells were labeled with 5 μM of CellTracker™Green CMFDA fluorescent dye (Thermo Fisher Scientific, USA, cat# C2925) as per the manufacturer’s protocol. Then 5×104 labelled BC cells were gently added to the apical chambers of tight hCMEC/D3 monolayers. BC cells were allowed to transmigrate to the basal compartment containing complete growth medium for 24 hours. The remaining cells on the upper chamber were gently removed using a cotton swab, and BC cells that transmigrated to the basal compartment were lysed with RIPA 1× buffer for fluorescence quantification at a wavelength excitation/emission of 492/517 nm using a Varioskan Flash microplate reader (Thermo Fisher Scientific).
Cell Sorting and Western Blot
Pre-transfected MDA231-BrM2 cells were co-cultured for 24 hours with hCMEC/D3 monolayers, which were stably transduced with a lentivirus expressing a triple-fusion reporter (TGL) encoding firefly luciferase and green fluorescence protein (GFP).29 GFP-expressing cancer cells were separated from unlabeled hCMEC/D3 by fluorescence-activated cell sorting (FACS) using the BD FACSAria™ III cell sorter (BD Biosciences, USA). The collected hCMEC/D3 cells were then lysed for western blot analysis according to standard procedures and as previously described.13,15 The following primary antibodies were used: Anti-Claudin 5 (4C3C2) (35–2500) 1/500 (Thermo Fisher Scientific), Anti-VE-cadherin (ab33168) 1/1000, Anti-MMP-1 (ab38929) 1/5000 (Abcam), and Anti-GAPDH (ab9485) 1/7500 (Abcam) (as loading control).
Luciferase Assay
For miR-623 target validation, the wild-type (WT) putative binding site of miR-623 in the 3′UTR of MMP1 predicted by TargetScan (edition7.2) (position 420–426 of MMP1 3’UTR), and the mutant (MUT) 3ʹUTR of MMP1 with the seed region deleted, were cloned into pmirGLO Dual-Luciferase miRNA Target Expression Vector (pmirGLO-empty, Promega, USA) to create Luciferase reporter constructs.13,14 The following primers were used to clone 3′-UTR-MMP1 in pmirGLO vector:
miR623_MMPI_WT_F: 5ʹCTAGTGTGCAGTCACTGGTGTCACCCTGGATAGGCAAGGGATAACTCTTCTAACACAAAATAAGTGTTTTA3’;
miR623_MMPI_WT_R: 5ʹCTAGTAAAACACTTATTTTGTGTTAGAAGAGTTATCCCTTGCCTATCCAGGGTGACACCAGTGACTGCACA3’;
miR623_MMPI_Mut_F: 5ʹCTAGTGTGCAGTCACTGGTGTCACCCTGGATAGGTAACTCTTCTAACACAAAATAAGTGTTTTA3’;
miR623_MMPI_Mut_R: 5ʹCTAGTAAAACACTTATTTTGTGTTAGAAGAGTTACCTATCCAGGGTGACACCAGTGACTGCACA3’;
(100 ng) PmirGLO-MUT, (100 ng) pmirGLO-WT, (20 nM) miR-623 mimic, or negative scrambled control were co-transfected into MDA-MB-231 cells (ATCC) using the Lipofectamine 3000 reagent protocol. The Firefly and Renilla luciferase activities were assessed using the Dual-Luciferase® Re-porter Assay System analysis (cat# E2940, Promega, USA) after 24 hours of transfection according to the manufacturer’s recommendations, and the Firefly activities were subsequently normalized with Renilla luciferase.
Statistical Analysis
Results are presented as the mean±standard deviation (SD) from three to six independent experiments. Independent Student’s t-test was used to compare data between two groups. One-way ANOVA with Tukey post hoc test was used to compare three or four groups, and one-way ANOVA with Bonferroni post hoc test was used to compare five groups. Differences observed were considered statistically significant at probability levels of P<0.05. GraphPad-Prism 8.2.0 was used to analyze the data and generate the figures.
Results
Downregulation of miR-623 in BM Breast Cancer Cells with High BM Propensity
In our previous work, analysis of the top 250 miRNAs differentially expressed between the primary and BM breast tumors was performed from the microRNA array cohort data (GSE37407) using the GEO2R tool.26 Additionally, four prediction databases were used to identify the microRNAs predicted to target the mRNA encoding MMP1 in BC cells. Our analysis identified that three miRNAs (miR-202-3p, miR-623, and miR-145) targeting MMP1 were downregulated in BM tumors compared to breast primary tumors.20,27,28 These miRNAs therefore represented potential candidates for further investigation. In the current study, the role of miR-623 in the regulation of MMP1 in BC cells has been investigated. Comparative expression profile of miR-623 in primary breast tumors and the paired BM tumors extracted from microRNA array cohort data (GSE37407)26 and analyzed with the GEO2R tool23–25 are shown in Figure 1A. These data showed significant downregulation of miR-623 in BM tumor samples compared to samples from primary breast tumors (adjusted p value=0.009). To investigate the regulation of MMP1 by miR-623 in vitro, we used TNBC cell lines with different BM propensities obtained from Dr. Joan Massagué Laboratory (Memorial Sloan-Kettering Cancer Center, New York, USA): the MDA231 (having low BM propensity and expressing low levels of MMP1 expression)13 and its BM variant MDA231-BrM2 (having high BM propensity and expressing high levels of MMP1 expression)13 isolated from nude mice brains following repeated injections of parental MDA231 into the internal carotid artery.29 Specifically, to establish the BrM2 cell line, Bos et al inoculated MDA231 cells into the arterial circulation of immunodeficient female mice. The resulting cell populations in the brain were subjected to a second round of in vivo selection, yielding BrM2 cell populations that showed a significant increase in brain metastatic activity compared to parental MDA231 cells.29 miR-623 expression in the two TNBC cell lines was assessed by real-time PCR. Our results were in accordance with the data obtained from patients’ samples and showed that BM cells express significant lower levels of miR-623 compared to the parental cells (p<0.001) (Figure 1B).
Regulation of MMP1 Expression by miR-623 in TNBC Cells
As a next step, the effect of miR-623 modulation on MMP1 expression in TNBC cells was investigated. Levels of miR-623 were modulated in BC cells by transfection. To induce miR-623, BMBC MDA231-BrM2 cells expressing low levels of miR-623 and high levels of MMP1 were transfected with a synthetic double-stranded miR-623 mimic that stimulates expression of mature miR-623. This transfection significantly increased miR-623 expression in MDA231-BrM2 (p=0.0113, Figure 2A) and this upregulation was concomitant with a significant downregulation of MMP1 gene (fold change=0.13, p=0.005, Figure 2B) and protein expression (fold change=0.03, p=0.0143, Figure 2C) compared to scrambled. miR-623 expression was then downregulated in MDA231 cells expressing higher levels of miR-623 and low levels of MMP1. MDA231 cells were then transfected using miR-623 inhibitor. And this transfection significantly reduces miR-623 expression in MDA231 cells (Figure 2D). This downregulation was associated with upregulation of the gene (fold change=1.6, p=0.04, Figure 2E) and protein expression (fold change=6.7, p=0.0452, Figure 2F) of MMP1. This gain-and-loss of function approach showed that miR-623 regulates MMP1 expression in TNBC where that loss of miR-623 induces MMP1, while the ectopic expression of miR-623 in BM TNBC cells reduces MMP1 levels.
Direct Targeting of the 3ʹUTR of MMP1 by miR-623 in TNBC Cells
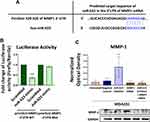
As miR-623 was previously reported to target the 3ʹUTR of MMP1 in pancreatic cancer cells,27 we were interested to examine whether the suppressive effect of miR-623 on MMP1 expression is controlled by direct targeting of the 3ʹUTR of MMP1 mRNA in TNBC cells. To this purpose, a luciferase reporter assay was conducted where co-transfected MDA231 cells with miR-623 mimic and luciferase constructs containing the putative (wild-type WT) or mutated (MUT) binding site of miR-623 in the 3′UTR of MMP1 were designed and prepared. Figure 3A shows the predicted target sequence of miR-623 in the 3ʹUTR of MMP1 mRNA using TargetScan 7.2. In this assay, MDA231 cells with no endogenous firefly luciferase activity were used. Results showed that transfection of cells with miR-623 mimic and pmiRGlo-MMP1-3ʹUTR-WT construct decreased the luciferase activity by approximately 62% (p=0.0036, Figure 3B) compared to scrambled control, while co-transfection of miR-623 with a luciferase construct containing the deletion mutant (pmiRGlo-MMP1-3ʹUTR-Mut) did not affect the luciferase activity (Figure 3B). These results were found similar to previous study conducted on pancreatic cancer cells27 and showed indeed that miR-623 directly targets the 3ʹUTR of MMP1 and regulates its expression in breast cancer cells.
Furthermore, the effect of miR-623 on MMP1 was confirmed by rescue experiments. MDA231 cells were co-transfected with miR-623 inhibitor and MMP1 siRNA and the expression of MMP1 was assessed by western blot. To determine the optimum inhibitory conditions, three concentrations of MMP1 siRNA (5 nM, 15 nM and 30 nM) and three times (12 h, 24 h and 48 h) were initially tested. MMP1 expression was then assessed by real-time PCR. The highest reduction in MMP1 gene expression was observed with the concentration 30 nM (48 h). Results of the rescue experiments showed that MDA231 co-transfected cells prevented the upregulation of MMP1 caused by miR-623 inhibitor excluding off-target effects and therefore confirming the direct regulation of MMP1 by miR-623 (Figure 3C).
BE Extravasation is Attenuated by Restoration of miR-623 Expression in BM Breast Cancer Cells
Since MMP1 plays a key role in BM by promoting extravasation of TNBC cells through the BE,11–15 the effect of miR-623 on the extravasation of TNBC cells was evaluated in an in-vitro model of the brain endothelial barrier cultured on transwell. The hCMEC/D3 cell line known to retain the characteristic of primary brain endothelial cells was used.30,31 Results showed that transfection of MDA-BrM2 cells having high transmigratory capacity13–15 with miR-623 mimic reduces the transmigration by 76% (p=0.0341, Figure 4A). MMP1 is known to promote transmigration by degrading the inter-endothelial junctions of the brain endothelial barrier and increasing its permeability. To this purpose, the effect of miR-623 restoration on the integrity of the hCMEC/D3 monolayers and on the expression of the two inter-endothelial junctions considered as major contributors to the barrier integrity, claudin-5 and VE-cadherin, was evaluated. Integrity of the hCMEC/D3 monolayers was examined by TEER measurement. Our results showed that control monolayers had TEER values above 60 Ω.cm2. MDA231-BrM2 transfected with scrambled microRNA reduced the monolayer’s TEER by 59% (p=0.0022) while MDA231-BrM2 transfected with miR-623 mimic did not cause significant reduction in the TEER compared to hCMEC/D3 monocultures (Figure 4B). Western blot analysis of claudin-5 and VE-cadherin showed a significant reduction in their expression by MDA231-BrM2. Transfection of MDA231-BrM2 with miR-623 mimic restored normal expression of VE-cadherin (Figure 4C), and partially restored claudin-5 expression (Figure 4D) suggesting therefore that restoration of miR-623 expression in BM TNBC reduced transmigration through the BE and preserved the endothelial barrier integrity. miR-623 could be therefore used as a novel therapeutic target to suppress extravasation of TNBC during BM.
Discussion
miR-623/MMP1 Axis as a Molecular Target for the Prediction and Targeted Prevention of BM on TNBC
A better understanding of the molecular mechanisms and markers that regulate BM of TNBC enables the development of novel targeted and personalized predictive and preventive therapeutic options that improves individual outcomes and could be applicable to the secondary care to protect against potential development of metastatic disease.5–7 In the current study, we provided first-time evidence about the regulation of MMP1 by miR-623 in triple-negative BMBC cells. miR-623 expression is reduced in BM cells compared to parental cells. Downregulation of miR-623 induces MMP1 while ectopic expression of miR-623 reduces MMP1 expression in BM cells and attenuates their transmigration through the BE. These findings provide additional understanding about the molecular mechanisms driving extravasation of TNBC cells, a key step in BM, and shed light on miR-623 as molecular target to predict and prevent BM. Importantly, the present study helps in unraveling a brain metastasis-specific microRNA signature in TNBC that can be used as a guide to a personalized preventive therapy and metastasis prediction.
BM of breast cancer cells is a multi-phase process, starting with tumor cell detachment from primary tumors, followed by intravasation into the blood, survival in the blood circulation, arrest and adhesion to the BE, extravasation through the endothelial barrier and brain colonization.10 Normally, the brain is protected from substances and cells circulating in the blood by physiological barriers, particularly the blood–brain barrier (BBB).35 The brain endothelial cells (BECs) are key components forming the BBB. These cells are tightly linked to each other by adherens and tight junctional complexes, restricting substances and cells paracellular movement.36 The tight junctional complexes are composed of transmembrane proteins (Occludin, Claudins, and Junction-Associated Molecules) connected to the actin cytoskeleton through scaffolding proteins (Zonula-Occludens ZO1, ZO2, ZO3) which are in their turn linked to the cellular actin/myosin cytoskeletal system via cingulin dimers. The adherens junctional complexes are composed of cadherins, transmembrane proteins that are linked to the actin cytoskeleton via specific intermediary cytoplasmic proteins like plakoglobin and β-catenin.37 During BM, BC cells release MMP1 that degrades the inter-endothelial junctions, allowing BC cells to transmigrate through the brain endothelial barrier and enter the brain.11 Wu et al previously analyzed the clinical significance of 21 matrix metalloproteinases on breast cancer patients’ BM-free survival and found that only MMP1 is significantly correlated with BM.11 It was found that MMP1 degrades claudin-5 and occludin which promotes the extravasation through the BBB and subsequently BM.11–15
Due to MMP1ʹs pro-metastatic role in tumor progression, synthetic inhibitors have been developed and trialed in several types of cancer, however these trials in patients with advanced metastatic disease were unable to prove a survival benefit.38 An explanation for the failure of these synthetic MMPs inhibitors in clinical trials was their lack of selectivity and ability to target multiple MMPs, resulting in severe side effects. It is also crucial to highlight that while MMP1 is involved in the early stages of metastasis, the MMP1 inhibitors were tested in patients with advanced metastatic diseases. In this context, further investigations are needed to understand the molecular mechanisms behind the upregulation of MMP expression, particularly MMP1 in BM in order to develop more specific and efficient MMP inhibitors.
MicroRNA, Key Regulators of MMP1 Expression, as Potential Biomarkers for Cancer Metastasis
In the last years, several evidence has suggested microRNAs as interesting biomarkers in cancer because of their prominent role in the molecular pathogenesis of cancer development and metastasis.16 Interestingly, increasing numbers of studies have demonstrated that endogenous microRNAs play a significant role in regulating MMP1 expression. MMP1 was shown to be targeted and regulated by miR-222 in fibroblasts and tongue squamous cell carcinoma,21,39 miR-526b in skin cells, miR-675 in chondrocytes,17 miR-330-5p in esophageal adenocarcinoma,18 miR-202-3p in scleroderma fibrosis,20 miR-361-5p and miR-202-3p in BC,13,19 and by miR-623 in pancreatic cancer cells.27 In BM, breast metastatic tumors were shown to have altered expressions of microRNAs compared to primary tumors, and these alterations are suggested to play a key role in BM.13–15,23,26,40 In search of the microRNAs that could be involved in MMP1 upregulation in BM tumors, we performed a bioinformatic analysis of the clinical microRNA array cohort data (GSE37407) with the GEO2R tool.23–25 This analysis revealed three microRNAs, specifically miR-202-3p, miR-145, and miR-623, are known to target MMP1 and shown to be downregulated in BM tumors compared to primary tumors in patients.13,23,26 We previously proved that silencing miR-202 in TNBC cells induces MMP1 expression and promotes a brain invasive phenotype.13 However, the role of miR-623 and miR-145 in the regulation of MMP1 in TNBC cells and in BM remains unexplored.
In the current study, the effect of miR-623 on MMP1 regulation in TNBC cells was investigated. miR-623 was previously shown to directly suppress MMP1 in pancreatic cancer cells and to attenuate their metastasis.27 The anti-metastatic role of miR-623 has also been demonstrated in other types of cancers. For instance, in glioblastoma, miR-623 overexpression attenuated the migration and invasion of glioblastoma cells.41 miR-623 was also shown to suppress invasion of BC in vitro.42 In this study, we demonstrated that BM TNBC cells express lower levels of miR-623 compared to their parental cells. Silencing miR-623 in TNBC cells with low BM propensity induces MMP1 expression. Importantly, restoring miR-623 expression in BM TNBC reduces MMP1 and attenuates their transmigration through an in-vitro model of the BE. These findings suggest that loss of miR-623 could be a critical event leading to MMP1 upregulation in BC tumors and miR-623 restoration could serve as a therapeutic strategy to prevent upregulation of MMP1 in BM tumors and therefore to prevent extravasation of BC cells into the brain. However, further in vivo studies are needed to confirm the protective role of miR-623 against BM.
Conclusion
The current study demonstrates for the first time the involvement of miR-623 in the regulation of MMP1 and the control of the transmigration of TNBC through the BE. Exogenous miR-623 restoration was found to inhibit MMP1 expression and decrease metastatic TNBC cells' trans-endothelial migration. Taken together, these findings elucidate the crucial role of miR-623 as an MMP1 direct regulator in BM breast cancer cells providing new insights into the regulation of cancer cell extravasation through the brain endothelium, a key step in the BM cascade. miR-623 could therefore be exploited as a novel therapeutic target to predict and prevent brain metastasis in TNBC. Importantly, the presents study helps in unraveling a brain metastasis-specific microRNA signature in TNBC which enables the development of novel personalized predictive and preventive approaches tailored to the individualized patient profile that improves individual outcomes and could be applicable to the secondary care to protect against potential development of metastatic disease.
Funding
This research was funded by the Terry Fox Foundation’s International Run Program, grant number I1032 and the University of Sharjah Competitive Grant, grant number 210111350.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71(3):209–249. doi:10.3322/caac.21660
2. Aversa C, Rossi V, Geuna E, et al. Metastatic breast cancer subtypes and central nervous system metastases. Breast. 2014;23(5):623–628. doi:10.1016/j.breast.2014.06.009
3. Kodack DP, Askoxylakis V, Ferraro GB, Fukumura D, Jain RK. Emerging strategies for treating brain metastases from breast cancer. Cancer Cell. 2015;27(2):163–175. doi:10.1016/j.ccell.2015.01.001
4. Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. JCO. 2010;28(20):3271–3277. doi:10.1200/JCO.2009.25.9820
5. Mazurakova A, Koklesova L, Samec M, et al. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 2022;13(2):315–334. doi:10.1007/s13167-022-00277-2
6. Golubnitschaja O, Liskova A, Koklesova L, et al. Caution, “normal” BMI: health risks associated with potentially masked individual underweight-EPMA position paper 2021. EPMA J. 2021;12(3):243–264. doi:10.1007/s13167-021-00251-4
7. De Francesco EM, Cirillo F, Vella V, Belfiore A, Maggiolini M, Lappano R. Triple-negative breast cancer drug resistance, durable efficacy, and cure: how advanced biological insights and emerging drug modalities could transform progress. Expert Opin Ther Targets. 2022;26:513–535. doi:10.1080/14728222.2022.2094762
8. Yerukala Sathipati S, Ho SY. Identifying a miRNA signature for predicting the stage of breast cancer. Sci Rep. 2018;8(1):16138. doi:10.1038/s41598-018-34604-3
9. Torres Crigna A, Link B, Samec M, Giordano FA, Kubatka P, Golubnitschaja O. Endothelin-1 axes in the framework of predictive, preventive and personalised (3P) medicine. EPMA J. 2021;12(3):265–305. doi:10.1007/s13167-021-00248-z
10. Custódio-Santos T, Videira M, Brito MA. Brain metastasization of breast cancer. Biochim Biophys Acta. 2017;1868(1):132–147. doi:10.1016/j.bbcan.2017.03.004
11. Wu K, Fukuda K, Xing F, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–9854. doi:10.1074/jbc.M114.602185
12. Liu H, Kato Y, Erzinger SA, et al. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer. 2012;12(1):583. doi:10.1186/1471-2407-12-583
13. Harati R, Hafezi S, Mabondzo A, Tlili A. Silencing miR-202-3p increases MMP-1 and promotes a brain invasive phenotype in metastatic breast cancer cells. PLoS One. 2020;15(10):e0239292–e0239292. doi:10.1371/journal.pone.0239292
14. Harati R, Mohammad MG, Tlili A, El-Awady RA, Hamoudi R. Loss of miR-101-3p promotes transmigration of metastatic breast cancer cells through the brain endothelium by inducing COX-2/MMP1 signaling. Pharmaceuticals. 2020;13(7):144. doi:10.3390/ph13070144
15. Harati R, Mabondzo A, Tlili A, Khoder G, Mahfood M, Hamoudi R. Combinatorial targeting of microRNA-26b and microRNA-101 exerts a synergistic inhibition on cyclooxygenase-2 in brain metastatic triple-negative breast cancer cells. Breast Cancer Res Treat. 2021;187(3):695–713. doi:10.1007/s10549-021-06255-y
16. Crigna AT, Samec M, Koklesova L, et al. Cell-free nucleic acid patterns in disease prediction and monitoring—hype or hope? EPMA J. 2020;11(4):603–627. doi:10.1007/s13167-020-00226-x
17. Kim KH, Jung JY, Son ED, Shin DW, Noh M, Lee TR. miR-526b targets 3′ UTR of MMP1 mRNA. Exp Mol Med. 2015;47(8):e178–e178. doi:10.1038/emm.2015.52
18. Bibby BAS, Miranda CS, Reynolds JV, Cawthorne CJ, Maher SG. Silencing microRNA-330-5p increases MMP1 expression and promotes an invasive phenotype in oesophageal adenocarcinoma. BMC Cancer. 2019;19(1):784. doi:10.1186/s12885-019-5996-3
19. Ma F, Zhang L, Ma L, Zhang Y, Zhang J, Guo B. MiR-361-5p inhibits glycolytic metabolism, proliferation and invasion of breast cancer by targeting FGFR1 and MMP-1. J Exp Clin Cancer Res. 2017;36(1):158. doi:10.1186/s13046-017-0630-1
20. Zhou B, Zhu H, Luo H, et al. MicroRNA-202-3p regulates scleroderma fibrosis by targeting matrix metalloproteinase 1. Biomed Pharmacother. 2017;87:412–418. doi:10.1016/j.biopha.2016.12.080
21. Liu X, Yu J, Jiang L, et al. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genom. 2009;9:131–139.
22. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi:10.1038/nrg1379
23. Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23(14):1846–1847. doi:10.1093/bioinformatics/btm254
24. Smyth GK. Limma: linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005:397–420. doi:10.1007/0-387-29362-0_23
25. Smyth GK. Linear models and Empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3(1):1–25. doi:10.2202/1544-6115.1027
26. Gravgaard KH, Lyng MB, Laenkholm AV, et al. The miRNA-200 family and miRNA-9 exhibit differential expression in primary versus corresponding metastatic tissue in breast cancer. Breast Cancer Res Treat. 2012;134(1):207–217. doi:10.1007/s10549-012-1969-9
27. Chen Y, Peng S, Cen H, et al. MicroRNA hsa-miR-623 directly suppresses MMP1 and attenuates IL-8-induced metastasis in pancreatic cancer. Int J Oncol. 2019. doi:10.3892/ijo.2019.4803
28. Azmi AS, Li Y, Muqbil I, et al. Exportin 1 (XPO1) inhibition leads to restoration of tumor suppressor miR-145 and consequent suppression of pancreatic cancer cell proliferation and migration. Oncotarget. 2017;8(47):82144–82155. doi:10.18632/oncotarget.19285
29. Bos PD, Zhang XHF, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi:10.1038/nature08021
30. Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10(1):16. doi:10.1186/2045-8118-10-16
31. Poller B, Gutmann H, Krähenbühl S, et al. The human brain endothelial cell line hCMEC/D3 as a human blood-brain barrier model for drug transport studies. J Neurochem. 2008;107(5):1358–1368. doi:10.1111/j.1471-4159.2008.05730.x
32. Harati R, Hammad S, Tlili A, Mahfood M, Mabondzo A, Hamoudi R. miR-27a-3p regulates expression of intercellular junctions at the brain endothelium and controls the endothelial barrier permeability. PLoS One. 2022;17(1):e0262152. doi:10.1371/journal.pone.0262152
33. Hammad S, Mabondzo A, Hamoudi R, Harati R. Regulation of P-glycoprotein by miR-27a-3p at the Brain Endothelial Barrier. J Pharm Sci. 2021;S0022354921005621. doi:10.1016/j.xphs.2021.10.021
34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
35. Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi:10.1101/cshperspect.a020412
36. Greene C, Campbell M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers. 2016;4(1):e1138017. doi:10.1080/21688370.2015.1138017
37. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
38. Winer A, Adams S, Mignatti P. Matrix metalloproteinase inhibitors in cancer therapy: turning past failures into future successes. Mol Cancer Ther. 2018;17(6):1147–1155. doi:10.1158/1535-7163.MCT-17-0646
39. Zhang Y, Lin X, Zhang L, Hong W, Zeng K. MicroRNA-222 regulates the viability of fibroblasts in hypertrophic scars via matrix metalloproteinase 1. Exp Ther Med. 2017. doi:10.3892/etm.2017.5634
40. Xing F, Sharma S, Liu Y, et al. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-α. Oncogene. 2015;34(37):4890–4900. doi:10.1038/onc.2014.412
41. Cui D, Wang K, Liu Y, Gao J, Cui J. MicroRNA-623 inhibits epithelial-mesenchymal transition to attenuate glioma proliferation by targeting TRIM44. Onco Targets Ther. 2020;13:9291–9303. doi:10.2147/OTT.S250497
42. Wang C, Wang J, Zhang J, et al. MicroRNA-623 inhibits tumor progression and is a predictor of poor prognosis of breast cancer. Oncol Lett. 2020;20(6):386. doi:10.3892/ol.2020.12249
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.