Back to Journals » OncoTargets and Therapy » Volume 11
miR-517a promotes Warburg effect in HCC by directly targeting FBP1
Authors Zhang D, Li Z, Li T, Luo D , Feng X, Liu Y, Huang J
Received 24 April 2018
Accepted for publication 9 August 2018
Published 13 November 2018 Volume 2018:11 Pages 8025—8032
DOI https://doi.org/10.2147/OTT.S172084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianmin Xu
Delin Zhang,1,* Zhu Li,2,* Tao Li,1 Dan Luo,1 Xinfu Feng,1 Yan Liu,1 Jianzhao Huang1
1Department of Hepatobiliary Surgery, Guizhou People’s Hospital, Guiyang 550002, China; 2Department of Hepatobiliary Surgery, Affiliated Hospital of Guizhou Medical University, Guiyang 550002, China
*These authors contributed equally to this work
Purpose: Hepatocellular carcinoma (HCC) is one of the most aggressive malignancies worldwide. Our aim is to explore the expression and biological function of miR-517a in HCC.
Materials and methods: We performed qRT-PCR to detect the expression of miR-517a in clinical samples and cell lines. CKK-8 assay and colony formation assay were employed to detect the miR-517a regulated cell proliferation. Glucose uptake and lactate production were examined to determine the Warburg effect. We also performed ECAR assay using Seahorse system. Luciferase acitivy assay was used to examine the binding of FBP1 3’UTR by miR-517a.
Results: miR-517a was upregulated in HCC samples in both genomic and mRNA levels. Moreover, overexpression of miR-517a promoted cell proliferation and Warburg effect. Mechanically, miR-517a could directly target the 3′-UTR of FBP1. In addition, restoring the expression of FBP1 inhibited cell growth.
Conclusion: We demonstrated that miR-517a acts as an oncogene to promote Warburg effect in HCC, favoring tumor growth, and miR-517a/FBP1 could be a novel target for HCC treatment.
Keywords: miR-517a, HCC, FBP1, Warburg effect
Introduction
Hepatocellular carcinoma (HCC) is the fifth most frequently occurring cancer and the third leading cause of cancer-related death.1,2 Most patients are found in the advanced stage, and the prognosis of HCC is extremely poor due to the frequent metastasis, relapse, and chemotherapy resistance.3–5 Therefore, understanding the molecular mechanisms underlying the initiation and development of HCC may lead to the improvement of clinical treatment.
Tumor cells have a rapid proliferation rate, requiring the unique metabolism pattern, which is well recognized as “aerobic glycolysis” or “Warburg effect”.6,7 It is characterized by cells metabolizing glucose to lactate irrespective of oxygen availability and producing intermediary metabolites to support the cell dividing. The Warburg effect is observed in various types of cancers, such as HCC, colorectal carcinoma, breast cancer, and ovarian cancer.8–11 Numerous evidence demonstrates that Warburg effect is critical for the growth of tumor cells and metastasis.12 Fructose-1,6-bisphosphatase (FBP1), a limiting enzyme of gluconeogenesis, is well established to be involved in metabolic regulation.13 In basal-like breast cancer (BCLC), FBP1 could be epigenetically silenced by Snail and therefore promotes tumor growth and metastasis of BLBC cells.14 FBP1 has also been shown to be functionally lost in lung cancer and renal clear cell carcinoma.15,16 However, whether FBP1 contributes to the Warburg effect in HCC has not been explored yet.
microRNAs (miRNAs) can modulate various cellular functions by posttranscriptionally regulating target genes. Recent studies have reported that several miRNAs have potential roles in aerobic glycolysis. For instance, miR-199a-5p targets hexokinase 2 and regulates glycolysis in HCC.17 miR-34a targets lactate dehydrogenase A in cervical cancer.18 miR-122 regulates PKM2 and is involved in breast cancer metastasis.19 In the present study, our aim was to investigate the new relationship between miRNA and key enzyme of Warburg effect in HCC. We demonstrated for the first time that miR-517a was dominantly overexpressed in HCC tissues and FBP1 directly targeted miR-517a. Inhibition of miR-517a or restoring the expression of FBP1 suppressed HCC cell proliferation. These results provided a new insight into the role of miR-517a in deregulated cellular metabolism of HCC and implied a strategy for inhibiting HCC development.
Materials and methods
Ethics statements and patient samples
The study was approved by the ethics committee of Guizhou People’s Hospital. In all, 167 cases of HCC tissue samples and matched adjacent normal samples were collected from the Department of Hepatobiliary Surgery, Guizhou People’s Hospital, between June 2014 and August 2016. All patients provided written consent forms. The clinicopathological features of 167 cases of HCC patients are given in Table 1.
  | Table 1 Clinicopathological features of 167 HCC patients |
Cell lines and reagents
The cell lines HEK293T and LO2 and HCC cell lines Huh7, SK-hep1, SMMC7721, and PLC/PRF/5 were from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and antibiotics (penicillin, streptomycin). Cells were maintained under 5% CO2 atmosphere and 37°C.
The miR-517a mimic, inhibitor, and controls were purchased from RiboBio (Guangzhou, China). The sequences of miR-517a mimic and inhibitor were as follows: AUCGUGCAUCCCUUUAGAGUGU and ACACUCUAAAGGGAUGCACGAU. FBP1 siRNAs were purchased from GenePharma (Shanghai, China). Expression plasmid with FBP1 ORF was purchased from GeneCopoeia (Guangzhou, China).
Cell transfection
Transfection of miR-517a mimics, inhibitors, and vectors was performed using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol as described previously.20 Cells were tested in 48 hours after transfection.
Western blot
Cells were lysed in RIPA buffer (Pierce, USA, Rockford, IL, USA) with protease inhibitor and phosphorylase inhibitor (Hoffman-La Roche Ltd., Indianapolis, IN, USA). Total protein concentration was measured by bicinchoninic acid (BCA) method (Boster, Wuhan, China) according to the protocol. In all, 60 μg protein was loaded and separated by 10% SDS-PAGE and then transferred onto polyvinylidenefluoride (PVDF) membrane. The membrane was incubated with primary antibody at 4°C overnight and (HRP)-conjugated secondary antibody for 1 hour. The band was visualized by sensitive electrochemiluminescence (ECL) method. The primary antibodies anti-β-tubulin (#ab6046), anti-PCNA (#ab29), and anti-FBP1 (#ab109020) were from Abcam (USA, Cambridge, MA, USA).
Quantitative real-time PCR
Total RNA isolation, reverse transcription, and miRNAs’ real-time PCR were performed as described previously.21 Real-time PCR for mRNA detections was performed by SYBR Green methods (Takara, Dalian, China). The samples were analyzed by ABI-7300 System (Thermo Fisher Scientific). The relative expressions of miRNA and mRNA were measured by the comparative 2−ΔΔCt method as described previously.22 The primers used were as follows: FBP1, 5′-CGCGCACCTCTATGGCATT-3′ and 5′-TTCTTCTGACACGAGAACACAC-3′ and β-tubulin, 5′-TGGACTCTGTTCGCTCAGGT-3′ and 5′-TGCCTCCTTCCGTACCACAT-3′.
Cell proliferation and colony formation assays
The cell proliferation assay was performed by the CCK-8 method (Boster). Cells were seeded in the 96-well plates and measured by adding 10 μL CCK-8 per well following the protocol. For colony formation assay, indicated cells were plated in the six-well plates and cultured for other 2 weeks. Cells were stained with 0.1% crystal violet and calculated using the microscope.
Luciferase reporter assay
Cells were plated in 48-well plates and co-transfected with miR-517a mimics, firefly luciferase reporter containing wild-type or mutant 3′ UTR of FBP1, and Renilla reporter. Cells were harvested after 48 hours and measured by Dual-Luciferase® Reporter Assay (Promega Corporation, Fitchburg, WI, USA) according to the protocol.
Glucose uptake and lactate production assay
Cells were calculated and seeded in six-well plates. The medium was replaced with fresh complete medium and incubated for additional 48 hours. The medium was collected for glucose uptake and lactate production assay by measuring the concentration of glucose and lactate. Glucose levels were measured using Glucose Assay Kit (#510A; Sigma-Aldrich, St Louis, MO, USA). Lactate levels were measured by Lactate Assay Kit (#ab65331; Abcam) according to the protocol. The data were normalized to the sum of total cellular protein as described previously.23–25
Extracellular acidification rate (ECAR) assay
The ECAR was measured by the Seahorse XF24 Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA) according to the manufacturer’s instruction. Briefly, indicated cells were seeded in the plate and measured for the glycolytic rate by adding glucose, oligomycin, and 2-deoxyglucose subsequently. The data were normalized to cell number.
Statistical analyses
All statistical analyses were performed using SPSS 21.0 and visualized with GraphPad Prism 6.0. The Mann–Whitney U test was performed to measure the difference in miR-517a and FBP1 expressions between normal and HCC tissues. Pearson’s correlation analysis was used to measure the association between miR-517a and FBP1 expressions. Unpaired Student’s t-test (two tailed) was used to measure the difference between each group in the cells’ experiments. One-way ANOVA analysis was used to measure the difference between more than two groups. Data are shown as mean±standard error of measurement (SEM). A P-value less than 0.05 was considered as statistically significant.
Results
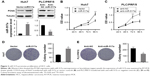
miR-517a is upregulated in HCC tissues and cell lines
To assess the potential functions of miR-517a in HCC, we initially explored the expression of miR-517a in HCC tissues both from public databases and our study samples. As shown in Figure 1A, gene miR-517a was amplified in HCC samples both in The Cancer Genome Atlas (TCGA) dataset and Asan Medical Center (AMC) dataset (cBioPortal, http://www.cbioportal.org). Then, in 167 cases, we found that miR-571a expression was significantly higher in HCC tissues compared to matched adjacent normal tissues (Figure 1B) by real-time PCR (RT-PCR) analysis. Moreover, miR-517a expression was also higher in four HCC cell lines compared to the immortal liver cell line LO2 (Figure 1C). Taken together, these data suggested that miR-517a might play as an oncogene in HCC.
miR-517a promotes cell proliferation
To further assess the functional role of miR-517a in HCC, we performed gain and loss function assays. We used Huh7 cell with lower miR-517a to perform miR-517a overexpression and PLC/PRF/5 cell line with a higher miR-517a expression to perform knocking down. We noticed that the expression of PCNA, a marker for cell proliferation, was elevated after miR-517a overexpression but decreased after miR-517a inhibition (Figure 2A, upper panel). The efficacy of miR-517a overexpression and inhibition was also confirmed (Figure 2A, lower panel). We found that overexpression of miR-517a induced a significantly rapid cell growth rate, measured by CCK-8 assay and colony formation assay (Figure 2B and D). Similarly, using miR-517a inhibitor showed converse results (Figure 2C and E). These data suggested that miR-517a promoted proliferation of HCC cells.
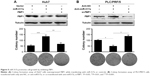
miR-517a enhances Warburg effect in HCC cells
Recent studies indicated that Warburg effect could lead to cell proliferation in a number of solid tumors. We sought to determine whether miR-517a affected Warburg effect. Compared with the control group, overexpression of miR-517a resulted in an increased glucose uptake and lactate production, while knocking down of miR-517a decreased these effects (Figure 3A and B). These results indicated that miR-517a could significantly enhance cell aerobic glycolysis. More importantly, we tested the cell ECAR, which is a key indicator of aerobic glycolysis. We found that miR-517a promoted the rate of extracellular acidification (Figure 3C), while inhibition of miR-517a decreased the ECAR (Figure 3D). These data suggested that miR-517a might be a key regulator of aerobic glycolysis in HCC cells.
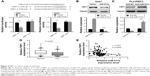
FBP1 is a direct target of miR-517a
Given the observation earlier, we investigated the molecular mechanisms underlying the function of miR-517a. We explored the potential target of miR-517a by TargetsScan (www.targetscan.org). In addition, the target might better be involved in the Warburg effect. We found that FBP1 was a potential target of miR-517a (Figure 4A, upper panel). To verify this finding, a dual-luciferase reporter assay was first performed. Co-transfection of miR-517a significantly suppressed the luciferase activity with only wild-type 3′ UTR of FBP1 but not that of the mutant type, both in 293 T cells and HCC cells, indicating that miR-517a could directly bind to the 3′ UTR of FBP1 (Figure 4A, lower panel). Furthermore, overexpression of miR-517a showed a decreased mRNA and protein expression of FBP1 (Figure 4B), and knockdown of miR-517a increased the FBP1 expression (Figure 4C). To further verify the FBP1 expression in clinical samples, we used RT-PCR to test the mRNA level of FBP1 in HCC tissues and matched normal tissues. We found that FBP1 expression was significantly higher in normal tissues compared to that in HCC tissues (Figure 4D). More importantly, we noticed the inverse correlation between miR-517a and FBP1 expression levels, suggesting that FBP1 was more likely decreased in patients with a higher expression of miR-517a (Figure 4E). Taken together, these data suggested that FBP1 was a direct target of miR-517a in HCC.
FBP1 is involved in miR-517a-induced cell proliferation
To further verify whether miR-517a regulates the Warburg effect through FBP1, we subsequently explored the FBP1 ectopic expression and miR-517a overexpression. The FBP1 expression was examined by Western blot. FBP1 expression was decreased after miR-517a overexpression, and restored after ectopic FBP1 expression (Figure 5A upper panel). Notably, overexpression of FBP1 attenuated miR-517a-induced cell proliferation (Figure 5A, lower panel). Knocking down of FBP1 reversed the inhibition of cell proliferation caused by miR-517a inhibition (Figure 5B). Collectively, these data suggested that miR-571a regulated the Warburg effect and cell proliferation by targeting FBP1.
Discussion
miR-517a was first identified as placental-specific miRNA in maternal circulation to diagnose pregnancy.26,27 However, its biological role in cancer needs to be further addressed. miR-517a is reported to accelerate lung cancer cell invasion by inhibiting FOXJ3 expression.28 Recently, Toffanin et al29 reported that miR-517a from chromosome 19q13.42 was overexpressed and HCC tissues and miR-517a promoted tumorigenesis and metastatic dissemination in vivo. However, the precise mechanisms remained largely unknown. In the present study, we focused on the potential role of miR-517a in regulating Warburg effect and contributing to the metabolic reprogramming.
According to the public database, our data demonstrated that miR-517a was gnomically amplified in HCC tissues. Although the amplification rate was less than 1%, it could partially explain the overexpression of miR-517a. Moreover, the DNA methylation of miR-517a promoter regions requires further investigation by us, which might also contribute to the upregulation of miR-517a. We further found that miR-517a promoted cell proliferation by the gain and loss function analysis, which was consistent with a previous report.29 We noticed that miR-517a enhanced the Warburg effect, and induced the acidic tumor microenvironment.
FBP1 is a direct target of miR-517a, which has not been mentioned previously. Dai et al30 demonstrated that FBP1 also was a target of miR-21, and they observed negative correlation between miR-21 and FBP1 in non-small-cell lung cancer samples. As miRNAs could exert their functions through multiple target genes, a gene could be regulated by multiple miRNAs as well. Furthermore, we examined the FBP1 expression in HCC tissues and normal tissues and found the inverse expression relationship between FBP1 and miR-517a in HCC tissues (r=−0.21, P=0.0064). These results could verify the regulation pattern of miR-517a and FBP1 in clinical samples. Subsequently, we observed that enhancement of the aerobic glycolysis and cell proliferation by miR-517a were dependent on FBP1, as restoration of FBP1 expression abrogated the effect of overexpression of miR-517a. FBP1 is a key regulator of glycolysis and gluconeogenesis. It was found to be downregulated or functionally lost in many types of tumors, such as pancreatic cancer and lung cancer.15,31 This might explain the enhanced Warburg effect in tumor cells. In addition, the methylation levels of FPB1 promoter could be an independent prognostic factor of gastric cancer.32 Here, our study suggested that FBP1 could be posttranscriptionally regulated by miRNAs as well.
Conclusion
By exploring the clinical tissue samples and cell lines, we have identified a regulatory mechanism of Warburg effect and a novel role of miR-517a. These results suggested that targeting miR-517a or restoring FBP1 could be a potential strategy to reverse the Warburg effect and aggressive phenotype in HCC.
Disclosure
The authors report no conflicts of interest in this work.
References
Brahmania M, Ahmed O, Kelley M, et al. Wait Time for Curative Intent Radio Frequency Ablation is Associated with Increased Mortality in Patients with Early Stage Hepatocellular Carcinoma. Ann Hepatol. 2017;16(5):765–771. | ||
Wu W, Fang D, Shi D, Bian X, Li L. Effects of marital status on survival of hepatocellular carcinoma by race/ethnicity and gender. Cancer Manag Res. 2018;10:23–32. | ||
Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150(4):835–853. | ||
Zhu K, Dai Z, Pan Q, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2011;17(23):7294–7302. | ||
Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. | ||
vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. | ||
Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. | ||
Iansante V, Choy PM, Fung SW, et al. PARP14 promotes the Warburg effect in hepatocellular carcinoma by inhibiting JNK1-dependent PKM2 phosphorylation and activation. Nat Commun. 2015;6:7882. | ||
Schell JC, Olson KA, Jiang L, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56(3):400–413. | ||
Takeda S, Okazaki H, Kudo T, et al. Bongkrekic Acid as a Warburg Effect Modulator in Long-term Estradiol-deprived MCF-7 Breast Cancer Cells. Anticancer Res. 2016;36(10):5171–5182. | ||
Caneba CA, Yang L, Baddour J, et al. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014;5:e1302. | ||
Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2 Pt A):156–164. | ||
Zaragoza O, Gancedo JM. Elements from the cAMP signaling pathway are involved in the control of expression of the yeast gluconeogenic gene FBP1. FEBS Lett. 2001;506(3):262–266. | ||
Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23(3):316–331. | ||
Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411(1–2):331–340. | ||
Li B, Qiu B, Lee DS, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513(7517):251–255. | ||
Guo W, Qiu Z, Wang Z, et al. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology. 2015;62(4):1132–1144. | ||
Zhang R, Su J, Xue SL, et al. HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 2016;6(2):312–320. | ||
Fong MY, Zhou W, Liu L, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. | ||
Qian Y, Wang B, Ma A, et al. USP16 Downregulation by Carboxyl-terminal Truncated HBx Promotes the Growth of Hepatocellular Carcinoma Cells. Sci Rep. 2016;6:33039. | ||
Matsumura T, Sugimachi K, Iinuma H, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer. 2015;113(2):275–281. | ||
Feng X, Liu N, Deng S, Zhang D, Wang K, Lu M. miR-199a modulates cisplatin resistance in ovarian cancer by targeting Hif1α. Onco Targets Ther. 2017;10:5899–5906. | ||
Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115(4):449–459. | ||
Zhao YH, Zhou M, Liu H, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28(42):3689–3701. | ||
Bernier M, Paul RK, Martin-Montalvo A, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286(22):19270–19279. | ||
Kotlabova K, Doucha J, Hromadnikova I. Placental-specific microRNA in maternal circulation – identification of appropriate pregnancy-associated microRNAs with diagnostic potential. J Reprod Immunol. 2011;89(2):185–191. | ||
Hromadnikova I, Kotlabova K, Doucha J, Dlouha K, Krofta L. Absolute and relative quantification of placenta-specific microRNAs in maternal circulation with placental insufficiency-related complications. J Mol Diagn. 2012;14(2):160–167. | ||
Jin J, Zhou S, Li C, et al. MiR-517a-3p accelerates lung cancer cell proliferation and invasion through inhibiting FOXJ3 expression. Life Sci. 2014;108(1):48–53. | ||
Toffanin S, Hoshida Y, Lachenmayer A, et al. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology. 2011;140(5):1618–1628.e16. | ||
Dai Q, Li N, Zhou X. Increased miR-21a provides metabolic advantages through suppression of FBP1 expression in non-small cell lung cancer cells. Am J Cancer Res. 2017;7(11):2121–2130. | ||
Zhu Y, Shi M, Chen H, et al. NPM1 activates metabolic changes by inhibiting FBP1 while promoting the tumorigenicity of pancreatic cancer cells. Oncotarget. 2015;6(25):21443–21451. | ||
Liu X, Wang X, Zhang J, et al. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29(3):442–450. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.