Back to Journals » Journal of Inflammation Research » Volume 16
miR-146a-5p Promotes the Inflammatory Response in PBMCs Induced by Microcystin-Leucine-Arginine
Authors Zhang H, Chen D, Ji Q, Yang M, Ding R
Received 23 January 2023
Accepted for publication 3 May 2023
Published 9 May 2023 Volume 2023:16 Pages 1979—1993
DOI https://doi.org/10.2147/JIR.S403945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Huiying Zhang,1,* Daojun Chen,1,2,* Qianqian Ji,1 Meiyan Yang,1 Rui Ding1
1Department of Occupational Health and Environmental Health, School of Public Health, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China; 2School of Medical Technology, Anhui Medical College, Hefei, Anhui, 230601, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Daojun Chen, Email [email protected]
Background: Microcystin-leucine-arginine (MC-LR) is the most abundant and most toxic variant of microcystin isomers. Various experiments have clearly shown that MC-LR has hepatotoxicity and carcinogenicity, but there are relatively few studies on its immune damage effect. In addition, numerous studies have shown that microRNAs (miRNAs) are involved in a wide range of biological processes. Do miRNAs also play a role in inflammatory response caused by microcystin exposure? This is the question to be answered in this study. Moreover, this study can also provides experimental evidence for the significance of miRNA applications.
Objective: To investigate the effect of MC-LR on the expressions of miR-146a and pro/anti-inflammatory cytokines in human peripheral blood mononuclear cells (PBMCs) and to further explore the role of miR-146a in the inflammatory responses caused by MC-LR.
Methods: Serum samples from 1789 medical examiners were collected and detect the concentrations of MCs, and 30 serum samples with concentrations of MCs around P25, P50, and p75 were randomly selected for the detection of inflammatory factors. PBMCs from fresh peripheral blood extracted from these 90 medical examiners were subsequently tested for relative miR-146a expression. In vitro, the MC-LR were exposed to the PBMCs to detect the levels of inflammatory factors as well as the relative expression of miR-146a-5p. Then, a miRNA transfection assay was performed to verify the regulation of inflammatory factors by miR-146a-5p.
Results: In population samples, the expression of inflammatory factors and miR-146a-5p increased with increasing MCs concentration. In vitro experiments showed that the expression of inflammatory factors and miR-146a-5p in PBMCs increased with MC-LR exposure time or exposure dose too. In addition, inhibiting the expression of miR-146a-5p in PBMCs reduced inflammatory factor levels.
Conclusion: miR-146a-5p exerts a promoting effect on the MC-LR-induced inflammatory response by positively regulating inflammatory factor levels.
Keywords: microcystin-leucine-arginine, MC-LR, human peripheral blood, mononuclear cells, PBMCs, miR-146a, inflammatory cytokines
Introduction
In recent decades, cyanobacterial blooms have become a serious threat to water quality and human health and have been listed as an emerging health problem by the World Health Organization (WHO) and the European Food Safety Authority
(EFSA).1,2 Eutrophication of water caused by human activities can cause cyanobacterial blooms to produce microcystins (MCs).3,4 MCs is a class of cyclic heptathiopeptidine compounds with biological activity. It is the most widely distributed hepatotoxin and has considerable stability. It strongly inhibits protein phosphatase activity and is a strong promoter of liver tumors. At present, more than 100 MC subtypes have been detected, among which microcystin-leucine-arginine (MC-LR) is the most abundant and most toxic variety of MCs.5 In vitro and in vivo experiments and epidemiological investigations have shown that MC-LR has strong hepatotoxicity and carcinogenicity, therefore, we must try to reduce the harm caused by MCs as much as possible. However, the conventional disinfection process of drinking water in daily life makes it difficult to effectively remove MCs, which can enter the human body through drinking water, consumption of food, aquatic organisms and other ways, resulting in the exposure of human cells, skin, and genes to toxicity.
The hepatotoxicity and tumor-promoting potential of MCs have been widely studied and reported in many studies.3,6–8 In recent decades, there has been a gradual increases of studies on the effects of MCs on the immune system, and the effects of MCs on the immune system have been demonstrated.9 However, in general, there is still relatively little research on the immunotoxicity of MCs. It is known that the immune response is often accompanied by the occurrence of inflammation,10 while the inflammatory response is often mediated by cytokines. Cell factor is produced by lymphocytes and other kinds of small molecular peptides or glycoproteins, has a variety of functions and participates in a variety of biological processes, such as inflammation. In various cell types previously studied,11–13 changes in inflammation-related cytokines can be detected by exposure to MC-LR. Among inflammatory cytokines, IL-1β is the fastest and strongest inflammatory cytokine in the biological body’s response to foreign bodies,14 TNF-α is a multifunctional cytokine with a variety of biological activities that can induce pro-inflammatory responses to exogenous substances,15 while IL-6 has long been shown to be a typical cytokine with pleiotropic effects on inflammatory immune responses and hematopoiesis.16 The production of IL-4 involves the type 2 immune response,17 and it has also been studied as a classical anti-inflammatory cytokine.18 Likewise, IL-10 is also an anti-inflammatory cytokine that plays an important role in the prevention of inflammation and autoimmune pathology.19 Therefore, in this study, human peripheral blood mononuclear cells (PBMCs) were exposed to different doses of MC-LR to observe whether the expression of inflammation-related cytokines can also be changed.
Since the discovery of the founding members of the microRNAs (miRNAs) family, lin4 and let-7,20–22 researchers have discovered hundreds of miRNAs in plants, animals, and viruses through molecular cloning and bioinformatics approaches.23,24 miRNAs downregulate gene expression by base-pairing with the 3′ -untranslated regions (3′ UTRs) of the target messenger RNA (mRNAs).20–22 These findings suggest that this class of noncoding RNA molecules form a new layer of regulation for gene expression programs in many organisms. A single miRNA can target hundreds of mRNAs and affect the expression of multiple genes, while multiple miRNAs can also regulate a specific target gene.25 Studies have found that under microcystin exposure, the expression changes of a variety of miRNAs can also be detected in a variety of cell models.26–29 miRNAs are small single-stranded noncoding RNAs averaging twenty-two nucleotides, and as novel molecular modulators of various genes and pathways, they are involved in the normal immune response, autoimmune diseases and the expression of inflammatory factors.26,30,31 In addition, by regulating the expression of protein-coding genes, miRNAs are involved with almost every biological process in eukaryotes.32
Among many miRNAs, miR-146a has been shown to regulate the innate immune inflammatory response and other pathways,33 miR-146a can inhibit the secretion of proinflammatory cytokines in dendritic cells and regulate the expression of the Interleukin-1 receptor-associated kinase 1 (IRAK1) gene in THP-1 (human leukemia monocytic cell line) cells to inhibit the activation of NF-kB and the production of proinflammatory cytokines.34,35 At the same time, it can also improve the hemoglobin-induced microglial inflammatory response through the TLR4/ IRAK1/ TRAF6-related pathway.36 In general, miR-146a is associated with the expression of various inflammatory factors, and it can promote or inhibit the occurrence of inflammation by regulating the expression of inflammatory factors. Therefore, this study explored the role of miR-146a in MC-LR-induced inflammatory response in PBMCs through a population and in vitro experiments.
Materials and Methods
Population Sample Collection and MC Concentration Detection
A total of 1789 serum samples from medical examiners (healthy subjects, including 880 males and 909 females, ranging in age from 23 to 70 years) were collected from Ikang Zhuoyue Physical Examination Center with informed consent. These samples were numbered, packaged, and stored at −20 °C, and all of them were used for the Enzyme-linked immunosorbent assay (ELISA) kit to measure MC concentration, while three group of samples with different MC concentration gradients were selected for the determination of inflammatory factor concentrations after the determination of MC concentrations.
Cells Culture, Main Reagents, and Equipment
PBMCs were extracted from the peripheral blood of 15 healthy volunteers (7 males and 8 females, mean 25 years. And these healthy volunteers belong to a group other than 90 subjects selected from MCs quantification), and cultured in RPMI1640 cell culture medium (Beijing Solarbio) containing 10% Fetal bovine serum (Hangzhou Tianhang), 100 U/mL Penicillin- streptomycin solution (Shanghai Beyotime) in a 5% CO2 incubator at 37 °C (Thermo Scientific Company). The MC-LR standard (95% purity, Beijing Puhuashi) was diluted (0.1, 1.0, 10.0 µg/mL) and exposed to PBMCs for 6 or 24 h. Cell Counting Kit-8 (CCK-8, Shanghai Beyotime) was used to detect cell viability. TRIzol reagent (Invitrogen, Cat#15596026) was used to extract cellular total RNA. Reagents required for quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) of miR-146a and inflammation-related cytokine mRNA were purchased from GeneCopoeia, USA (All-in-OneTM miRNA qRT-PCR Detection Kit, Cat. No. QP015) and Takara, Japan (PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time), Code No. RR047A and TB Green® Premix Ex TaqTM II (Tli RNaseH Plus), Code No. RR820A). Human TNF-α, IL-1β, IL-6, IL-10, and IL-4 ELISA kits (Katalog names are CK-E10110H, CK-E10120H, CK-E10130H, CK-E10140H, and CK-E10150H) were purchased from Suzhou Calvin Biotechnology Company. Inverted microscope (Olympus Company); SW-CJ-2F purification workbench (Suzhou).
Cell Counting Kit-8 (CCK-8) Assay
The cells were inoculated in a 96-well plate with 8×103 cells per well and exposed to MC-LR at 0, 0.1, 1.0, 10.0 µg/mL for 6 or 24 h. At the appointed time points, the absorbance value at 450 nm (450 nm filter has the highest detection sensitivity) of each sample was recorded using the CCK-8 kit for plotting the viability curves. Cell viability % = (OD value of intervention group - OD value of blank group) / (OD value of control group - OD value of blank group) * 100.
ELISA Detects the Concentration of Inflammatory Factors in Human Serum and the Supernatant of Cell Culture Medium
In the population experiments, ELISA was performed on randomly selected ninety serum samples exposed to different concentrations of MCs to detect the expression of pro/anti-inflammatory cytokines according to the kit instructions. In the in vitro experiments, the supernatant of the cell culture medium of the PBMCs was collected after 6 and 24 h of MC-LR treatment. The OD (450) values of inflammatory factors in the cell supernatants were determined by the conventional double-antibody sandwich ELISA method as required by the corresponding kit. The concentration of inflammatory cytokines was calculated according to the linear regression curve of the standard. The inter and intra-assay CV for the kits were less than 15%, and the sensitivity was the lowest detection concentration less than 1.0 pg/mL.
Extraction of Total RNA
Total RNA was extracted from PBMCs according to TRIzol reagent instructions. The concentration as well as the purity of RNA (OD260/ OD280 between 1.8 and 2.0 represents better purity), were determined by NanoDrop One, and the integrity of RNA was detected by agarose gel electrophoresis to evaluate the quality of RNA extraction. (Electrophoresis can show whether there is genomic DNA contamination and RNA degradation (28S, 18S bands). The quality of total RNA can be preliminarily evaluated by visual measurement of the ratio of 28S to 18S bands and it is generally believed that 28S:18S > =2 can be judged to have good total RNA integrity).
qRT-PCR Detecting the Expression of miR-146a
Based on literature references18,36,37 as well as the input of NF-kB/ IRAK1/ TRAF6, the key protein of signaling pathways involved in regulating the expression of inflammatory factors, in databases such as miRMap, miRWalk, and miRDB, we found that miR-146a indeed had a close association with the expression of inflammatory factors according to target rank and target score. Therefore, we subjected miR-146a to real-time PCR in the population as well as in vitro assays to check for differential expression. Using U6snRNA as an internal reference, reverse transcription of miRNA was performed by tailing according to the miRNA reverse transcription kit to generate single-stranded cDNA. miR-146a and one internal reference primer design, synthesis, and validation work were commissioned by the American GeneCopoeia corporation. Due to the company’s confidentiality treaty restrictions, only partial primer-related information can be provided to the investigator, no specific primer design parameters can be provided, and related information is shown in Table 1.
 |
Table 1 The Related Information of miRNA Primer |
Polymerase chain reaction (PCR) was performed using a fluorometric quantitation Kit with a total of 20 µL. The volume of the 2×All-in-One™ qPCR Mix is 10 µL; the volume of the All-in-One™ miRNA qPCR Primer (2µM) is 2 µL; The universal Adaptor PCR Primer (2µM) volume is 2 µL; First-strand cDNA (diluted 1:5) volume is 2 µL; without adding the ROX Reference Dye and adding double-distilled of 4 µL to the final volume of 20 µL. In addition, the relative expression of target genes is usually calculated by F=2−ΔΔCt method. ΔCT (test) = CT (target, test) - CT (ref, test), ΔCT (calibrator) = CT (target, calibrator) - CT (ref, calibrator); ΔΔCT = ΔCT (test) – ΔCT (calibrator).
The Expression of Inflammation Factors mRNA Was Measured by qRT-PCR
The first-strand cDNA was synthesized according to the PrimerScriptTM RT reagent Kit with gDNA Eraser operating manual. Quantitative PCR reactions were completed on a LightCycler System. The primer sequences of the inflammation- related cytokines and the reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), are shown in Table 2. The reaction system is 20 µL. The PCR reaction solution included 10 µL of TB Green Premix Ex Taq II, 2 µL of cDNA template, 0.8 µL of forward and reverse primers, and 6.4 µL of sterilized water.
 |
Table 2 The Related Information of mRNA Primer |
Hsa-miR-146a-5p Inhibitor Was Transfected into PBMCs
Cells were inoculated in 24-well plates, cultured to a resting state, and transfected with the corresponding miRNA Inhibitor and negative control (General Biol, Shanghai, China) using Lipofectamine 3000 (Invitrogen, USA). Then, the transfected cells were harvested for qRT-PCR experiments after 48 h.
Statistical Analysis
Statistical Product and Service Solutions (SPSS) 23.0 and GraphPad Prism 8.0 software were used for data analyses. The results of each group are expressed as the mean ± standard deviation. In population samples, a one-way analysis of variance (ANOVA) was conducted for multiple group comparisons. In vitro, unpaired t-test was used for comparison between the two groups, and multiple group comparisons were analyzed by ANOVA. A Pearson correlation test was performed to determine the
correlation between inflammatory cytokines and miR-146a-5p. P < 0.05 was considered statistically significant.
Results
Measuring Concentration of MC and Sample Grouping
The procedure was performed according to the instructions of the ELISA kit. By measuring the concentration of MCs, it was found that the lowest exposure concentration of MCs in the collected human serum samples was 2.762 ng/mL, and the highest was 223.521 ng/mL (P25 = 5.267, P50 = 25.449, P75 = 125.021 ng/mL. P25, P50 and P75 are the quartiles. Arranging a set of data from the smallest (left) to the largest (right), P25 represents the concentration from 25% of the sample, P50 is the median, and P75 is the concentration from 75% of the sample). Therefore, 30 serum samples of each group with exposure concentrations ranging from 4~6, 20~30, and 100~150 ng/mL were randomly selected for subsequent detection of inflammatory factors, and the concentration of MCs was subsequently set as the mean value of 5, 25, and 125 ng/mL in each group. The basic characteristics of each group are shown in Table S1.
Effects of MCs on the Expression of Pro/Anti-Inflammatory Cytokines and miR-146a-5p in Human Samples and the results of Correlation Analysis Between Them
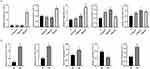
As shown in Figure 1, the expression of TNF-α, IL-1β, IL-6, IL-10 and IL-4 was increased in a concentration-dependent manner, and the difference was statistically significant (P < 0.05). In addition, an additional 2 mL of fresh peripheral blood from the ninety individuals was extracted, and PBMCs were extracted within two hours for miR-146a detection. By examining the expression of miR‐146a, we found that it also increased with the concentration of MCs, but there was no significant difference between the 25 ng/mL group and the 5 ng/mL group (P > 0.05). The results are presented in Figure 2.
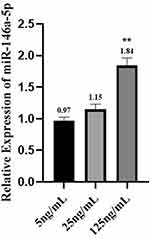 |
Figure 2 Expression of miR-146a in population samples. Data were analyzed in one-way ANOVA. **p < 0.01 vs 5ng/mL. |
As shown in Figure 3, the correlation of miR-146a expression with inflammatory cytokines expression in the population samples was analyzed by Pearson correlation analysis in SPSS 23.0, and the results showed that miR-146a was moderately and highly correlated with the expression of multiple inflammatory factors (P < 0.001).
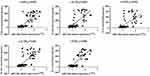 |
Figure 3 Association analysis between miR-146a expression and inflammatory cytokines expression in population samples. |
Effects of MC-LR on miR-146a-5p Expression in vitro
PBMCs were induced with 0, 0.1, 1.0, or 10 µg/mL MC-LR for 6 or 24 h. The results showed that the expression of miR-146a-5p was increased at 24 h compare with 6 h (on the left picture) regardless of the dose group. When the exposure time was 6 h, the expression of miR-146a-5p was compared between the different dose groups (on the right picture), and it was found that miR-146a-5p only increased significantly at a dose of 10.0 µg/mL compared with the 0 µg/mL. While at 24 h, the expression of miR-146a-5p was increased in a concentration-dependent way and peaked at 10.0 µg/mL (P < 0.05). The results are shown in Figure 4.
 |
Figure 4 Relative expression of miR-146a-5p in vitro experiment. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs 6 h or 0 µg/mL. |
Effects of MC-LR on the Concentration and mRNA Level of Inflammatory Factors in vitro and the Results of Correlation Analysis Between Expressions of Inflammatory Factors and Expressions of miR-146a-5p
After treatment with 0, 0.1, 1.0, or 10 µg/mL MC-LR for 6 h or 24 h, the contents of TNF-α, IL-1β, IL-6, IL-10 and IL-4 were detected by ELISA kits, as shown in Figure 5. The results showed that the expression of TNF-α in the 10 µg/mL dose group was significantly higher than that in the control group at either 6 h or 24 h (P < 0.05); However, the expressions of IL-1β, IL-6, IL-10 and IL-4 were not different at any dose at 6 h (P > 0.05), but at 24 h, the expressions of IL-1β, IL-6, and IL-4 were increased at 1.0 and 10 µg/mL, and IL-10 increased at 0.1 and 1.0 µg/mL compared with the control (P < 0.05). The expression of IL-10 was decreased at 10 µg/mL compared with the control, but the difference was not significant (P > 0.05). In addition, the expression of TNF-α, IL-1β, IL-6 and IL-4 increased with increasing exposure time (P < 0.05). The expression of IL-10 increased with time at 0.1 and 1.0 µg/mL and decreased with time at 10 µg/mL. All these differences were statistically significant (P < 0.05).
Inflammatory factor mRNA levels are shown in Figure 6. The results (Figure 6a) showed that after 24 h of exposure to MC-LR, TNF-α and IL-1β mRNA expression increased significantly at 10 µg/mL compared with the control (P < 0.05); IL-6 and IL-4 mRNA expression increased at 1.0 and 10 µg/mL compared with the control, while IL-10 mRNA expression increased at 0.1 and 1.0 µg/mL compared with the control (P < 0.05). As shown in Figure 6b, 10 µg/mL MC-LR treatment increased the mRNA expression of TNF-α, IL-1β, IL-6 and IL-4 in a time-dependent manner (P < 0.05), but the expression of IL-10 mRNA decreased with increasing exposure time (P < 0.05). The qRT-PCR results were consistent with the ELISA results.
As shown in Figure 7, results showed that miR-146a-5p was highly correlated with the expression of various inflammatory factors (P < 0.001).
 |
Figure 7 Correlation analysis between expression of miR-146a and expression of inflammatory factors in vitro. |
Hsa-miR-146a-5p Inhibitor Transfection Results
As shown in Figure 8, the miRNA transfection reagent, as well as the hsa-miR- 146a-5p inhibitor, and hsa-miR-146a-5p inhibitor negative control, were added to the cell culture medium, 48 h after transfection, cells were harvested, RNA was extracted, and the expression of hsa-miR-146a-5p was detected. The results showed that hsa-miR-146a-5p was significantly inhibited (P < 0.05), which demonstrated successful transfection.
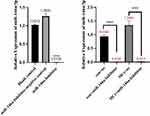 |
Figure 8 Hsa-miR-146a-5p inhibitor transfection results. Con+nc: control+miR-146a inhibitor negative control; MCs+nc: MCs+miR-146a inhibitor negative control. ****p < 0.0001. |
Relative Expression of Each Inflammatory Factor After Transfection with Hsa-miR-146a-5p Inhibitor versus Transfection with Hsa-miR-146a-5p Inhibitor Negative Control
As shown in Figure 9, when the exposure time of MC-LR was 24 h and the exposure dose was 0.1 µg/mL, the expression of IL-10 and IL-4 were decreased compared with the no inhibitor group, but the expression of TNF-α, IL-1β and IL-6 were increased compared with the no inhibitor group (P < 0.05). When the dose was 1.0 µg/mL, the expression of IL-10, IL-4 and TNF-α were decreased compared with the noninhibitor group, while the expression of IL-1β and IL-6 were still increased compared with the noninhibitor group (P < 0.05). When the dose was 10 µg/mL, the expression of IL-10, IL-4, TNF-α, and IL-6 were significantly decreased, and the expression of IL-1β was slightly increased compared with the group without inhibitor (P < 0.05).
When the exposure dose of MC-LR was 10 µg/mL and the exposure time was 6 h, the expression of TNF-α and IL-10 were decreased compared with the group with the negative control (P < 0.05), while the expression of IL-1β was significantly higher than that of the negative control group (P < 0.05). When the time was 24 h, the expression of TNF-α, IL-6, IL-10, and IL-4 were reduced compared with that in the group without the inhibitor (P < 0.05), IL-1β expression was still elevated compared with the noninhibitor group (P < 0.05). These results demonstrated that miR-146a could promote the inflammatory response induced by MC-LR in PBMCs.
Discussion
This study is to investigate the effect of MC-LR on the expressions of miR-146a and pro/anti-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, IL-4) in PBMCs and to further explore the role of miR-146a in the inflammatory responses caused by MC-LR. The results showed that exposure to MCs increased miR-146a expression both in vivo and in vitro. In addition, through detected the serum samples containing MCs showed that the expressions of both pro-inflammatory and anti-inflammatory cytokines increased with the increase of MCs concentration. The results of in vitro detection of inflammatory cytokines showed that the expression of IL-4 decreased with high MC-LR exposure, and other inflammatory cytokines increased with the increase of MC-LR exposure concentration. Moreover, correlation analysis showed that miR-146a was highly positively correlated with the expression of various inflammatory cytokines, and this correlation was preliminarily proved by transfection of miR-146a inhibitors. In this study, MC-LR induced a rapid elevation of proinflam- matory cytokines, which was similar to the results of LPS stimulation and was consistent with previous in vivo and in vitro studies.38–40 At the same time, miR-146a expression was also similar to the results after LPS stimulation. LPS resulted in a rapid elevation of the expression of miR-146a in human monocytic THP-1 cells and BV2 cells.41,42
Algal bloom incidence has brought the topic of cyanobacteria into the spotlight. Cyanobacteria are gram-negative bacteria found in fresh and eutrophic waters globally.43 Microcystins are cyanotoxins produced by cyanobacteria genera such as Microcystis, Nostoc, Dolichospermum (formerly Anabaena), Oscillatoria and Planktothrix.44 There is already evidence45 that microcystins have negative effects on human health. The toxic effects of microcystins include chronic hepatocellular carcinoma genicity and oxidative stress, as well as immunosuppressive effects by inhibiting IFN production and cytokine synthesis.9 This suggests that the immune system is easily exposed and sensitive to toxic agents. Although several phycotoxin studies have addressed the immune-related endpoint question46–48 using various cellular models, there is a general lack of studies on the effects of MC-LR on immune cells and on the underlying molecular mechanisms or responsible signaling pathways in immune cells. It was previously mentioned that the expression changes of miRNAs can be detected by microcystin exposure in multiple types of cell models,27–29 at the same time, miR-146a has also been found to be involved in regulating inflammatory response in several studies.49–51 Therefore, this study explored the role of miR-146a in MC-LR-induced changes of inflammatory cytokines in PBMCs through population and in vitro experiments.
miRNA is involved in a wide range of biological processes and has multiple biological functions. One study summarized the evidence regarding the 27 miRNAs regulating inflammatory cytokine signaling pathways.52 However, abnormal expression of some miRNAs can also lead to an impaired immune response and immunopathological consequences.25 miR-146a has been shown to regulate the expression of several pro/anti-inflammatory cytokines in several studies,18,53,54 and in this study, miR-146a was also shown to be involved in regulating the expression of inflammation-related cytokines in PBMCs. In the population experiments, it was found that the expression of pro/anti-inflammatory cytokines was positively correlated with the expression of miR-146a. To investigate whether in vitro exposure of MC-LR alone to human PBMCs can also cause similar changes, PBMCs were extracted from fresh human peripheral blood for in vitro culture and MC-LR stimulation. In addition, to determine the noncytotoxic concentrations of MC-LR, we used the CCK-8 kit to assess the effect of increased MC-LR concentrations (0.1 to 10.0 µg/mL) exposure for 6 and 24 h on peripheral blood mononuclear cell viability. The results showed that 0.1 to 10.0 µg/mL were all at noncytotoxic concentrations, so in the following experiments, PBMCs were exposed to nontoxic concentrations of MC-LR to detect changes in miR-146a and inflammation-related cytokines.
In the in vitro experiments, the results of the correlation analysis were similar to those from the population, and the expression of miR-146a was highly correlated with the expression of inflammatory factors. Later, when the exposure concentration of MC-LR was 10 µg/mL and the exposure time was 24 h, the regulatory role of miR-146a in the expression of inflammatory factors was verified by in vitro transfection experiments. However, the expression of IL-1β did not decrease with the inhibition of miR-146a, but increased instead, which may be because IL-1β is regulated by multiple essential signals, for example, the priming signal and the triggering signal.55 In addition, assembly of the inflammasome complex leads to the activation of caspase-1, which can also promotes the maturation and release of the inflammatory cytokines IL-1β.56 As mentioned above, IL-1β regulation is a complex process57–59 that is not only regulated by miR-146a.
The immune system is a highly evolved biological system that is susceptible to environmental influences and responsible for immunologic surveillance and defense, and immunoregulation. The immune system is a complex network of immune organs, immune cells and immune-active substances, in which immune cells communicate with each other through direct interaction or soluble cytokines.60 The coordination of the immune system achieved the removal of senescent cells and defense against invading pathogens.61 Investigation of the human immune system is a key factor in understanding the inflammatory response. Human PBMCs, including lymphocytes (B cells, T cells, and NK cells), monocytes, and dendritic cells, are frequently used to assess the immune response.62 In addition, there are many studies examining expression in human PBMCs to characterize the response of human health conditions to environmental, behavioral, or pathological factors.63 It has been mentioned earlier that studies on the effects of MCs on the immune system are not comprehensive enough, therefore, this study explored the effects of MC-LR on the immune system by exposing MC-LR to human peripheral blood mononuclear cells.
Cytokines are proteins with specific roles in the human immune response that play an important role in the immune response of the immune system, such as cell-mediated and antibody-mediated immune, inflammatory reactions, autoimmunity and hypersensitivity reactions,15 The cytokines involved in this study, such as IL-6 and TNF-α, were previously reported to have increased levels of lipopolysaccharide (LPS)-stimulated PBMCs compared with unstimulated PBMCs.64 Another study also observed that the production of the anti-inflammatory cytokines IL-4 and IL-10 in PBMCs stimulated by LPS was also significantly increased.65 An imbalance between pro-inflammatory and anti-inflammatory cytokines or uncontrolled cytokine production can lead to an inflammatory response.52
In this study, it was found that miR-146a positively regulated the expression of inflammatory cytokines, while most previous studies have found that miR-146a negatively regulates various pathways,18,33,35 such as innate immunity and the inflammatory response, which can inhibit the secretion of pro-inflammatory cytokines. Although the results of this study on miR-146a were inconsistent with previous studies, such positive correlation was verified by in vitro miRNA transfection experiments. Therefore, this finding indicates the possibility of a different response due to the differences in cell types and stimulation and also provides a experimental evidence that miR-146a positively regulates inflammatory response. Overall, this study demonstrates that miRNAs play an important role in the regulation of MC-LR induced inflammatory response.
However, this study still has some shortcomings. Firstly, there were only ninety population samples in the population experiment in this study, a relatively small number of individuals and larger population data are needed for further verification in the future. Secondly, due to limited experimental conditions, this study only extracted PBMCs from peripheral blood of 15 healthy volunteers for in vitro experiments, and the representativeness of the results may not be good. More PBMCs from healthy volunteers of different ages and genders should be extracted later to prove the conclusion of this study. Thirdly, the exact mechanism by which miR-146a regulates the expression of inflammatory factors is still unclear, and both the underlying mechanism and the transcriptional signal need to be further investigated in the future.
Conclusion
In summary, this study demonstrated that exposure of human PBMCs to MC-LR can cause the expression of miR-146a and inflammation-related cytokines increased and miR-146a exerts a promoting effect on the MC-LR-induced inflammatory response by positively regulating inflammatory factor levels.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The ethical approval of the Biomedical Ethics Committee of Anhui Medical University (Approved Number: 2021H037) and the informed consent of the participants were obtained before the study began. This study complies with the Declaration of Helsinki.
Acknowledgments
This study was supported by Funds from Natural Science Research Project of colleges and Universities in Anhui Province [2022AH052336] and Chen Daojun 2018 PhD Research Grant [0307012103] and Grants from Wang Jianhua scientific research and innovation team project of Anhui Medical College [WJH2022004t].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Natural Science Research Project of colleges and Universities in Anhui Province [2022AH052336] and Chen Daojun 2018 PhD Research Grant [0307012103] and Grants from Wang Jianhua scientific research and innovation team project of Anhui Medical College [WJH2022004t].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Manganelli M, Scardala S, Stefanelli M, et al. Emerging health issues of cyanobacterial blooms. Ann Ist Super Sanita. 2012;48(4):415–428. doi:10.4415/ANN_12_04_09
2. Testai E, Buratti FM, Funari E, et al. Review and analysis of occurrence, exposure and toxicity of cyanobacteria toxins in food. EFSA Support. Publ. 2016;13:998E.
3. Buratti FM, Manganelli M, Vichi S, et al. Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch Toxicol. 2017;91(3):1049–1130. doi:10.1007/s00204-016-1913-6
4. Bormans M, Amzil Z, Mineaud E, et al. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France. Harmful Algae. 2019;87:101639. doi:10.1016/j.hal.2019.101639
5. Niedermeyer T. Microcystin congeners described in the literature. figshare. 2014. doi:10.6084/m9.figshare.880756.v5
6. Shi L, Du X, Liu H, et al. Update on the adverse effects of microcystins on the liver. Environ Res. 2021;195:110890. doi:10.1016/j.envres.2021.110890
7. Fischer WJ, Altheimer S, Cattori V, et al. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol Appl Pharmacol. 2005;203(3):257–263. doi:10.1016/j.taap.2004.08.012
8. Svirčev Z, Drobac D, Tokodi N, et al. Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Arch Toxicol. 2017;91(2):621–650. doi:10.1007/s00204-016-1921-6
9. Palikova M, Ondrackova P, Mares J, et al. In vivo effects of microcystins and complex cyanobacterial biomass on rats (Rattus norvegicus var. alba): changes in immunological and haematological parameters. Toxicon. 2013;73:1–8. doi:10.1016/j.toxicon.2013.06.016
10. Secombes CJ, Wang T, Hong S, et al. Cytokines and innate immunity of fish. Dev Comp Immunol. 2001;25(8–9):713–723. doi:10.1016/S0145-305X(01)00032-5
11. Ma J, Li Y, Duan H. Chronic exposure of nanomolar MC-LR caused oxidative stress and inflammatory responses in HepG2 cells. Chemosphere. 2018;192:305–317. doi:10.1016/j.chemosphere.2017.10.158
12. Ma Y, Wang J, Xu D, et al. Chronic MC-LR exposure promoted Aβ and p-tau accumulation via regulating Akt/GSK-3β signal pathway. Sci Total Environ. 2021;794:148732. doi:10.1016/j.scitotenv.2021.148732
13. Cao L, Huang F, Massey IY, et al. Effects of microcystin-LR on the microstructure and inflammation-related factors of jejunum in mice. Toxins. 2019;11(9):482. doi:10.3390/toxins11090482
14. Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8(4):253–265. doi:10.1016/S1359-6101(97)00023-3
15. Ksontini R, MacKay SL, Moldawer LL. Revisiting the role of tumor necrosis factor alpha and the response to surgical injury and inflammation. Arch Surg. 1998;133:558–567. doi:10.1001/archsurg.133.5.558
16. Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi:10.1016/s0065-2776(08)60532-5
17. Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–435. doi:10.1126/science.1221064
18. Liu GJ, Zhang QR, Gao X, et al. MiR-146a ameliorates hemoglobin-induced microglial inflammatory response via TLR4/IRAK1/TRAF6 associated pathways. Front Neurosci. 2020;14:311. doi:10.3389/fnins.2020.00311
19. O’Garra A, Barrat FJ, Castro AG, et al. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–131. doi:10.1111/j.1600-065X.2008.00635.x
20. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi:10.1016/0092-8674(93)90529-Y
21. Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. doi:10.1038/35002607
22. Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862. doi:10.1016/0092-8674(93)90530-4
23. Berezikov E, Cuppen E, Plasterk RH. Approaches to microRNA discovery. Nat Genet. 2006;38(Suppl):S2–S7. doi:10.1038/ng1794
24. Ruby JG, Jan C, Player C, et al. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell. 2006;127(6):1193–1207. doi:10.1016/j.cell.2006.10.040
25. Chen M, Wang F, Xia H, et al. MicroRNA-155: regulation of immune cells in sepsis. Mediators Inflamm. 2021;2021:8874854. doi:10.1155/2021/8874854
26. Carissimi C, Fulci V, Macino G. MicroRNAs: novel regulators of immunity. Autoimmun Rev. 2009;8:520–524. doi:10.1016/j.autrev.2009.01.008
27. Ma J, Li Y, Yao L, et al. Analysis of MicroRNA expression profiling involved in MC-LR-induced cytotoxicity by high-throughput sequencing. Toxins. 2017;9(1):23. doi:10.3390/toxins9010023
28. Yang S, Chen L, Wen C, et al. MicroRNA expression profiling involved in MC-LR-induced hepatotoxicity using high-throughput sequencing analysis. J Toxicol Environ Health A. 2018;81(5):89–97. doi:10.1080/15287394.2017.1415580
29. Zhou Y, Wang H, Wang C, et al. Roles of miRNAs in microcystin-LR-induced Sertoli cell toxicity. Toxicol Appl Pharmacol. 2015;287(1):1–8. doi:10.1016/j.taap.2015.05.008
30. Iborra M, Bernuzzi F, Invernizzi P, et al. MicroRNAs in autoimmunity and in flammatory bowel disease: crucial regulators in immune response. Autoimmun Rev. 2012;11:305–314. doi:10.1016/j.autrev.2010.07.002
31. Scrivo R, Vasile M, Bartosiewicz I, et al. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev. 2011;10:369–374. doi:10.1016/j.autrev.2010.12.006
32. Friedman RC, Farh KK, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105.
33. Li L, Chen XP, Li YJ. MicroRNA-146a and human disease. Scand J Immunol. 2010;71(4):227–231. doi:10.1111/j.1365-3083.2010.02383.x
34. Chen T, Li Z, Jing T, et al. MicroRNA-146a regulates the maturation process and pro-inflammatory cytokine secretion by targeting CD40L in oxLDL-stimulated dendritic cells. FEBS Lett. 2011;585(3):567–573. doi:10.1016/j.febslet.2011.01.010
35. Zhou C, Zhao L, Wang K, et al. MicroRNA-146a inhibits NF-kB activation and pro-inflammatory cytokine production by regulating IRAK1 expression in THP-1 cells. Exp Ther Med. 2019;18(4):3078–3084. doi:10.3892/etm.2019.7881
36. Sanada T, Sano T, Sotomaru Y, et al. Anti-inflammatory effects of miRNA-146a induced in adipose and periodontal tissues. Biochem Biophys Rep. 2020;22:100757. doi:10.1016/j.bbrep.2020.100757
37. Wang J, Cui Z, Liu L, et al. MiR-146a mimic attenuates murine allergic rhinitis by downregulating TLR4/TRAF6/NF-kB pathway. Immunotherapy. 2019;11(13):1095–1105. doi:10.2217/imt-2019-0047
38. Gram M, Sveinsdottir S, Ruscher K, et al. Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J Neuroinflammation. 2013;10:100.
39. Kwon MS, Woo SK, Kurland DB, et al. Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci. 2015;16(3):5028–5046. doi:10.3390/ijms16035028
40. Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi:10.1186/1742-2094-9-46
41. Rey C, Nadjar A, Buaud B, et al. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun. 2016;55:249–259. doi:10.1016/j.bbi.2015.12.013
42. Taganov KD, Boldin MP, Chang KJ, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103(33):12481–12486. doi:10.1073/pnas.0605298103
43. Campos A, Vasconcelos V. Molecular mechanisms of microcystin toxicity in animal cells. Int J Mol Sci. 2010;11:268–287. doi:10.3390/ijms11010268
44. Redouane EM, El Amrani Zerrifi S, El Khalloufi F, et al. Mode of action and fate of microcystins in the complex soil-plant ecosystems. Chemosphere. 2019;225:270–281. doi:10.1016/j.chemosphere.2019.03.008
45. Chorus Ingrid BJ. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring, and Management. London: Taylor & Francis; 1999.
46. Christen V, Meili N, Fent K. Microcystin-LR induces endoplasmatic reticulum stress and leads to induction of NF-kB, interferon-alpha, and tumor necrosis factor-alpha. Environ Sci Technol. 2013;47(7):3378–3385. doi:10.1021/es304886y
47. Chen L, Zhang X, Chen J, et al. NF-kB plays a key role in microcystin-RR induced HeLa cell proliferation and apoptosis. Toxicon. 2014;87:120–130. doi:10.1016/j.toxicon.2014.06.002
48. Zhang XX, Fu Z, Zhang Z, et al. Microcystin-LR promotes melanoma cell invasion and enhances matrix metalloproteinase-2/-9 expression mediated by NF-κB activation. Environ Sci Technol. 2012;46(20):11319–11326. doi:10.1021/es3024989
49. Essandoh K, Li Y, Huo J, et al. MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock. 2016;46(2):122–131. doi:10.1097/SHK.0000000000000604
50. Fei Y, Chaulagain A, Wang T, et al. MiR-146a down-regulates inflammatory response by targeting TLR3 and TRAF6 in Coxsackievirus B infection. RNA. 2020;26(1):91–100. doi:10.1261/rna.071985.119
51. Yan F, Wufuer D, Ding J, et al. MicroRNA miR-146a-5p inhibits the inflammatory response and injury of airway epithelial cells via targeting TNF receptor-associated factor 6. Bioengineered. 2021;12(1):1916–1926. doi:10.1080/21655979.2021.1927545
52. Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med. 2012;18(5):736–749. doi:10.1038/nm.2754
53. Garo LP, Ajay AK, Fujiwara M, et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat Commun. 2021;12(1):2419. doi:10.1038/s41467-021-22641-y
54. Chen L, Yu L, Zhang R, et al. Correlation of microRNA-146a/b with disease risk, biochemical indices, inflammatory cytokines, overall disease severity, and prognosis of sepsis. Medicine. 2020;99(22):e19754. doi:10.1097/MD.0000000000019754
55. Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi:10.4049/jimmunol.0901363
56. Monteleone M, Stow JL, Schroder K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine. 2015;74:213–218. doi:10.1016/j.cyto.2015.03.022
57. Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and its receptors in the regulation of inflammatory response. Int J Mol Sci. 2017;18(5):1028. doi:10.3390/ijms18051028
58. Yi XM, Li M, Chen YD, et al. Reciprocal regulation of IL-33 receptor-mediated inflammatory response and pulmonary fibrosis by TRAF6 and USP38. Proc Natl Acad Sci USA. 2022;119(10):e2116279119. doi:10.1073/pnas.2116279119
59. Daskalaki MG, Tsatsanis C, Kampranis SC. Histone methylation and acetylation in macrophages as a mechanism for regulation of inflammatory responses. J Cell Physiol. 2018;233(9):6495–6507. doi:10.1002/jcp.26497
60. Kumar A, Sharma N, Singh S, et al. Oral vaccine antigen induced immune response signalling pathways: current and future perspectives. J Vaccines Vaccin. 2014;5(3):1–6.
61. Viana I, Roussel S, Defrêne J, et al. Innate and adaptive immune responses toward nanomedicines. Acta Pharm Sin B. 2021;11(4):852–870. doi:10.1016/j.apsb.2021.02.022
62. Bolen CR, Uduman M, Kleinstein SH. Cell subset prediction for blood genomic studies. BMC Bioinform. 2011;12:258. doi:10.1186/1471-2105-12-258
63. Mohr S, Liew CC. The peripheral-blood transcriptome: new insights into disease and risk assessment. Trends Mol Med. 2007;13:422–432. doi:10.1016/j.molmed.2007.08.003
64. Lechner J, Chen M, Hogg RE, et al. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J Neuroinflammation. 2017;14(1):42. doi:10.1186/s12974-017-0820-y
65. Ramírez-Pérez S, Hernández-Palma LA, Oregon-Romero E, et al. Downregulation of inflammatory cytokine release from IL-1β and LPS-stimulated PBMC orchestrated by ST2825, a MyD88 dimerisation inhibitor. Molecules. 2020;25(18):4322. doi:10.3390/molecules25184322
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.