Back to Journals » OncoTargets and Therapy » Volume 12
miR-125a restrains cell migration and invasion by targeting STAT3 in gastric cancer cells
Authors Yang L , Zhang S, Guo K , Huang H , Qi S , Yao J , Zhang Z
Received 16 March 2018
Accepted for publication 18 November 2018
Published 24 December 2018 Volume 2019:12 Pages 205—215
DOI https://doi.org/10.2147/OTT.S168454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
This paper has been retracted.
Liu Yang, 1,* Shuguang Zhang, 2,* Kai Guo, 3,* Hu Huang, 4,* Shuai Qi, 5 Jie Yao, 6 Zhihong Zhang 7
1Department of Cancer Biotherapy Center, Hubei Cancer Hospital, Wuhan, Hubei 430079, China; 2Department of Clinical Laboratory, Liaocheng People’s Hospital, Liaocheng, Shandong 252000, China; 3Department of Gastroenterology, The 161th Hospital of PLA, Wuhan, Hubei 430010, China; 4Department of Oncology, The 161th Hospital of PLA, Wuhan, Hubei 430010, China; 5Department of Pharmacy, The 161th Hospital of PLA, Wuhan, Hubei 430010, China; 6Department of Urological Surgery, Zhongnan Hospital of Wuhan University, Wuhan, Hubei 430071, China; 7Department of Oncology, Gong’an County People’s Hospital, Jingzhou, Hubei 434000, China
*These authors contributed equally to this work
Background: Recently, many microRNAs have been found to be involved in the cancer progression including miR-125a. However, the underlying mechanisms of miR-125a in gastric cancer (GC) remain to be completely elucidated.
Objective: The study was to investigate the functional role of miR-125a and the expression relevance of signal transducer and activator of transcription 3 (STAT3) and hyaluronan synthase 1 (HAS1).
Method: CCK-8 assay, scratch wound healing and transwell assay were conducted to identify the functional role of miR-125a in GC. In addition, using bioinformatics analysis, the target regulation relationship was found in STAT3 and miR-125a. To confirm the relationship, luciferase reporter assay was performed. More importantly, quantitative polymerase chain reaction and western blot assay were carried out to determine the association among miR-125a, STAT3 and HAS1 in GC cells.
Results: Overexpressed miR-125a inhibited the migration and invasion of GC cells through scratch wound healing and transwell assay, and its knockdown displayed adverse effects, but the viability of GC cells did not show significant difference using CCK-8 assay. In addition, we identified that the knockdown of STAT3 or HAS1 remarkably suppressed the migration and invasion abilities of GC cells. Using bioinformatics analysis, miRTar, in particular, indicated that the 3'-untranslated region of STAT3 binds to miR-125a with a high score. Subsequently, we also verified that STAT3 was a target of miR-125a via luciferase reporter assay. Furthermore, we found that upregulated miR-125a expression could conspicuously constrain STAT3 expression at both protein and mRNA levels in MKN45 and NCI-N87 cells using quantitative polymerase chain reaction and Western blot assay, but no significant difference had been found in SGC 7901 cells. To further identify the regulatory relationship between miR-125a and STAT3, downregulation of miR-125a in MKN45 and NCI-N87 cells was carried out, which showed that the protein and mRNA expression levels of STAT3 were declined in two cell lines. Finally, we observed that upregulated miR-125a could lead to the decrease of HAS1 at protein and mRNA levels, whereas its knockdown revealed opposite effects. Meanwhile, we noticed that overexpression of STAT3 could induce the escalation of HAS1 at protein and mRNA expression levels and its knockdown exhibited the adverse outcomes.
Conclusion: These findings indicated that miR-125a may control the HAS1 expression in GC progression by targeting STAT3, which is likely to facilitate a better understanding of the regulation mechanisms of miR-125a in GC.
Keywords: miR-125a, STAT3, HAS1, gastric cancer
Introduction
Gastric cancer (GC), as the third most common cancer and the second leading cause of death, is still a major public health problem worldwide.1,2 Despite recent progress in the detection and management of early GC, most cases diagnosed at advanced stages along with aggressive invasion or lymphatic metastasis are confronted with rather low efficiency treatment, commonly operated by traditional therapies such as surgery, radiotherapy, chemotherapy, or chemotherapy integrated with Chinese medicine.3–5 Therefore, a good knowledge of the molecular pathogenesis of this disease is urgently needed and the identification of novel molecular biomarkers is crucial to improve the current therapies for GC.
The microRNAs (miRNAs) are a class of small noncoding RNAs with 18–24 nucleotides in length, which are famous for regulating the gene expression by pairing with the 3′-untranslated region (3′-UTR) of their target mRNAs.6,7 A class of miRNAs recently discovered is involved in important biological processes.8–10 On the one hand, several miRNAs apparently regulate cancer stemness of all stripes, such as miR-203 for breast cancer,11 miR-124a for non-small-cell lung cancer,12 and miR-7 for prostate cancer.13 On the other hand, accumulating evidence strongly suggests that aberrant miRNAs expression could play a role in the growth and metastasis of various human cancers including GC.14–18 miR-125a is well known in a variety of human cancers, including hepatocellular carcinoma,19 non-small-cell lung cancer,20 and so forth. As for GC, miR-125a was reported to inhibit tumor proliferation and angiogenesis by targeting human epidermal growth factor receptor-221 and vascular endothelial growth factor A.22 Obviously, it is probable that other target genes of miR-125a are involved in mediation of GC progression. It is worth noting that previous studies have identified that miR-125a could directly target signal transducer and activator of transcription 3 (STAT3) in cervical23 and lung carcinoma.24 STAT3, as a member of the STAT protein family, is a transcription factor, encoded by the STAT3 gene in humans.25,26 Existing reports have revealed that STAT3 governs the progression of GC, such as cell proliferation, migration, metastasis, and invasion.27–29 It was, therefore, tempting to speculate that the roles of miR-125a in GC may be related to STAT3. In other respect, STAT3 expression level has been found to be closely involved in CD44 in GC,30 which is connected with hyaluronan synthase 1 (HAS1).31 HAS1 pertains to one of three hyaluronic acid (HA) synthases that synthesize HA32 and has been widely reported to serve as a modulator in tumors.33–35 As recently reported, HA-coated nanoparticles could significantly inhibit tumor growth in GC stem cells.36 Nevertheless, little is known about the relationship between STAT3 and HAS1 in GC. Therefore, it is of great interest to investigate regulation associations among miR-125a, STAT3, and HAS1 in GC cells, probably providing novel insights into the molecular mechanism underlying the miR-125a-induced suppression of tumorigenic properties in GC cells. In our study, we investigated the role of miR-125a targeting STAT3 in GC cell lines and the relationship between STAT3 and HAS1. Primarily, the transfection concentration of miR-125a mimic in GC cell lines was optimized. After that, the functional role of miR-125a in the viability, migration, and invasion of GC cells was determined. Through overexpression or knockdown experiments in miR-125a, STAT3, and HAS1, respectively, miR-125a targeting 3′-UTR of STAT3 was also verified by dual luciferase reporter assay, and their regulatory relationship was also analyzed in GC cells through quantitative polymerase chain reaction (qPCR) and Western blot assays. In addition, we further investigated the association between miR-125a and HAS1, so did the STAT3 and HAS1 via qPCR and Western blot assay.
Materials and methods
Cell lines and cell culture
Human GC cell lines, MKN45, SGC7901, and NCI-N87, were purchased from Conservation Genetics Chinese Academy of Sciences Cell Bank (Shanghai, China). All the cell lines were cultured in DMEM (Hyclone, Logan, UT, USA). Both media contained 10% FBS (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and cells were maintained at 37°C in a humidified atmosphere with 5% CO2.
Transfection
The chemically synthesized miR-125a mimics or inhibitor, and negative control (NC) were purchased from RiboBio (Guangzhou, China). The siRNA of STAT3 or HAS1, and NC were also obtained from RiboBio. The pcDNA-STAT3 was obtained from Sino Biological Inc (Beijing, China) and pcDNA 3.1 from Promega (Madison, WI, USA). Then, transfection with the indicated plasmids was mediated using Lipofectamine 2000 according to the manufacturer’s protocol. The cell post transfection was then prepared for the following assays.
RNA extraction and qPCR analysis
Total RNA was extracted from cultured cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer’s instructions. The RNAs were reversely transcribed into complementary DNA (cDNA) using the RevertAidTM H Minus First Strand cDNA synthesis Kit (Takara, Otsu, Japan). As for miRNAs, the cDNA was synthesized using miScript Reverse Transcription Kit (Qiagen). qPCR was performed using the SYBR PrimeScript qPCR kit (Takara) in a CFX Connect™ qPCR Detection System (BIO-RAD Laboratories, Inc., Berkeley, CA, USA) according to the manufacturer’s instructions. U6 for miR-125a and GAPDH for STAT3 and HAS1 were separately used as internal control. The specific primers were as follow: miR-125a forward 5′-GCGACTCCCTGAGACCCTTTAA-3′ and universal primer 5′-GCGAGCACAGAATTAATACGAC-3′; U6, forward 5′-CTCGCTTCGGCAGCACA-3′ and universal primer 5′-GCGAGCACAGAATTAATACGAC-3′; STAT3, forward 5′-GGAGGAGGCATTCGGAAAG-3′ and reverse, 5′-TCGTTGGTGTCACACAGAT-3′; HAS1, forward 5′-GTAGGGGCTGTTGGTGGGGAC-3′ and reverse 5′-TGAGCATGCGGTTGGTGAGGT-3′; GAPDH, forward 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. All reactions were performed in triplicate.
The optimization of transfected concentrations of miR-125a mimic
The optimization of transfected concentrations of miR-125a mimic was performed in MKN45 cells using qPCR. Briefly, cells were separately transfected with miR-125a mimic at concentrations of 0, 10, 30, 50, and 70 nM. After 48 hours of transfection, the relative expression of miR-125a was determined and calculated by qPCR.
Cell viability assay
Cell viability was measured using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) following the manufacturer’s protocol. Cells were transfected with miR-125a mimic at an optimal concentration or miR-125a inhibitor, and the corresponding NC mimic or NC inhibitor. The culture medium was replaced with DMEM containing 20% CCK-8 solution at 48 hours post transfection. Then, the optical density was measured at a wavelength of 450 nm using Multiskan FC (Thermo Fisher Scientific, Inc.). Based on the calculated number of viable cells, the growth curve was obtained.
Scratch wound healing assay
MKN45, SGC7901, and NCI-N87 cells were grown on plastic six-well plates at the density of 5×105 cells per well and cultured for 12 hours. Uniform wounds were scraped by a sterile pipette tip after transfection with miR-125a mimic or miR-125a inhibitor, and NC mimic or NC inhibitor for 24 hours. The wound closure was observed by microscope and photographed at 0, 24, and 48 hours after scratching.
Cell invasion assay
Transwell assay was performed to observe the invasive property of GC cells. Cells were cultured in serum-free DMEM after transfection for 48 hours. Cells were plated into the upper chamber that consisted of transwell-precoated Matrigel membrane filter (8 μm) and inserted pore in culture plates. After incubation for 48 hours, the cells remaining on the upper membrane were removed with cotton swabs, whereas those that had invaded through the membrane were fixed in 4% polyformaldehyde and stained with 0.1% crystal violet for 20 minutes at 4°C. The numbers of invaded cells on the lower chamber were calculated using photographic images. All experiments were performed at least three times independently.
Western blot analysis
Protein concentration was determined by Western blot analysis. Total cells were rinsed with ice-cold phosphate-buffered saline (cas. no P0013, Beyotime Biotechnology, Shanghai, China) and were boiled in SDS-sample buffer. Proteins from each sample (80 μg) were resolved by electrophoresing on 10% SDS-PAGE gels and then transferred onto polyvinylidene fluoride (PVDF) membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked for 60 minutes with 5% skim milk at room temperature, incubated with the primary antibody STAT3 (cas. no 9139s, 1:1,000, CST), GAPDH (cas. no AC001, 1:1,000, ABclonal) overnight at 4°C, and then incubated with corresponding secondary antibody (rabbit anti-mouse, cas. no 72293, 1:10,000, ABclonal) at 37°C for 1 hour. The PVDF membrane was developed using Immobilon western chemiluminescent HRP substrate (cas. no WBKLS0100, Millipore). Finally, Bio-Rad Gel Doc XR + system (Bio-Rad, Hercules, CA, USA) was employed to visualize the band.
Luciferase activity assay
Luciferase reporter assay was performed in MKN45, SGC7901, and NCI-N87 cells to verify if STAT3 was a direct target of miR-125a. The wild-type (WT) and mutant-type (MT) vectors of STAT3 3′-UTRs were separately cloned into a PGL-3 control vector (Promega). The sequences were listed as follow: WT 3′-UTR, forward 5′-CGGGGTACCTCCTTTGTAATGTATTGGCC-3′ and reverse 5′-CCGCTCGAGCACAGAAACTCTGATCAGCTG-3′; MT 3′-UTR, forward 5′-CGACGTGTCTGGTTGAGAATATGGTTCTTAGCCAGTTTC-3′ and reverse 5′-GGCTAAGAACCATATTCTCAACCAGACACGTCGCTGGG-3′. Cells were transfected with pGL3-WT-3′-UTR-STAT3 or pGL3-Mut-3′-UTR-STAT3 together with miR-125a mimic, miR-125a inhibitor, or control vector (VT). The pRL-TK vector was used as an internal control to normalize the transfection efficacy. After 48 hours of co-transfection, luciferase activity was measured using a dual-luciferase reporter assay system (Promega) according to the manufacturer’s instructions.
Statistical analysis
All in vitro experiments were performed in triplicate. Two-way ANOVA was applied for luciferase reporter assay. Unless otherwise mentioned, one-way ANOVA was employed to analyze the difference in multiple groups (>2). All statistical calculations and analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as the mean ± SD. P<0.05 was considered to indicate a statistically significant difference.
Results
The optimal transfection concentration of miR-125a mimic in MKN45 cells
In MKN45 cells, we observed that the expression of miR-125a reached the top when miR-125a mimic at a concentration of 50 nM among different concentrations (Figure 1, P<0.05).
miR-125a inhibited the migration and invasion of MKN45, SGC7901, and NCI-N87 cells without altering the cells viability in vitro
Our data identified that miR-125a did not significantly affect the viability of MKN45, SGC7901, and NCI-N87 cells in vitro through CCK-8 assay (Figure 2A). Reciprocally, wound healing and transwell assay suggested that the migration and invasion abilities were obviously suppressed in the three cell lines transfected with miR-125a mimic when compared with NC group, but advanced in cells treated with miR-125a inhibitor. In addition, in order to investigate if miR-125a showed GC suppressive effect by STAT3, we examined these functional assays in cells treated with si-STAT3. Cells with si-STAT3 evidently attenuated the migration and invasion compared with NC siRNA group, which showed similar phenotype to the results in miR-125a mimic group, so was the case in si-HAS1 group (Figure 2B and C). Thereinto, the confirmation of HAS1 reduction in si-HAS1 experiment at mRNA and protein levels is presented in Figure S1.
STAT3 was a target of miR-125a in GC cells
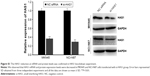
Based on the bioinformatics analysis, miR-125a target genes were identified using four web-based bioinformatics algorithms: microRNA.org (http://www.microrna.org/microrna/home.do), miRDB (http://mirdb.org/), TargetScan Human (http://www.targetscan.org/vert), and miRTar (http://mirtar.mbc.nctu.edu.tw/human/), which predict miRNA-binding sites based on complementarity to the nucleotide sequence of the miRNA. The algorithms used identified highly complementary sites. The results of miRTar indicated that the 3′-UTR of STAT3 binds to miR-125a with a high score. Beyond this, previous studies have evidenced that miR-125a could directly target STAT3.23,24 Our luciferase activity assay was also consistent with previous results. As shown in Figure 3A, the putative miR-125a could bind to the sequence of STAT3 in the 3′-UTR. Furthermore, our results indicated that luciferase activity of miR-125a mimic group was obviously reduced in WT 3′-UTR of STAT3 group, and miR-125a inhibitor group was dramatically increased (Figure 3B, P<0.01). Nevertheless, it was not significantly altered in cells with MT 3′-UTR of STAT3 group. In addition, qPCR and Western blot assay suggested that mRNA and protein expression levels of STAT3 were decreased in MKN45 and NCI-N87 cells treated with miR-125a mimic groups compared with the NC mimic groups, but no significant difference had been found in SGC7901 cells (Figure 3C, P<0.05). To further verify the relationship between miR-125a and STAT3, MKN45, and NCI-N87 were chosen and transfected with miR-125a inhibitor, separately. Compared with the NC group, we observed that the mRNA expression and protein level of STAT3 were significantly upregulated in two cell lines (Figure 3D, P<0.05). In the meantime, as an additional support, we found that STAT3 overexpression contributed to the reduction of miR-125a expression in MKN45 and NCI-N87 cells, implying that STAT3 could rescue the effect of miR-125a (Figure S2). These results suggested that miR-125a could inversely regulate the expression of STAT3.
STAT3 upregulates HAS1 expression
To identify the association between STAT3 and HAS1, MKN45 and NCI-N87 cell lines were selected since the outcomes of STAT3 in SGC7901 cells was not significant in overexpression experiment of miR-125a. Primarily, our data suggested that mRNA and protein levels of HAS1 in two cell lines were obviously reduced after transfection of miR-125a mimic, while increased after transfection of miR-125a inhibitor (Figure 4A and B, P<0.05). Further experiments were carried out with overexpression of STAT3 in two cell lines and the results showed that mRNA and protein levels of HAS1 were promoted (Figure 4C, P<0.05), whereas inverted results were obtained with the knockdown of STAT3 in MKN45 and NCI-N87 cells as displayed in Figure 4D (P<0.05). Conjointly, it could be concluded that STAT3 expression was linked with HAS1 expression and overexpression of STAT3 promoted HAS1 expression in GC cells, but its knockdown showed opposite effects.
Discussion
Important studies have been made in cancer treatments like surgery, radiotherapy, chemotherapy, or Chinese medicine treatments,3,5,37,38 but current treatments for GC still remains to be improved because patients often suffer from tumor recurrence.39 It is therefore important to determine the pathogenesis of GC. To date, numerous miRNAs have been discovered as important regulators in biological processes by binding to the 3′-UTR of their target genes.8–10 Many miRNAs have been reported to play essential roles in the tumorigenesis and development of various human cancers.14–18 Previous studies have reported that miR-125a is commonly aberrantly expressed in a variety of human cancers.19,20,40–43 In particular, miR-125a has also been found to be closely connected with GC. For example, Nishida et al revealed that miR-125a could inhibit GC development by targeting human epidermal growth factor receptor-2.21 Another example is that miR-125a probably targets vascular endothelial growth factor A to influence the progression of GC.22 STAT3 is a well-characterized transcription factor that has been identified as target gene of miR-125a in certain cancers, such as cervical cancer and lung carcinoma.23,24 However, little research has been conducted to show the roles of miR-125a targeting STAT3 in GC. Additionally, STAT3 has been reported to be closely related to CD44 in GC,30 and an existing study suggested that CD44 is influenced by HAS1.31 HAS1 pertains to one of three HA synthases that synthesize HA,32 which is widely reported as a modulator in tumors.33–35 Existing evidence has shown that upregulated HA-coated nanoparticles could promote the progression of GC stem cells.36 It was thus conjectured that regulation association among miR-125a, STAT3, and HAS1 was one of the contributors to GC cells, which promoted us to conduct this work.
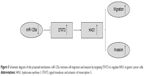
In our study, we primarily determined the optimal transfection concentration of miR-125a mimic in GC cells. The functional assays suggested that despite without altering the viability of GC cells, overexpression of miR-125a constrained the migration and invasion abilities of GC cells. Meanwhile, its knockdown facilitated those abilities. In fact, some evidence has indicated that ectopic expression of miR-125a-5p substantially inhibited the GC progression.21,44 More importantly, we identified that the knockdown of STAT3 or HAS1 in GC cells obviously inhibited the migration and invasion abilities. Using bioinformatics analysis, we found that the 3′-UTR of STAT3 binds to miR-125a with a high score. To further identify the association, luciferase assay was conducted, which demonstrated that miR-125a could directly target 3′-UTR of STAT3. Our result was also in accordance with previous studies.23,24 In addition, the overexpression of miR-125a resulted in the reduction of STAT3 in MKN45 and NCI-N87 cells treated with miR-125a mimic groups, but no significant difference had been found in SGC7901 cells. In order to further identify the relationship between miR-125a and STAT3, MKN45 and NCI-N87 were chosen and transfected with miR-125a inhibitor, which indicated that the STAT3 mRNA expression was significantly upregulated as well as at protein level in two cell lines. These findings may suggest that miR-125a could inversely regulate the STAT3 expression in GC cells. In addition, the association of miR-125a and HAS1 was analyzed in MKN45 and NCI-N87 cells using qPCR and Western blot assays, which indicated that the mRNA and protein levels of HAS1 were decreased in cells transfected with miR-125a mimic groups, while increased in miR-125a knockdown experiment. Furthermore, the relationship between STAT3 and HAS1 was explored. We observed that HAS1 expression was obviously enhanced in cells transfected with pcDNA STAT3 and reduced after transfection of STAT3 siRNA, which suggested that STAT3 was positively related with HAS1 expression. Collectively, as presented in Figure 5, our findings outlined possibilities that miR-125a may influence cell migration and invasion via targeting STAT3 to interact with HAS1 in GC progression. Of course, we have to acknowledge the existing limitations of this work, for instance: 1) the functional role of HAS1 in GC is not investigated; 2) it is not clear how reduced expression of STAT3 and subsequently HAS1 impact GC progression; 3) are there more cancers other than GC that HAS1 also acts on? Further work should therefore be included for exploring these issues.
Conclusion
In conclusion, these findings provide a novel insight into the mechanism of miR-125a associated with STAT3 and HAS1 in GC cells. To the best of our knowledge, this work might serve as evidence for the primary regulation association among miR-125a, STAT3, and HAS1 in GC cells. Our findings highlight that miR-125a could restrain cell migration and invasion by targeting STAT3 to regulate HAS1 in GC cells.
Acknowledgment
This work was supported by the PLA Youth Training Project for Medical Science (15QNP093).
Disclosure
The authors report no conflicts of interest in this work.
References
Lin X, Zhao Y, Song WM, Zhang B. Molecular classification and prediction in gastric cancer. Comput Struct Biotechnol J. 2015;13:448–458. | ||
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. | ||
Coburn NG. Lymph nodes and gastric cancer. J Surg Oncol. 2009;99(4):199–206. | ||
Liu X, Xiu LJ, Jiao JP, et al. Traditional Chinese medicine integrated with chemotherapy for stage IV non-surgical gastric cancer: a retrospective clinical analysis. J Integr Med. 2017;15(6):469–475. | ||
Pan HW, Li SC, Tsai KW. MicroRNA dysregulation in gastric cancer. Curr Pharm Des. 2013;19(7):1273–1284. | ||
Li L, Liu Y. Diverse small non-coding RNAs in RNA interference pathways. Methods Mol Biol. 2011;764:169–182. | ||
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. | ||
Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA. 2005;11(12):1753–1761. | ||
Shruti K, Shrey K, Vibha R. Micro RNAs: tiny sequences with enormous potential. Biochem Biophys Res Commun. 2011;407(3):445–449. | ||
Stewart CR, Deffrasnes C, Foo CH, Bean AGD, Wang LF. A functional genomics approach to henipavirus research: the role of nuclear proteins, microRNAs and immune regulators in infection and disease. Curr Top Microbiol Immunol. Epub 2017 Jul 4. | ||
Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7(36):58595–58605. | ||
Yu F, Liu JB, Wu ZJ, et al. Tumor suppressive microRNA-124a inhibits stemness and enhances gefitinib sensitivity of non-small cell lung cancer cells by targeting ubiquitin-specific protease 14. Cancer Lett. 2018;427:74–84. | ||
Chang YL, Zhou PJ, Wei L, et al. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6(27):24017–24031. | ||
Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. | ||
Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs – the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9(4):293–302. | ||
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. | ||
Qu Y, Zhang H, Sun W, et al. MiR-155 promotes gastric cancer growth and invasion by negatively regulating transforming growth factor beta receptor 2. Cancer Sci. 2018;109(3):618–628. | ||
Narita Y, Muro K. Challenges in molecular targeted therapy for gastric cancer: considerations for efficacy and safety. Expert Opin Drug Saf. 2017;16(3):319–327. | ||
Potenza N, Mosca N, Zappavigna S, et al. MicroRNA-125a-5p is a downstream effector of Sorafenib in its antiproliferative activity toward human hepatocellular carcinoma cells. J Cell Physiol. 2017;232(7):1907–1913. | ||
Zang Z, Guan W, Chen D, Han Y, Shi Z, Zhou J. Association between microRNA-125a rs12976445 C>T polymorphism and 18F-Fluorodeoxyglucose (18FDG) uptake: clinical and metabolic response in patients with non-small cell lung cancer. Med Sci Monit. 2016;22:4186–4192. | ||
Nishida N, Mimori K, Fabbri M, et al. MicroRNA-125a-5p is an independent prognostic factor in gastric cancer and inhibits the proliferation of human gastric cancer cells in combination with trastuzumab. Clin Cancer Res. 2011;17(9):2725–2733. | ||
Dai J, Wang J, Yang L, Xiao Y, Ruan Q. miR-125a regulates angiogenesis of gastric cancer by targeting vascular endothelial growth factor A. Int J Oncol. 2015;47(5):1801–1810. | ||
Fan Z, Cui H, Xu X, et al. MiR-125a suppresses tumor growth, invasion and metastasis in cervical cancer by targeting STAT3. Oncotarget. 2015;6(28):25266–25280. | ||
Zhong L, Sun S, Shi J, Cao F, Han X, Chen Z. MicroRNA-125a-5p plays a role as a tumor suppressor in lung carcinoma cells by directly targeting STAT3. Tumour Biol. 2017;39(6):1010428317697579. | ||
Zhang L, Li J, Wang Q, et al. The relationship between microRNAs and the STAT3-related signaling pathway in cancer. Tumour Biol. 2017;39(7):1010428317719869. | ||
Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. | ||
Zhou J, Wu A, Yu X, Zhu J, Dai H. SIRT6 inhibits growth of gastric cancer by inhibiting JAK2/STAT3 pathway. Oncol Rep. 2017;38(2):1059–1066. | ||
Tuo H, Shu F, She S, et al. Sorcin induces gastric cancer cell migration and invasion contributing to STAT3 activation. Oncotarget. 2017;8(61):104258–104271. | ||
Ma DH, Li BS, Liu JJ, et al. miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett. 2017;408:23–32. | ||
Wei B, Sun X, Geng Z, et al. Isoproterenol regulates CD44 expression in gastric cancer cells through STAT3/MicroRNA373 cascade. Biomaterials. 2016;105:89–101. | ||
Siiskonen H, Kärnä R, Hyttinen JM, Tammi RH, Tammi MI, Rilla K. Hyaluronan synthase 1 (HAS1) produces a cytokine- and glucose-inducible, CD44-dependent cell surface coat. Exp Cell Res. 2014;320(1):153–163. | ||
Nguyen N, Kumar A, Chacko S, Ouellette RJ, Ghosh A. Human hyaluronic acid synthase-1 promotes malignant transformation via epithelial-to-mesenchymal transition, micronucleation and centrosome abnormalities. Cell Commun Signal. 2017;15(1):48. | ||
Ghosh A, Kuppusamy H, Pilarski LM. Aberrant splice variants of HAS1 (Hyaluronan Synthase 1) multimerize with and modulate normally spliced HAS1 protein: a potential mechanism promoting human cancer. J Biol Chem. 2009;284(28):18840–18850. | ||
Yamada Y, Itano N, Narimatsu H, et al. Elevated transcript level of hyaluronan synthase1 gene correlates with poor prognosis of human colon cancer. Clin Exp Metastasis. 2004;21(1):57–63. | ||
Adamia S, Reichert AA, Kuppusamy H, et al. Inherited and acquired variations in the hyaluronan synthase 1 (HAS1) gene may contribute to disease progression in multiple myeloma and Waldenstrom macroglobulinemia. Blood. 2008;112(13):5111–5121. | ||
Yang W, Zhang H, Xin L. A novel design of HA-coated nanoparticles co-encapsulating plasmid METase and 5-Fu shows enhanced application in targeting gastric cancer stem cells. Biol Chem. 2018;399(3):293–303. | ||
Shen SJ, Zhang YH, Gu XX, Jiang SJ, Xu LJ. Yangfei Kongliu Formula, a compound Chinese herbal medicine, combined with cisplatin, inhibits growth of lung cancer cells through transforming growth factor-β1 signaling pathway. J Integr Med. 2017;15(3):242–251. | ||
Lin WF, Lu JY, Cheng BB, Ling CQ. Progress in research on the effects of traditional Chinese medicine on the tumor microenvironment. J Integr Med. 2017;15(4):282–287. | ||
Kunisaki C, Makino H, Kimura J, et al. Impact of lymphovascular invasion in patients with stage I gastric cancer. Surgery. 2010;147(2):204–211. | ||
Qin X, Wan Y, Wang S, Xue M. MicroRNA-125a-5p modulates human cervical carcinoma proliferation and migration by targeting ABL2. Drug Des Devel Ther. 2016;10:71–79. | ||
Chen H, Xu Z. Hypermethylation-associated silencing of miR-125a and miR-125b: a potential marker in colorectal cancer. Dis Markers. 2015;2015:345080–345087. | ||
Yin F, Zhang JN, Wang SW, et al. MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PLOS One. 2015;10(1):e0116759. | ||
Chen D, Li Y, Su Z, et al. Identification of miR-125a-5p as a tumor suppressor of renal cell carcinoma, regulating cellular proliferation, migration and apoptosis. Mol Med Rep. 2015;11(2):1278–1283. | ||
Xu Y, Huang Z, Liu Y. Reduced miR-125a-5p expression is associated with gastric carcinogenesis through the targeting of E2F3. Mol Med Rep. 2014;10(5):2601–2608. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.