Back to Journals » OncoTargets and Therapy » Volume 13
MiR-101-3p and Syn-Cal14.1a Synergy in Suppressing EZH2-Induced Progression of Breast Cancer
Authors Jiang H, Li L, Zhang J, Wan Z, Wang Y, Hou J, Yu Y
Received 5 June 2020
Accepted for publication 3 August 2020
Published 28 September 2020 Volume 2020:13 Pages 9599—9609
DOI https://doi.org/10.2147/OTT.S264600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Huabo Jiang1 ,* Li Li2 ,* Jingjing Zhang,3 Zhong Wan,4 Yuanyuan Wang,5 Jingjing Hou,6 Yongsheng Yu1
1Clinical and Translational Research Center, Shanghai First Maternity and Infant Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China; 2Assisted Reproduction Technology Center, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 3Department of Plastic Surgery, Zhongshan Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China; 4Urologic Medical Center, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 5Department of Health Medicine, Zhongshan Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China; 6Department of Gastrointestinal Surgery, Institute of Gastrointestinal Oncology, Zhongshan Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingjing Hou; Yongsheng Yu Email [email protected]; [email protected]
Objective: EZH2 is the catalytic subunit of the polycomb repressive complex 2 (PRC2) and has been documented as an oncogene in breast cancer. The microRNA (miR)-101-3p can suppress breast cancer progression by targeting with EZH2. Syn-cal14.1a, a synthetic peptide derived from Californiconus californicus (Cal14.1a), can decrease the cell viability and activate the cell apoptosis in cancer. In this study, we explored whether the synergy of miR-101-3p mimic and syn-cal14.1a could inhibit the expression of EZH2. We also investigated this binding treatment’s effects on the suppression of breast cancer cells.
Methods: MiR-101-3p mimic was transfected and syn-cal14.1a was added in SK-BR-3 and MCF-7 breast cancer cells. The expression of EZH2 protein level was determined. Then, cell proliferation, migration, invasion, and apoptosis were observed.
Results: MiR-101-3p and syn-cal14.1a, when applied together, exerted a synergistic anti-EZH2 expression in breast cancer cells. The combination of miR-101-3p and syn-cal14.1a synergistically suppressed the EZH2-induced breast cancer cell migration, invasion, and proliferation. In parallel, this synergy treatment was able to promote the apoptosis of breast cancer cells. To our knowledge, this is the first report describing inhibition of EZH2 in human breast cancer cell lines by syn-cal14.1a.
Conclusion: The anti-EZH2 roles of miR-101-3p and/or syn-cal14.1a could provide an effective therapeutic strategy in breast cancer. These data provide significant insights into molecular mechanisms of breast cancer and may have benefits in clinical therapeutics for breast cancer.
Keywords: breast cancer, miR-101-3p, conotoxins, synthetic peptide, EZH2, synergism
Introduction
Breast cancer (BC) is one of the leading causes of cancer-related death in women. Although significant progress has been made in the early detection and treatment of breast cancer, it has caused 571,000 deaths each year and ranked first among all female tumors.1,2 It is often diagnosed at a relatively young (around 55 years) age, and surgery is the preferred treatment for non-metastatic breast cancer; assisted radiotherapy or chemotherapy can effectively reduce tumor recurrence,3 while metastasis and recurrence are still the major causes of high mortality rates of breast cancer patients.4 Hence, it is vital to understand the molecular mechanisms that can benefit in the design of better and more available therapeutic strategies to improve breast cancer patients’ outcomes.
EZH2 is a Polycomb Group (PcG) protein homologous to Drosophila Enhancer of Zeste, and is one of the most studied histone methyltransferases.5 It serves as the catalytic subunit of the polycomb repressive complex 2 (PRC2). PRC2, one of the two major classes of Polycomb complexes, can control gene silencing extensively through tri-methylation of lysine 27 on histone H3 (H3K27me3), and plays a critical role in the process of cell proliferation, apoptosis, and carcinogenesis.6–8 Alterations in EZH2, which antagonize polycomb function, have been described in multiple cancer subtypes including breast cancer.9 Kleer et al 10 were the first to find that EZH2 is aberrantly overexpressed in breast cancer, and the upregulated expression of EZH2 is closely related with poor prognosis and aggressive biological level.
MicroRNAs (miRs) are a class of endogenous, small non-coding RNA molecules (containing 20 to 25 nucleotides). These miRNAs can bind to the 3ʹ-untranslated region of the target gene (3ʹ-UTR), resulting in degradation of the mRNA or inhibition of the expression of protein, thus to regulate downstream physiological functions and pathological processes.11,12 Interestingly, most human miRs are associated with carcinogenesis.13,14 Among them, miR-101 is a tumor suppressor and closely related to the occurrence and development of tumors. MiR-101 has shown a strong anti-cancer effect in multiple tumors such as prostate cancer, liver cancer, and lung cancer. It has been elucidated that EZH2 is a target gene for miR-101 in prostate and bladder cancer.15,16 EZH2 has been found to regulate the development of breast cancer cells through interacting with miR-101, but the regulatory mechanism of EZH2 expression and its relationship with miR-101 in the development of breast cancer have not been fully elucidated.16,17
Venom peptides isolated from predatory marine cone snails (Conus) have been developed for treating several human diseases.18 The genus Conus are composed of 500–700 species19,20 and each Conus species generates 100–400 venom toxins.21,22 The family of α-conotoxins is the biggest group of characterized Conus peptides. Due to their disulfide bond of these Conus peptides, the compact loop structures are stabilizable and in charge of receptor subtype selectivity and resistance to proteases. A 17 amino acid synthetic peptide, based on a native toxin (cal14.1a) derived from the Californiconus californicus, obtains the high receptor affinity and the α-conotoxins activity because of its amino acid residues and cysteine pattern. This synthetic peptide is active against acetylcholine nicotinic receptors and decreases the viability and induces the apoptosis of lung cancer cells via reducing expression of the pleiotropic transcription factor NF-κB1.23
In the present study, we investigated the effect of the binding of miR-101-3p and syn-cal14.1a onto EZH2 during breast cancer development. According to the IHC and Western Blot assay, EZH2 was overexpressed in breast cancer. Significantly, we found that the combination of miR-101-3p and syn-cal14.1a synergized the inhibition of breast cancer cells through suppressing the expression of EZH2. To our knowledge, this is the first report describing inhibition of EZH2 in human breast cancer cell lines by syn-cal14.1a. These findings suggested that EZH2 plays a critical role in breast cancer carcinoma, and the synergy treatment provides a new experimental clue for the application of breast cancer treatments.
Materials and Methods
Tissue Specimens
Paired human breast cancer tissues and adjacent non-tumor breast tissues were collected from patients who underwent surgery at the Shanghai First Maternity and Infant Hospital (China) and had not received any local or systemic treatments. Normal breast tissues were collected from patients receiving benign breast tumors resection. Tumor tissues and adjacent non-tumor breast tissues were confirmed by histological method. This study provides written informed consent, which is in accordance with the Helsinki Declaration of 1975. Clinicopathological features are shown in Supplementary Table 1.
The ethics committee of the Shanghai First Maternity and Infant Hospital (China) approved this study (approval number KS18183). This study was performed in accordance with the Declaration of Helsinki.
Immunohistochemistry
The breast cancer and normal breast tissues were fixed in 10% buffered formalin and embed in paraffin. All sections were stained routinely with hematoxylin-eosin (HE) for diagnosis and revision of malignancy grade. IHC reactions were performed on 4 μm thick sections by using a BenchMark XT IHC Autostainer module (Roche, Switzerland). Immunohistochemical scores (HIS) were performed according to the intensity of staining in the previous study:24 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). According to the percentage of positive cells: 0 (≤5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), or 4 (>75%); the combination of the two scores, a final immunoreactivity score <3 is divided into negative (-), and >3 is divided into positive (+), (++), or (+++) by using a single blind method for reading. The antibody EZH2 was used (CST5246, Cell Science Technology, USA) for Western blot assay.
Cell Culture
Normal human epithelial mammary cell lines MCF-10A were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Human breast cancer SK-BR-3 and MCF-7 were obtained from the Cell Bank of Clinical and Translational Research Center (Shanghai, China). The miR-101-3p mimic (miR-mimic) and negative control (mimic-NC) were designed according to the miRBase database and purchased from RiboBio (Guangzhou, China). The sequences of synthesized RNA oligo are shown in Supplementary Table 2. MCF-7 and SK-BR-3 cells were grown in 6-well plates to 70–80% confluence overnight. The relative group cells were transfected with miR-101-3p mimic or mimic-NC at the concentration of 100 nM by Lipofectamine 2000 reagents (Life Technology, USA) according to the product’s instructions. After 24 hours transfection, the relative group cells were treated by syn-cal14.1a or negative control peptides at the final concentration of 30 μM for another 24 hours. The peptides were synthesized by Shanghai Apeptide Biological Technology Co., Ltd (Shanghai China), and purity was over 95%. All the experiments in vitro such as Western Blot, proliferation, wound healing, migration, invasion, and apoptosis assay cells were treated according to the above describe procedures. (Blank: Treated by PBS; NC: Treated by mimic-NC miRNA and negative control peptide; miR-mimic: Treated by miR-101-3p mimic miRNA; syn-cal14.1a: Treated by syn-cal14.1a peptide; synergy: Treated by miR-101-3p mimic miRNA and syn-cal14.1a peptide).
Cell Proliferation Analysis
The Cell Counting Kit-8 (CCK-8, SAINT-BIO, China) was used according to the manufacturer’s protocol to test cell proliferation ability. Five thousand cells per well were seeded into 96-well plates at a volume of 100 μL of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS). All the cells were treated as described in 4.3. Then, 10 μL CCK-8 was added to each well of the plate. The plates were incubated for 1–4 hours at 5% CO2 and 37°C. The absorbance was measured at 450 nm by using a microplate reader.
Wound Healing Assay
Next, 2.5–5×105 cells were added to each well of a 6-well plate (the principle of inoculation was 100% after overnight). After miRNAs transfection for 24 hours, an artificial wound was scratched into a monolayer of each well with a sterile plastic 200 μL micropipette. Then the relative group cells were treated by peptides. After wounding, the cells were washed three times with sterile PBS, and fresh serum-free medium was replaced. The cells were cultured in a 37°C, 5% CO2 incubator. The medium were then removed at 0 and 24 hours. The width of the scratches was observed and measured under a microscope and photographed.
Cell Migration and Invasion Assay
The migratory and invasive ability of breast cancer cells were conducted by using Transwell chambers (polycarbonate, 8 μm pores, BD Biosciences, USA) according to the manufacturer’s protocol. For the cell migration assay, cells (5–10×103) in 100 μL of serum free DMEM were seeded into the upper chamber after 48 hours transfection. For the invasion assay, the inserts were pre-coated with 40 µL Matrigel (1:6 dilution; BD Biosciences). Then the cells were treated as the cell migration assay. Then 10% FBS of DMEM was added into the lower chamber and used as a chemoattractant to induce breast cancer cells migration or invasion walk through the membrane. All the cells were treated as describe in 4.3. The migrated or invaded cells across the Transwell membrane were stained with crystal violet and counted under an optical microscope.
Apoptosis Assay by Flow Cytometry Analysis
All the cells were treated as described in 4.3. Cells were collected by centrifugation (2000 rpm for 5 minutes). Then, 1–5×105 was collected after being washed twice with PBS. Then, 500 μL of binding buffer and 5 μL of Annexin V-FITC were added into the suspension. The suspension cells were treated with 5 μL of propidium iodide at room temperature for 5–15 minutes and then analyzed through a flow cytometer within 1 hour.
Western Blot
All the cells were treated as described in 4.3. Protein samples were separated by 10% SDS PAGE gel and transferred to a PVDF membrane by constant 250 mA electrophoresis. The membranes were blocked in 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween-20 (TBST) for 1 hour at room temperature and incubated overnight at 4°C with the primary rabbit antibodies, EZH2 antibody (1:1000 dilution; Cat:4950S; Cell signaling technology), PARP antibody (1:1000 dilution; Cat:9532S; Cell signaling technology), Cleaved PARP antibody (1:1000 dilution; Cat:5625T; Cell signaling technology), and GAPDH antibody (1:1000 dilution; Cat:5174S; Cell signaling technology). After several rinses in TBST, the membranes were incubated for 1 hour at room temperature with the appropriate volume of secondary antibody. Immunoreactivity was detected by enhanced chemiluminescence (ECL, Millipore, USA).
Statistical Analysis
The Loewe additivity model was performed to confirm the inhibitors interaction between miR-mimic and syn-cal14.1a in suppressing cell growth. In this model, the combination index (CI) was defined as CI=d1/D1+d2/D2, where D1 and D2 were the doses of miR-mimic and syn-cal14.1a that produced an inhibition rate of SK-BR-3 or MCF-7 cells growth when used alone, d1 and d2 were the doses of miR-mimic and syn-cal14.1a in combination in the present study. If the CI is equal, less than or more than 1, the combination dose (d1, d2) is termed as additive, synergistic, or antagonistic, respectively.
The data analysis of this study was performed using the GraphPad Prism5 software (La Jolla, CA, USA). One-way ANOVA with Bonferroni’s post-test was used for multiple comparisons and the Student’s t-test (two-tailed) was used for pair-wise comparisons. P<0.05 indicates that the difference was statistically significant.
Results
EZH2 Is Significantly Upregulated in Human Breast Cancer Clinical Samples and Cells
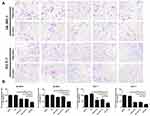
We collected 10 clinical breast cancer tissue samples and performed an immunohistochemistry experiment to explore the relationship between the expression of EZH2 and the breast cancer. It was found that all breast cancer tissue samples were positive, but negative in normal breast tissues or adjacent tissues (Figure 1A–F) and closely related to lymph node metastasis and prognosis in breast cancer. As shown in Supplementary Table 1, lymph node status was absent in five of the 10 and distant metastasis was found in all 10 of the human gastric cancer patients. Significant upregulation of EZH2 protein was observed in two breast cancer cell lines SK-BR-3 and MCF-7 compared with human non-tumorigenic epithelial cell line MCF-10A (Figure 2A and B). Thus, we chose SK-BR-3 and MCF-7 cells for the following experiments.
Synergy of MiR-101-3p and Syn-Cal14.1a in Down Regulation of EZH2
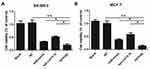
EZH2 was predicted a potential target of miR-101-3p, as previously described.15,25,26 We used Western Blot assay to reveal the combination of miR-101-3p mimic and syn-cal14.1a (GDCPPWCVGARCRAEKC)23 against the expression of EZH2 in SK-BR-3 cells. The protein expression of EZH2 is inhibited after transfection with miR-101-3p mimic after 48 hours. Interestingly, the same result was obtained in the cells treated by syn-cal14.1a at the final concentration 30 μM after 24 hours. Compared with the other groups, treatment of breast cancer SK-BR-3 cells with synergy of miR-101-3p and syn-cal14.1a can remarkably suppress the expression of EZH2 (Figure 3A and B). It indicates miR-101-3p combined with syn-cal14.1a exerted a synergistic effect on the expression of oncogene EZH2 in breast cancer cells. As far as we know, this is the first report introducing inhibition of the expression of EZH2 in breast cancer cells by syn-cal14.1a isolated from Conus californicus.
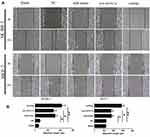
Anti-Spreading and Proliferation Effects of Synergy on Breast Cancer Cells
To determine the anticancer activity, the cytotoxic effect of the combination of miR-101-3p and syn-cal14.1a on cell spreading and proliferation of breast cancer cells was evaluated. For the scratch wound healing assay, the cancer cells would spread into the scratch area after 24 hours of treatment. Results showed treatment of miR-101-3p or syn-cal14.1a alone did not significantly stop the cells from closing the gap, but the synergy treatment significantly reduced the widths of the scratch, indicated by the wider distance of the two cell fronts (Figure 4A and B). This indicates that the synergy treatment can decrease the mobility of breast cancer cells and thereby prevent the cells spreading out, presumably through the interaction with EZH2. Next we examined the anti-proliferation effect of synergy treatment of SK-BR-3 and MCF-7 cells with CCK8 assay. Compared with other groups, the combined treatment significantly suppressed the proliferation of the two breast cancer cells. Note that the anti-proliferation effect is different from the toxicity effect. Prolonged treatment by synergy under the condition of cell proliferation (48 hours growth) showed a significant anti-proliferative effect (Figure 5A and B); indicating that this effect may be due to the suppression of cell proliferation through the blockage of an EZH2-mediated proliferation signal. When the miR-101-3p-mimic (d1:100nM) and syn-cal14.1a (d2:30μM) were used together, the inhibition rate was about 90.2% or 91.5% for SK-BR-3 or MCF-7, respectively. SK-BR-3 and MCF-7 cells were treated with varying concentrations of miR-101-3p-mimic or syn-cal14.1a when used alone for 48 hours. When generating the same inhibition rate, the concentrations were as below: miR-101-3p-mimic (D1:165.05922 nM), syn-cal14.1a (D2:112.36742 μM) for SK-BR-3 and miR-101-3p-mimic (D1: 208.33173 nM), syn-cal14.1a (D2:101.21973 μM) for MCF-7. The CI value was 0.873 or 0.776, respectively. These clearly illustrated a synergistic inhibition in both two cells (Supporting information Figure 1A and 1B).
Anti-Migratory Effect of Synergy on Breast Cancer Cells
Cell migration and invasion is responsible for tumor metastasis of neoplasms.27 Hence, the suppression of migration and invasion is a useful therapeutic strategy for ablation of tumor progression.28 The migration of cancer cells via the lower side of a Transwell system serves as a first step in the process of metastasis.29 A Matrigel-coated filter with Transwell chamber was widely used to mimic cancer's natural condition and evaluate the invasive ability of cancer cell lines. We used Transwell chambers to evaluate the migratory and invasive ability of breast cancer cells. Although treatment of miR-101-3p or syn-cal14.1a alone inhibits the migration and invasion of SK-BR-3 and MCF-7 cells, the inhibition rate is not significant. However, the synergy treatment greatly reduced the above behaviors of breast cancer cells (Figure 6A and B). This indicates miR-101-3p combined with syn-cal14.1a exerted a synergistic effect on the migration and invasion inhibition of breast cancer cells.
Apoptotic Effect of Synergy on Breast Cancer Cells
To confirm the apoptotic effect of the synergy treatment, firstly we carried out a flow cytometry assay to determine the apoptosis of SK-BR-3 and MCF-7 cells. The results showed a significant increase in synergy induced apoptosis compared with other groups. We found treatment of miR-101-3p or syn-cal14.1a alone caused an about 40% or 35% apoptotic rate, but the percentage of apoptotic cells induced by synergy treatment increased to 70% in SK-BR-3 cells. A similar result was obtained in MCF-7 cells (Figure 7A and B). Consistently, we performed Western Blot analysis to detect the cell-specific apoptosis marker (cleaved PARP) in breast cancer cells. It was highly significant that the use of a mixture of both miR-101-3p mimic in combination with syn-cal14.1a proved to be the most effective treatment, resulting in a dramatic increased expression of cleaved PARP (Figure 7C and D). This is probably due to the synergistic effect. Conversely, treatment of miR-101-3p or syn-cal14.1a alone produced much lower expression of cleaved PARP as compared with the synergy group. In summary, these data demonstrated that the synergy treatment enhanced EZH2 inducing apoptosis of breast cancer cells.
Discussion
Epigenetic dysregulation plays an important role in the cause of tumor progression.30 EZH2 belongs to the epigenetic regulatory factor PcG protein family which plays a transcriptional repressive role in the form of mutually inhibitory complex. Several clinical studies have demonstrated that overexpression of EZH2 positively correlated with histological grade, clinical stage, lymph node metastasis, as well as poor prognosis in breast cancer.31 The abnormal expression of PcG protein is closely related to tumor occurrence and development. EZH2 is an attractive target for developing therapeutic inhibitors to treat breast cancer patients. Knockdown or knockout of EZH2 expression significantly inhibits breast cancer cell proliferation and promotes apoptosis in vitro, and also produces anti-tumor effects in vivo.32 Fortunately, multiple EZH2 inhibitors have been produced,33 and some of these specific inhibitors have entered Phase I–II clinical trials. For instance, Song et al32 recently found a highly specific small molecule EZH2 inhibitor - ZLD1039, which can exert strong tumor inhibition in breast cancer xenograft mice. It has predominant oral bioavailability and is a suitable in vivo experiment.
MicroRNAs (miRs) are small non-coding RNAs and about 20 nucleotides in length. Due to the properties of being highly conserved and endogenous, miRs are widely used as suppressors of cancer cells. MiR-101-3p has been reported to be a cancer inhibitor through targeting of multiple oncogenes. Although miR-101-3p was confirmed to regulate EZH2 expression in breast cancers in our experiments, other studies found that EZH2 was not only be epigenetically regulated by miR-101-3p but also modulated by several other tumor suppressor miRs, such as miR-340 and miR-138.34 Wu et al35 reported that the long noncoding RNA RC3H2 as a completive endogenous RNA sponging miR-101-3p targets EZH2 and facilitates the malignant behavior of oral squamous cell carcinoma (OSCC). Qiu et al36 demonstrated that long non-coding RNAs PSMA3-AS1 up-regulated EZH2 expression by competitively binding to miR-101 in Esophageal Squamous Cell Carcinoma (ESCC). The small peptides α-conotoxins, obtained from the venom of cone snails, can target nicotinic acetylcholine receptors (nAChRs) as the competitive antagonists. α-conotoxins are known as the smallest conopeptides derived from Conus sp. Syn-cal14.1a is a kind of α-conotoxin and its structure is highly conserved. This unique conserved pattern ensures the function of syn-cal14.1a for the inhibition of cancer cells. Oroz-Parra et al23, analyzed the effect of syn-cal14.1a on lung cancer cell viability, this α-conotoxins peptide has been verified to target nAChRs in lung cancer cells. The nicotinic acetylcholine receptors have been characterized to promote the growth and metastasis of tumors. Hence, they speculated that the synthetic peptide syn-cal14.1a induced the lung cancer cells death via acting on the expression of nAChRs. They also demonstrated that syn-cal14.1a caused a cytotoxic effect on the lung cancer through down regulating BAX and up regulating Bcl-2. Significantly, they made an announcement that this peptide caused the activation of caspase-3 and -−7, resulting in inducing the apoptosis of the lung cancer cells via reducing expression of the pleiotropic transcription factor NF-κB1.
The present study inferred that EZH2 was upregulated in breast cancer tissue and cells. The binding of miR-101-3p and syn-cal14.1a synergized the inhibition of EZH2-induced progression of the breast cancer cells. To our best knowledge, this is the first report describing inhibition of EZH2 in human breast cancer cell lines by syn-cal14.1a. Although the fact that the mechanism of miR-101-3p inhibits the EZH2-induced breast cells progression is clear, the syn-cal14.1a is currently unknown. A previous study has shown that EZH2 is a direct NF-κB target gene, and activation of NF-κB also induces EZH2 expression in CD40L stimulated cells from Chronic Lymphocytic Leukemia patients.37 Therefore, we suspect that treatment with syn-cal14.1a reduces the expression of NF-κB1, inducing the inhibition of EZH2 in breast cancer cells. In addition, this combinatorial synergy enhances the effects on EZH2 in breast cancer cells. However, it is possible that the mechanism of synergy may be explained by more than one mechanism. In conclusion, our findings demonstrated that EZH2 plays a critical role in breast cancer carcinoma, and the synergy of miR-101-3p and syn-cal14.1a provides a potentially therapeutic strategy to improve the therapeutic outcome of breast cancer.
Conclusions
To the best of our knowledge, this is the first report describing the synthetic peptide syn-cal14.1a isolated from Conus californicus inhibition of EZH2-induced growth of breast cancer cells. Consistently, we demonstrated the effect of the synergy of miR-101-3p and syn-cal14.1a on inhibiting the invasion and proliferation and promoting the apoptosis of the breast cancer. These findings provide significant insights into molecular mechanisms of EZH2 and the synergy treatment could lead to more effective therapies for breast cancer.
Funding
This research was funded by the National Natural Science Foundation of China (81902622 and 81902996), the Shanghai Pujiang Program (18PJ1409400), the Shanghai “Science and Technology Innovation Action Plan“ Hong Kong, Macao, and Taiwan Science and Technology Cooperation Project (20430760100), and the Scientific Research Foundation of Shanghai Municipal Commission of Health and Family Planning (20174Y0218). We thank Dr. Yanchun Meng from Shanghai Cancer Center of Fudan University for her assitance.
Disclosure
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi:10.1016/j.ejca.2012.12.027
2. Tun JO, Salvador-Reyes LA, Velarde MC, Saito N, Suwanborirux K, Concepcion GP. Synergistic Cytotoxicity of Renieramycin M and Doxorubicin in MCF-7 Breast Cancer Cells. Mar Drugs. 2019;17(9):536. doi:10.3390/md17090536
3. Harding C, Pompei F, Burmistrov D, Welch HG, Abebe R, Wilson R. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;175(9):1483–1489. doi:10.1001/jamainternmed.2015.3043
4. Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16(4):201. doi:10.1038/nrc.2016.25
5. Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011;17(9):2613–2618. doi:10.1158/1078-0432.CCR-10-2156
6. Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298(5595):1039–1043. doi:10.1126/science.1076997
7. Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. doi:10.1038/nature09784
8. Velichutina I, Shaknovich R, Geng H, et al. EZH2-mediated epigenetic silencing in germinal center B cells contributes to proliferation and lymphomagenesis. Blood J Am Soc Hematol. 2010;116(24):5247–5255.
9. Xu B, Konze KD, Jin J, Wang GG. Targeting EZH2 and PRC2 dependence as novel anticancer therapy. Exp Hematol. 2015;43(8):698–712. doi:10.1016/j.exphem.2015.05.001
10. Kleer CG, Cao Q, Varambally S, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc National Acad Sci. 2003;100(20):11606–11611. doi:10.1073/pnas.1933744100
11. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi:10.1016/S0092-8674(04)00045-5
12. Raitoharju E, Seppälä I, Oksala N, et al. Blood microRNA profile associates with the levels of serum lipids and metabolites associated with glucose metabolism and insulin resistance and pinpoints pathways underlying metabolic syndrome: the cardiovascular risk in Young Finns Study. Mol Cell Endocrinol. 2014;391(1–2):41–49. doi:10.1016/j.mce.2014.04.013
13. Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19(2):232–243. doi:10.1016/j.ccr.2011.01.001
14. Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012;33(6):1126–1133. doi:10.1093/carcin/bgs140
15. Friedman JM, Liang G, Liu -C-C, et al. The putative tumor suppressor microRNA-101 modulates the cancer epigenome by repressing the polycomb group protein EZH2. Cancer Res. 2009;69(6):2623–2629. doi:10.1158/0008-5472.CAN-08-3114
16. Varambally S, Cao Q, Mani R-S, et al. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322(5908):1695–1699. doi:10.1126/science.1165395
17. Qian K, Liu G, Tang Z, et al. The long non-coding RNA NEAT1 interacted with miR-101 modulates breast cancer growth by targeting EZH2. Arch Biochem Biophys. 2017;615:1–9. doi:10.1016/j.abb.2016.12.011
18. Akondi KB, Muttenthaler M, Dutertre S, et al. Discovery, synthesis, and structure–activity relationships of conotoxins. Chem Rev. 2014;114(11):5815–5847. doi:10.1021/cr400401e
19. Olivera B. Conus venom peptides: correlating chemistry and behavior. J Comparative Physiol. 1999;185(4):353–359. doi:10.1007/s003590050394
20. Zhou M, Wang L, Wu Y, et al. Characterizing the evolution and functions of the M-superfamily conotoxins. Toxicon. 2013;76:150–159. doi:10.1016/j.toxicon.2013.09.020
21. Pore MM, Hiltermann TJN, Kruyt FA. Targeting apoptosis pathways in lung cancer. Cancer Lett. 2013;332(2):359–368. doi:10.1016/j.canlet.2010.09.012
22. Thorburn A. Death receptor-induced cell killing. Cell Sign. 2004;16(2):139–144. doi:10.1016/j.cellsig.2003.08.007
23. Oroz-Parra I, Navarro M, Cervantes-Luevano KE, et al. Apoptosis activation in human lung cancer cell lines by a novel synthetic peptide derived from Conus californicus venom. Toxins. 2016;8(2):38. doi:10.3390/toxins8020038
24. Soumaoro LT, Uetake H, Higuchi T, Takagi Y, Enomoto M, Sugihara K. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10(24):8465–8471. doi:10.1158/1078-0432.CCR-04-0653
25. Zhang J-G, Guo J-F, Liu D-L, Liu Q, Wang -J-J. MicroRNA-101 exerts tumor-suppressive functions in non-small cell lung cancer through directly targeting enhancer of zeste homolog 2. J Thoracic Oncol. 2011;6(4):671–678. doi:10.1097/JTO.0b013e318208eb35
26. Zhang K, Zhang Y, Ren K, Zhao G, Yan K, Ma B. MicroRNA-101 inhibits the metastasis of osteosarcoma cells by downregulation of EZH2 expression. Oncol Rep. 2014;32(5):2143–2149. doi:10.3892/or.2014.3459
27. Yu Y, Liu M, Ng TT, et al. PDZ-reactive peptide activates ephrin-B reverse signaling and inhibits neuronal chemotaxis. ACS Chem Biol. 2016;11(1):149–158. doi:10.1021/acschembio.5b00889
28. Yu Y, Nie Y, Feng Q, et al. Targeted covalent inhibition of Grb2–Sos1 interaction through proximity-induced conjugation in breast cancer cells. Mol Pharm. 2017;14(5):1548–1557. doi:10.1021/acs.molpharmaceut.6b00952
29. Liu J, Qu J, Zhou W, et al. Syntenin-targeted peptide blocker inhibits progression of cancer cells. Eur J Med Chem. 2018;154:354–366. doi:10.1016/j.ejmech.2018.05.015
30. Piunti A, Pasini D. Epigenetic factors in cancer development: polycomb group proteins. Future Oncol. 2011;7(1):57–75. doi:10.2217/fon.10.157
31. Jang S-H, Lee JE, Oh M-H, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal a breast cancer. J Breast Cancer. 2016;19(1):53–60. doi:10.4048/jbc.2016.19.1.53
32. Song X, Gao T, Wang N, et al. Selective inhibition of EZH2 by ZLD1039 blocks H3K27methylation and leads to potent anti-tumor activity in breast cancer. Sci Rep. 2016;6(1):1–15. doi:10.1038/s41598-016-0001-8
33. McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108–112. doi:10.1038/nature11606
34. Au SLK, Wong CCL, Lee JMF, et al. Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology. 2012;56(2):622–631. doi:10.1002/hep.25679
35. Wu K, Jiang Y, Zhou W, et al. Long Noncoding RNA RC3H2 facilitates cell proliferation and invasion by targeting MicroRNA-101-3p/EZH2 Axis in OSCC. Mol Ther Nucleic Acids. 2020;20:97–110. doi:10.1016/j.omtn.2020.02.006
36. Qiu BQ, Lin XH, Ye XD, et al. Non-Coding RNA PSMA3-AS1 promotes malignant phenotypes of esophageal cancer by modulating the miR-101/EZH2 Axis as a ceRNA. Aging. 2020;12(2):1843–1856. doi:10.18632/aging.102716
37. Iannetti A, Ledoux AC, Tudhope SJ, et al. Regulation of p53 and Rb links the alternative NF-κB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014;10(9):9. doi:10.1371/journal.pgen.1004642
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.