Back to Journals » The Application of Clinical Genetics » Volume 16
Microspherophakic Angle Closure Glaucoma in a Patient with Coffin-Siris Syndrome: Case Report
Authors Rojananuangnit K , Rojnueangnit K
Received 17 June 2023
Accepted for publication 24 August 2023
Published 29 August 2023 Volume 2023:16 Pages 165—170
DOI https://doi.org/10.2147/TACG.S422312
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Kulawan Rojananuangnit,1,* Kitiwan Rojnueangnit2,*
1Glaucoma Unit, Department of Ophthalmology, Mettapracharak (Wat Rai Khing) Hospital, Nakhon Pathom, Thailand; 2Division of Genetics, Department of Pediatrics, Faculty of Medicine, Thammasat University, Pathumthani, Thailand
*These authors contributed equally to this work
Correspondence: Kitiwan Rojnueangnit, Division of Genetics, Department of Pediatrics, Faculty of Medicine, Thammasat University, 99/209 Moo 18, Phahonyothin Road, Pathumthani, 12120, Thailand, Tel +66-2-926-9514, Fax +66-2-926-9513, Email [email protected]
Background: Bilateral secondary angle closure glaucoma is a presenting symptom of microspherophakia and ectopia lentis. Characterizing the associated syndrome and confirmation by genetic testing can identify associated systemic abnormalities and provide appropriate genetic counseling.
Case Presentation: A 42-year-old woman with severe intellectual disability presented with light perception visual acuity and glaucoma, with intraocular pressure (IOP) in her right and left eyes of 69 and 70 mmHg, respectively. She underwent two sessions of 270-degree laser diode transscleral cytophotocoagulation treatment at a 6-month interval and was prescribed topical anti-glaucoma medication. Her family noticed a progressive decrease in her vision while on treatment for 2 years. She was diagnosed with apparent Weill-Marchesani syndrome, accompanied by angle closure glaucoma and microspherophakia. Cataract surgery and intraocular lens implantation were successful in both eyes and post-operative IOP was controlled with anti-glaucoma medication but her vision did not improve from severe glaucomatous optic neuropathy. Her underlying syndrome was investigated genetically by whole exome sequencing.
Results: Sequencing showed a pathogenic variant in ARID1B, c.3955dupC (p.Gln1319Profs*14), diagnostic of Coffin-Siris syndrome. This is the first report of Coffin-Siris syndrome associated with microspherophakia and angle closure glaucoma.
Conclusion: Bilateral angle closure glaucoma from ectopia lentis in patients with genetic syndromes could be an indicator of microspherophakia in adulthood. Ophthalmological surveillance is important in patients with Coffin-Siris syndrome.
Keywords: angle closure glaucoma, ARID1B, Coffin-Siris syndrome, ectopia lentis, intellectual disability, microspherophakia
Introduction
Microspherophakia is a rare cause of bilateral secondary angle closure glaucoma; its symptoms, however, can be overlooked in childhood.1,2 Pathophysiologically, this rare congenital order is caused by the arrested formation of the crystalline lens during the embryonal period. The small spherical crystalline lens and the weak tension of the zonular fibers results in failed development of secondary lens fibers. Presenting ocular signs and symptoms include bilateral spheres and small equatorial diameter of the lens, with the lens equator being visible when the pupil is fully dilated. The weak tension of the zonular fibers of the lens, along with secondary angle closure glaucoma and high myopia refraction, can result in mobile lens movement, ranging from subluxation to dislocation. Microspherophakia has been associated with several ocular and systemic conditions, including Weill-Marchesani syndrome, Marfan syndrome, homocystinuria, aniridia and Alport syndrome. These conditions are difficult to diagnose in patients with microspherophakia, as they require linking microspherophakia with the general appearance and associated abnormalities of each patient, with confirmation by genetic testing.
Coffin-Siris syndrome (CSS) is a rare disease characterized by a combination of intellectual disability; hypoplasia of the distal phalange of the fifth digits and nails; and subtle facial features, including a coarse face, thick eyebrows, long eyelashes, a wide mouth with thickened and everted vermilion lips, broad nasal bridge and tip, and hypertrichosis.3,4 Its prevalence remains unknown, as only 200 patients have been reported worldwide.4 Twelve genes have been reported to cause CSS.5 One of these genes, the gene encoding AT-rich interaction domain-containing protein 1b (ARID1B), has been identified in 40–60% of patients with CSS,3,4 whereas 11 other genes have been found in 20% of patients. In contrast, no genetic anomalies have been identified in 40% of patients with CSS.4
Visual problems, including nystagmus, refractive errors, strabismus and optic nerve hypoplasia, have been detected in 78% of patients with CSS-ARID1B variants.6 Most case reports on these patients have involved children, with few patients diagnosed as adults.6,7 The present study describes an adult who presented with microspherophakia and angle closure glaucoma and was found to have CSS, as confirmed by a pathogenic variant in ARID1B. To our knowledge, this study is the first to describe an adult with CSS who presented with microspherophakic angle closure glaucoma.
Case Presentation
The study was approved by the Human Research Ethics Committee of the Faculty of Medicine, Thammasat University No. 1 (MTU-EC-PE-0-289/65). Written informed consent was obtained from the sister of the patient, who was her legal guardian.
A 42-year-old woman developed movement difficulty and bumped into surrounding objects, which was noticed by her sister. The history of the patient consisted of a severe intellectual disability, making her unable to communicate vocally. She could care for herself, understand simple commands and communicate by gestures without speech.
Two years before presentation, she was evaluated by an ophthalmologist; at that time, her visual acuity was light perception. The reliability of assessing corrected visual acuity in our patient, however, was low. Corneal edema was present in both eyes. At initial presentation the intraocular pressure (IOP) was 69 mmHg in her right eye and 70 mmHg in her left eye. She was diagnosed with glaucoma and underwent two sessions of 270-degree laser diode transscleral cyclophotocoagulation in both eyes at a 6-month interval and was prescribed the topical anti-glaucoma medications dorzolamide/timolol and latanoprost. However, her vision worsened, as did her difficulty moving and bumping into objects.
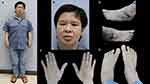
Physical examination upon presentation showed short stature, with a height of 140 cm (less than the third percentile). Behaviorally, she appeared gentle and sweet. Her face was coarse, with thick eyebrows, narrow and short palpebral fissures, broad nasal bridge and nasal tip, anteverted nares, thickened and everted vermillion lips, and down-turned corners of the mouth. Further physical examination showed small hands and feet, hypoplasia of the distal phalanges of the fifth fingers, and small nails of her second to fifth fingers and toes (Figure 1).
Due to her severe intellectual disability, a comprehensive ocular examination was not possible. She was tentatively diagnosed with Weill-Marchesani syndrome, accompanied by angle closure glaucoma. Because both of her lens were microspherophakic, she underwent intra-operative phacoemulsification. Evaluation of the ocular biometry parameters in her right and left eyes showed axial lengths of 33 and 34.3 mm, respectively; anterior chamber depths of 3.2 and 3.14 mm, respectively; K1 keratometry of 42.75 and 40.0 diopters (D), respectively; K2 keratometry of 43.75 and 44 D, respectively; IOL power (A-constant 118.3) of −3.0 and −4.0 D, respectively, and central corneal thicknesses of 660 and 667 microns, respectively. Cataract surgery and intraocular lens implantation were successful in both eyes and her postoperative IOP was controlled with anti-glaucoma medications. However, she did not regain her vision due to chronic elevated IOP with severe glaucomatous optic nerve damage.
Genetic Analysis
This possibility of an underlying genetic condition in our patient was investigated by whole exome sequencing. Peripheral blood was drawn from the patient and genomic deoxyribonucleic acid (DNA) was isolated from her peripheral blood leukocytes using the Puregene® DNA extraction kit. Whole exome sequencing was performed using the IIumina HiSeq platform (Macrogen, South Korea). All variants were analyzed by a standard protocol (Burrows-Wheeler Alignment tool),8 and the variants were filtered using a minor allele frequency (MAF) cutoff of <0.01 in the database for single nucleotide polymorphisms in 10,000 genomes. Genes targeted were those linked to the phenotypes of this patient, including micropherophakia (HP:0030961), glaucoma (HP:0000501), intellectual disability (HP:0001249), and short stature (HP:0004322). Genetic variants were classified based on the recommendations of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology criteria for variant classification.9 Evidence for the pathogenicity of identified variants was assessed using in silico predictive programs, including M-CAP (http://bejerano.stanford.edu/mcap), CADD (https://cadd.gs.washington.edu/snv), Mutation Taster (https://www.mutationtaster.org/), and PROVEAN (http://provean.jcvi.org/index.php). The novelty of the variants was determined by searching Clinvar (https://www.ncbi.nlm.nih.gov/clinvar), GnomAD (https://gnomad.broadinstitute.org), HGMD (http://www.biobase-international.com), and the in-house 3206 Thai whole exome sequencing database.10 The presence of a pathogenic variant was confirmed by Sanger sequencing.
Results
Whole exome sequencing of our patient identified a heterozygous one basepair duplication at nucleotide 3955 (c.3955dupC) in the ARID1B gene (NM_001374828.1). This insertion introduced a frameshift, resulting in a prematurely truncated protein (p.Gln1319Profs*14). The presence of this variant was confirmed by Sanger sequencing (Figure 2). This variant is classified as pathogenic, PVS1, PM2, PP4, and PP5, by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.9 This variant was not detected in 282,752 alleles in gnomAD (https://gnomad.broadinstitute.org) or in 3206 Thai exomes (6412 alleles).
Discussion
The etiology of bilateral secondary angle closure glaucoma can vary widely, complicating both correct diagnoses and proper treatment. Three major causes of secondary glaucoma have been identified. The first includes medically induced acute angle closure, such as pupil dilating medication in patients with compromised angle closure;11 alpha-adrenergic agonists in flu medications; the anticholinergic effects and serotonergic activities of antidepressants, including tricyclic antidepressants, selective serotonin reuptake inhibitors, and monoamine oxidase inhibitors; antihistamines; antiparkinsonian drugs; antipsychotic medications, and antispasmodic agents, such as sulfonamides, the anticonvulsant topiramate, and acetazolamide.12–14 A second major cause of secondary glaucoma is swelling of the ciliary body due to systemic inflammation, caused by, for example, systemic lupus erythematosus choroidopathy,15,16 or treatment with an extensive panretinal photocoagulation laser.1,17,18 A third major cause of secondary glaucoma is bilateral ectopia lentis associated with various systemic conditions.19 A correct diagnosis requires history taking, evaluation of the general appearance of the patient, a comprehensive ocular examination and a high index of suspicion.
The short stature, intellectual disability, microspherophakia and angle closure glaucoma observed in the present patient suggested an initial diagnosis of Weill-Marchesani syndrome.20,21 Most patients with Weill-Marchesani syndrome, however, have normal cognitive function, with some having mild intellectual impairment.22
The clinical presentation of the present patient, consisting of severe intellectual disability, an inability to speak, hypoplasia of the fifth digits and nails, short stature and unique facial features, was compatible with CSS. The pathogenic variant, c.3955dup C, in the ARID1B gene observed in the present patient, was previously reported in several patients with CSS.6,23,24 Although functional analysis has not been performed to assess the pathogenicity of this variant, the nucleotide duplication creates a frameshift, resulting in the disruption of amino acids synthesis.
The symptoms of microspherophakia in the present patient were secondary to angle closure glaucoma from subluxate lenses, a complication observed in nearly 50% of patients with microspherophakia.25 The pathophysiology of angle closure is due to the small size of the lens with laxity of zonular fibers, allowing the lens to move forward and causing a pupillary block. The biometry of the present patient differed from that in a previous case series, with the latter patients having deeper anterior chamber depth and longer axial length.25
Although the prevalence of visual impairment is higher in persons with intellectual disability than in the general population,26,27 comprehensive eye care is lacking in intellectually disabled persons, suggesting a need for improvement.28 In addition, ophthalmologic complications, including microspherophakia, may not be noticeable during early childhood. Knowledge and awareness of the complexity of ocular manifestations in these persons is the key to successful treatment.
No standard protocol has yet been developed to manage patients with microspherophakia. Treatment can be customized to fit individuals of specific ages and disease stages. These can include refractive correction during early childhood in patients with high lenticular myopia to prevent amblyopia, peripheral iridotomy to prevent pupillary block mechanism from subluxation and anti-glaucoma medications to control IOP. Definitive surgical treatments can include lens removal and intraocular lens (IOL) implantation, by procedures such as lens aspiration, phacoemulsification, lensectomy and vitrectomy, followed by in-the-bag IOL implantation, capsular tension ring and scleral fixation IOL. The association of these procedures with specific systemic conditions, such as cardiac and orthopedic conditions, should be evaluated.19,22
The management of secondary glaucoma may be more challenging, because its pathophysiology can consist of combinations of various mechanisms, such as developmental angle dysgenesis, chronic angle closure with peripheral anterior synechiae and trabecular dysfunction. IOP can be further controlled by treatments such as goniotomy, gonio-synechialysis, trabeculectomy and implantation of glaucoma drainage devices.
A definitive genetic diagnosis in adulthood can benefit patients, as it can guide proper management, monitoring and prognosis. Importantly, genetic counseling can advise families on the risk of family members having offspring with the same syndrome. Here, only one patient with CSS and microspherophakia has been reported, which is the main limitation of a case report. Further studies and more patients with CSS are required for better information on ophthalmologic findings in adulthood.
Conclusion
Determining the etiology of bilateral angle closure glaucoma can help determine the diagnosis and treatment of patients with this condition. Ectopia lentis and microspherophakia should be included in the differential diagnosis of patients with syndromic genetic diseases. Confirmation of a genetic diagnosis and proper genetic counseling are important elements of holistic care for patients with associated syndromes. Professional eye care teams should become aware of the importance of comprehensive eye care in vulnerable patients with intellectual disability.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The patient’s guardian provided informed consent for testing as well as for the use of anonymized data/material, including for publication. Institutional approval was provided (MTU-EC-PE-0-289/65).
Acknowledgments
The authors thank the patient and her family for participating in this study; clinical research center, Faculty of Medicine, Thammasat University and Ms Sam Ormond for assistance in professionally English editing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Parivadhini A, Lingam V. Management of secondary angle closure glaucoma. J Curr Glaucoma Pract. 2014;8(1):25–32. doi:10.5005/jp-journals-10008-1157
2. Senthil S, Rao HL, Hoang NTQ, et al. Glaucoma in microspherophakia: presenting features and treatment outcomes. J Glaucoma. 2014;23(4):262–267. doi:10.1097/IJG.0b013e3182707437
3. Mari F, Marozza A, Mencarelli MA, et al. Coffin-Siris and Nicolaides-Baraitser syndromes are a common well recognizable cause of intellectual disability. Brain Dev. 2015;37(5):527–536. doi:10.1016/j.braindev.2014.08.009
4. Schrier Vergano S, Santen G, Wieczorek D, et al. Coffin-Siris syndrome. In: Adam MP, Mirzaa GM, Pagon RA, editors. Genereviews(®). Seattle: University of Washington; 2021:1993–2023.
5. Online Mendelian Inheritance in Man (OMIM)® [homepage on the Internet]. Baltimore: Coffin-Siris syndrome, Phenotypic Series - PS135900; 2023. Available from: https://www.omim.org/phenotypicSeries/PS156200,PS135900.
6. van der Sluijs PJ, Jansen S, Vergano SA, et al. The ARID1B spectrum in 143 patients: from nonsyndromic intellectual disability to Coffin-Siris syndrome. Genet Med. 2019;21(6):1295–1307. doi:10.1038/s41436-018-0330-z
7. Määttänen L, Hietala M, Ignatius J, Arvio M. A 69-year-old woman with Coffin-Siris syndrome. Am J Med Genet A. 2018;176(8):1764–1767. doi:10.1002/ajmg.a.38844
8. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324
9. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi:10.1038/gim.2015.30
10. Shotelersuk V, Wichadakul D, Ngamphiw C, et al. The Thai reference exome (T-REx) variant database. Clin Genet. 2021;100(6):703–712. doi:10.1111/cge.14060
11. Wolfs RC, Grobbee DE, Hofman A, de Jong PT. Risk of acute angle-closure glaucoma after diagnostic mydriasis in nonselected subjects: the Rotterdam Study. Invest Ophthalmol Vis Sci. 1997;38(12):2683–2687.
12. Yang MC, Lin KY. Drug-induced acute angle-closure glaucoma: a review. J Curr Glaucoma Pract. 2019;13(3):104–109. doi:10.5005/jp-journals-10078-1261
13. Aminlari A, East M, Wei W, Quillen D. Topiramate induced acute angle closure glaucoma. Open Ophthalmol J. 2008;2(1):46–47. doi:10.2174/1874364100802010046
14. Stangler F, Prietsch RF, Fortes Filho JB. Glaucoma agudo bilateral em paciente jovem secundário ao uso de topiramato: relato de caso [Bilateral acute angle closure glaucoma in a young patient receiving oral topiramate: case report]. Arq Bras Oftalmol. 2007;70(1):133–136. doi:10.1590/s0004-27492007000100025
15. Nguyen QD, Uy HS, Akpek EK, Harper SL, Zacks DN, Foster CS. Choroidopathy of systemic lupus erythematosus. Lupus. 2000;9(4):288–298. doi:10.1191/096120300680199024
16. Han YS, Min Yang C, Lee SH, Shin JH, Moon SW, Kang JH. Secondary angle closure glaucoma by lupus choroidopathy as an initial presentation of systemic lupus erythematosus: a case report. BMC Ophthalmol. 2015;15(1):148. doi:10.1186/s12886-015-0144-6
17. Birinci H, Abidinoglu MR, Oge I. Anterior chamber depth and intraocular pressure following panretinal argon laser photocoagulation for diabetic retinopathy. Ann Saudi Med. 2006;26(1):73–75. doi:10.5144/0256-4947.2006.73
18. Kapoor B, Peh KK, Raman SV. An unusual case of acute angle closure glaucoma following argon laser pan retinal photocoagulation. BMJ Case Rep. 2010;2010(aug06 1):bcr1220092511. doi:10.1136/bcr.12.2009.2511
19. Kirandeep Kaur BG. Ectopia Lentis. In: StatPearls [Internet]. StatPearls Publishing; 2023.
20. Lim SH, Son JH, Cha SC. Acute angle-closure glaucoma in a highly myopic patient secondary to Weill-Marchesani syndrome: histopathologic lens features. Int Ophthalmol. 2016;36(6):921–924. doi:10.1007/s10792-016-0220-9
21. Al Motawa MNA, Al Shehri MSS, Al Buali MJ, Al Agnam AAM. Weill-Marchesani syndrome, a rare presentation of severe short stature with review of the literature. Am J Case Rep. 2021;22:e930824. doi:10.12659/AJCR.930824
22. Marzin P, Cormier-Daire V, Tsilou E, et al. Weill-marchesani syndrome. In: Adam MP, Mirzaa GM, Pagon RA, editors. Genereviews(®). Seattle: University of Washington; 2020:1993–2023.
23. Wieczorek D, Bögershausen N, Beleggia F, et al. A comprehensive molecular study on Coffin-Siris and Nicolaides-Baraitser syndromes identifies a broad molecular and clinical spectrum converging on altered chromatin remodeling. Hum Mol Genet. 2013;22(25):5121–5135. doi:10.1093/hmg/ddt366
24. Clinvar [homepage on the Internet]. Bethesda: National Library of Medicine; 2023. Available from: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000694703.14.
25. Yu X, Chen W, Xu W. Diagnosis and treatment of microspherophakia. J Cataract Refract Surg. 2020;46(12):1674–1679. doi:10.1097/j.jcrs.0000000000000334
26. Warburg M. Visual impairment in adult people with moderate, severe, and profound intellectual disability. Acta Ophthalmol Scand. 2001;79(5):450–454. doi:10.1034/j.1600-0420.2001.790504.x
27. van Splunder J, Stilma JS, Bernsen RM, Evenhuis HM. Prevalence of visual impairment in adults with intellectual disabilities in the Netherlands: cross-sectional study. Eye. 2006;20(9):1004–1010. doi:10.1038/sj.eye.6702059
28. Li JC, Wong K, Park AS, Fricke TR, Jackson AJ. The challenges of providing eye care for adults with intellectual disabilities. Clin Exp Optom. 2015;98(5):420–429. doi:10.1111/cxo.12304
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.