Back to Journals » OncoTargets and Therapy » Volume 11
MicroRNA 302b-3p/302c-3p/302d-3p inhibits epithelial–mesenchymal transition and promotes apoptosis in human endometrial carcinoma cells
Authors Li Y, Huo J, Pan X, Wang C, Ma X
Received 19 October 2017
Accepted for publication 6 December 2017
Published 6 March 2018 Volume 2018:11 Pages 1275—1284
DOI https://doi.org/10.2147/OTT.S154517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ingrid Espinoza
Yibing Li, Jianing Huo, Xin Pan, Cuicui Wang, Xiaoxin Ma
Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China
Background: Studies have shown that the microRNA miR-302 can affect the proliferation, migration and cell cycle progression of endometrial carcinoma (EC). miR-302 clusters have been shown to play an important role in the proliferation and differentiation of cancer cells and in their tumorigenicity.
Subjects and methods: In this study, we detected the expression of genes through quantitative reverse transcription polymerase chain reaction (qRT-PCR). We detected the expression of proteins through Western blot. The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) double-staining assay were used to detect the ability of miR-302b-3p/302c-3p/302d-3p to affect the cell apoptosis. The CCK-8 were used to detect the ability of miR-302b-3p/302c-3p/
302d-3p to affect the cell proliferation. The Cell cycle analysis were used to detect the ability of miR-302b-3p/302c-3p/302d-3p to affect the cell cycle. Finally, the wound healing assay was used to detect the ability of miR-302b-3p/302c-3p/302d-3p to impact cell migration.
Results: We found that miR-302b-3p/302c-3p/302d-3p of the miR-302 cluster was downregulated in EC, and it altered the epithelial–mesenchymal transition (EMT) process in the EC cell lines Ishikawa and HEC-1A. Western blot and the Annexin V- FITC/PI double-staining assay were used to detect the ability of miR-302b-3p/302c-3p/302d-3p to promote the apoptosis of Ishikawa and HEC-1A cells. In addition, qRT-PCR results showed that overexpression of miR-302b-3p/302c-3p/302d-3p significantly inhibited the expression of ZEB1, suppressed the expression of Bcl-2 and promoted the expression of BAX. The overexpression of miR-302b-3p/302c-3p/302d-3p inhibited the proliferation and migration of Ishikawa and HEC-1A cells. Cell cycle analysis showed that miR-302b-3p/302c-3p/302d-3p arrested cell cycle progression in the G0/G1 phase.
Conclusion: All results showed that miR-302b-3p/302c-3p/302d-3p can be used as a tumor suppressor in EC and is expected to be a new target for the treatment of EC.
Keywords: endometrial carcinoma, miR-302b-3p, miR-302c-3p, miR-302d-3p, EMT, cell apoptosis
Erratum for this paper has been published.
Introduction
Endometrial carcinoma (EC) is one of the most common malignancies of the female reproductive system. The incidence of this disease is high, and the mortality rate is second only to ovarian cancer and cervical cancer.1 The risk factors include long-term single estrogen stimulation, obesity, diabetes, hypertension, infertility and delayed menopause. For EC, early detection and early treatment are the key factors in improving its prognosis and increasing its cure rate.
miRNAs are noncoding RNAs that are involved in the posttranscriptional regulation of gene expression in multicellular organisms and can influence mRNA transcription and stability. miRNAs bind to the 3′ untranslated region (UTR) of the target gene, downregulate its expression at the posttranscriptional level by inducing the degradation of the target mRNA, inhibit the translation of the target mRNA and regulate various basic cellular functions such as proliferation, migration, invasion and epithelial–mesenchymal transition (EMT).2–6 For example, miR-874 inhibits the EMT process of hepatocellular carcinoma cells by targeting SOX12, miR-539 inhibits the EMT process of esophageal cancer cells and miR-630 inhibits the EMT process of gastric cancer cells by modulating FoxM1.7–9 In EC, miR-205 and miR-93 can promote the EMT process of EC cells, while miR-194 can inhibit the EMT process of EC cells.10–12
The miR-302 cluster was first found in human embryonic stem cells and includes miR-302a, miR-302b, miR-302c, miR-302d and miR-367. Recently, miR-302 clusters have been shown to play an important role in the proliferation and differentiation of cancer cells and in their tumorigenicity.13
In our study, we found that miR-302b-3p/302c-3p/302d-3p was downregulated in EC, inhibited the EMT process of the EC cells and promoted EC cell apoptosis. Cell Counting Kit-8 (CCK-8) and cell cycle experiments showed that miR-302b-3p/302c-3p/302d-3p can inhibit the proliferation of EC cells. Wound healing assays showed that miR-302b-3p/302c-3p/302d-3p suppressed the migration of EC cells. These results indicate that miR-302b-3p/302c-3p/302d-3p can provide a new target for the diagnosis and treatment of EC.
Materials and methods
Tissue samples
All tissue samples were collected from Shengjing Hospital of China Medical University, which included 30 cases of EC and 30 cases of normal endometrial tissue. All samples were frozen with liquid nitrogen and then stored at −80°C. Patients were not treated with radiotherapy, chemotherapy or hormone therapy before surgery. All patients with EC were diagnosed by pathology.
Cell line and culture
The human EC cell lines, Ishikawa and HEC-1A, obtained from the Shanghai Institute of Cell Biology of the Chinese Academy of Sciences (Shanghai, China), were cultured in the Roswell Park Memorial Institute 1640 culture medium and McCoy’s 5A culture medium (BioInd, Kibbutz Beit Haemek, Israel), respectively, supplemented with 10% fetal bovine serum (BioInd), 10 U/mL penicillin and 10 U/mL streptomycin (Corning Incorporated, Corning, NY, USA). All cells were cultured in an incubator at 37°C under 5% CO2.
Cell transfection
The overexpression and inhibition of miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells were established by using mimics and inhibitors from GenePharma (Shanghai, China). According to the manufacturer’s protocols for jetPRIME (Polyplus-transfection, Illkirch, France), cells were seeded onto plates, precultured to 70% confluency and transfected with mimics or inhibitors. The overexpression and inhibition of miR-302b-3p/302c-3p/302d-3p were verified by quantitative reverse transcription polymerase chain reaction (qRT-PCR).
qRT-PCR
Total RNA was isolated from tissues and from cultured Ishikawa and HEC-1A cells by TRIzol Reagent (Takara Biotechnology Inc., Dalian, China) according to the protocols. The relative expression levels of miR-302b-3p, miR-302c-3p and miR-302d-3p were quantified by PCR relative to the expression level of the endogenous control U6. The relative expression levels of ZEB1, Bcl-2 and BAX were quantified by PCR relative to the expression level of the endogenous control GAPDH. PCR conditions were as follows: denaturation for 30 s at 95°C, followed by 40 cycles for 3 s at 95°C and then DNA synthesis for 30 s at 60°C. After amplification, the real-time data were collected and analyzed. The relative quantitative results were calculated using 2−ΔΔct. The primer sequences (Sangon Biotech, Shanghai, China) used for miR-302b-3p/302c-3p/302d-3p were as follows: miR-302b-3p, forward 5′-GCG CGT AAG TGC TTC CAT GTT TTA GTA G-3′; miR-302c-3p, forward 5′-ccg TAA GTG CTT CCA TGT TTC AGT GG-3′; miR-302d-3p, forward 5′-gcg TAA GTG CTT CCA TGT TTG TGT GT-3′ and U6, forward 5′-CGG GTT TGT TTT GCA TTT CT-3′ and reverse 5′-AGT CCC AGC ATG AAC AGC TT-3′. The primer sequences (Sangon Biotech) were as follows: ZEB1, forward 5′-GAG GAA GAG GAG GAG GAG GA-3′ and reverse 5′-GCT TGA CTT TCA GCC CTG TC-3′; Bcl-2 forward, 5′-AGT ACC TGA ACC GGC ACC T-3′ and reverse 5′-CTT CAG AGA CAG CCA GGA GAA-3′; BAX, forward 5′-AGC GAC TGA TGT CCC TGT CT-3′ and reverse 5′-CTC AGC CCA TCT TCT TCC AG-3′ and GAPDH, forward 5′-GCA CCG TCA AGG CTG AGA AC-3′ and reverse 5′-TGG TGA AGA CGC CAG TGG A-3′.
Western blot
After a 48-hour transfection period, the cells were collected, washed twice with PBS, added to radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biotechnology, Shanghai, China) for 30 min on ice and centrifuged at 12,000 rpm for 15 min at 4°C. The total protein extracted was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking in 5% skim milk for 2 h, the membrane was incubated with primary antibodies at 4°C overnight. Next, the membrane was incubated with horseradish peroxidase-conjugated goat anti-ribbit secondary antibodies at 1:2,000 for 2 h. The proteins were visualized by BeyoECL Star (Beyotime Biotechnology). The primary antibodies for ZEB1, E-cadherin and N-cadherin were purchased from Cell Signaling Technology (Massachusetts, MA, USA). The primary antibodies for Bcl-2, Bcl-xL, Bax, caspase-3, caspase-8 and caspase-9 were purchased from Wanleibio (Shenyang, China).
CCK-8
A total of 1×104 cells/well were added to 96-well plates and incubated at 37°C and 5% CO2 for 24 h. After transfection and incubation for the appropriate time, 10 μL of CCK-8 test solution (KeyGen Biotech, Jiangsu, China) was added to each well. The cells were incubated at 37°C and 5% CO2 for 4 h, and the optical density at 450 nm was measured by a microplate reader.
Cell cycle analysis
The cells were collected after 48 h of transfection, centrifuged at 4°C and 1,800 rpm for 5 min and resuspended in ice-cold PBS. Again, the cells were centrifuged at 4°C and 1,800 rpm for 5 min, and the supernatant was discarded. Then, the cells were fixed in 70% ice-cold ethanol at 4°C overnight. After centrifugation at 4°C and 1,800 rpm for 5 min, the cells were resuspended in ice-cold PBS and recentrifuged. The cells were resuspended in 500 μL of PBS and 10 μL of RNase and 5 μL of propidium iodide (PI; Solarbio, Beijing, China) were added; the cells were incubated at 37°C for 30 min in the dark. Cell cycle was analyzed with a flow cytometer.
Annexin V-FITC/PI double-staining assay
The cells were collected after 48 h of transfection and suspended in PBS. Then, the cells were centrifuged at 4°C and 1,800 rpm for 5 min, and the supernatant was discarded. The cells were resuspended in 500 μL of binding buffer. Then, 5 μL of Annexin V-FITC and 5 μL of PI (KeyGen Biotech) were added, and the cells were incubated in the dark at room temperature for 15 min. Cell apoptosis was analyzed with a flow cytometer.
Wound healing assay
A 100-mL sterile pipette tip was used to scrape the plate, creating a wound, when the cell density reached ~90% confluency. The cell migration distance to the scraped area was recorded at 0, 24 and 48 h.
Statistical analysis
Data are expressed as the mean ± SD values. Statistical comparisons were made with t-tests. Statistical significance was defined as P-values <0.05. All statistical analyses were performed with the IBM SPSS Statistics 24.0 software (SPSS Inc., Chicago, IL, USA).
Ethics approval and informed consent
The study was approved by the medical research and new technology ethics committee of Shengjing Hospital of China Medical University (registered approval number: 2014PS75K). All the patients have been informed and offered written informed consent for using tissue samples for research.
Results
MiR-302b-3p/302c-3p/302d-3p was downregulated in EC
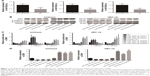
To study the expression level of miR-302b-3p/302c-3p/302d-3p in EC, we examined the expression of miR-302b-3p/302c-3p/302d-3p in EC and normal endometrial tissue by qRT-PCR. As shown in Figure 1A, the expression level of miR-302b-3p/302c-3p/302d-3p in EC was significantly lower than that in normal endometrial tissues, and the difference was statistically significant.
MiR-302b-3p/302c-3p/302d-3p inhibits the EMT process of EC cells and reduces the expression of ZEB1 in EC
To investigate the effect of miR-302b-3p/302c-3p/302d-3p on the EMT progress of EC cells, we examined the expression of EMT-related molecular markers to elucidate the effect of miR-302b-3p/302c-3p/302d-3p on the EMT progress. As shown in Figure 1B and C, the overexpression of miR-302b-3p/302c-3p/302d-3p increased the protein expression level of the epithelial marker E-cadherin in Ishikawa and HEC-1A cells and decreased the protein expression level of the mesenchymal marker N-cadherin. In contrast, low expression of miR-302b-3p/302c-3p/302d-3p reduced the protein expression level of the epithelial marker E-cadherin in Ishikawa and HEC-1A cells and increased the protein expression level of the mesenchymal marker N-cadherin. Meanwhile, we performed qRT-PCR for the expression of ZEB1 in Ishikawa and HEC-1A cells with miR-302b-3p/302c-3p/302d-3p overexpression and knockdown. As shown in Figure 1D, overexpressing miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells reduced the expression level of ZEB1. ZEB1 plays a key role in the EMT process. These results indicate that miR-302b-3p/302c-3p/302d-3p inhibits the EMT process of EC cells.
MiR-302b-3p/302c-3p/302d-3p induces the apoptosis of EC cells, suppresses the expression of Bcl-2 and promotes the expression of BAX
Apoptosis is an important feature of tumor cells. To investigate the effect of miR-302b-3p/302c-3p/302d-3p on the apoptosis of EC cells, we examined the expression of apoptosis-related markers first. As shown in Figure 2A and B, overexpressing miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells reduced the expression of the antiapoptotic proteins Bcl-2 and Bcl-xL and increased the expression levels of the pro-apoptotic protein BAX and the apoptosis-related markers caspase-3, caspase-8 and caspase-9. In contrast, knocking down the expression of miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells increased the expression levels of the antiapoptotic proteins Bcl-2 and Bcl-xL, while the expression levels of the pro-apoptotic protein BAX and apoptosis-related markers caspase-3, caspase-8 and caspase-9 were decreased. Among these proteins, Bcl-2 and BAX play important roles in the process of apoptosis. We performed qRT-PCR on the expression of Bcl-2 and BAX in Ishikawa and HEC-1A cells with miR-302b-3p/302c-3p/302d-3p overexpression or knockdown. Respectively, as shown in Figure 2C, overexpressing miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells reduced the expression level of the antiapoptotic gene Bcl-2 and increased the expression level of the pro-apoptotic gene BAX. In contrast, knocking down miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells increased the expression level of the antiapoptotic gene Bcl-2 and decreased the expression level of the pro-apoptotic gene BAX. Therefore, both the RNA and protein expression levels of Bcl-2 and BAX were altered after overexpression or knockdown of miR-302b-3p/302c-3p/302d-3p.
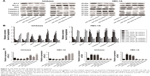
In addition, by overexpressing or knocking down miR-302b-3p/302c-3p/302d-3p and using the flow cytometer, we found that overexpression of miR-302b-3p/302c-3p/302d-3p increased the percentage of apoptotic cells and knockdown of miR-302b-3p/302c-3p/302d-3p suppressed the percentage of apoptotic cells in Ishikawa and HEC-1A cells (Figure 3A and B).
These results indicate that miR-302b-3p/302c-3p/302d-3p can facilitate the apoptosis of EC cells, inhibit the expression of Bcl-2 and promote the expression of BAX, suppressing the progression of EC.
MiR-302b-3p/302c-3p/302d-3p inhibits the proliferation of EC cells
We examined the effect of miR-302b-3p/302c-3p/302d-3p on the proliferation of EC cells at 0, 12, 24 and 48 h by CCK-8. As shown in Figure 4A, overexpression of miR-302b-3p/302c-3p/302d-3p inhibits the proliferation of EC cells. In a word, miR-302b-3p/302c-3p/302d-3p might act as a tumor suppressor in EC cells.
At the same time, to study the effect of miR-302b-3p/302c-3p/302d-3p on the cell cycle, we examined the cell cycle changes induced by miR-302b-3p/302c-3p/302d-3p in Ishikawa and HEC-1A cells by analyzing PI staining with flow cytometry. As shown in Figure 4B and C, cells in the G0/G1 phase were significantly increased in the miR-302b-3p/302c-3p/302d-3p-overexpressing cells compared to the normal control (NC) group, whereas the knockdown of miR-302b-3p/302c-3p/302d-3p reduced the percentage of G0/G1 phase cells in Ishikawa and HEC-1A cells. Therefore, miR-302b-3p/302c-3p/302d-3p can inhibit cell growth by increasing the number of cells in the G0/G1 phase of the cell cycle in EC cells.
MiR-302b-3p/302c-3p/302d-3p suppresses the migration of EC cells
To clarify the effect of miR-302b-3p/302c-3p/302d-3p on the migration of EC cells, we performed wound healing experiments in Ishikawa and HEC-1A cells. The results are shown in Figure 5; overexpression of miR-302b-3p/302c-3p/302d-3p can inhibit the migration of EC cells compared with the NC group. In contrast, inhibition of miR-302b-3p/302c-3p/302d-3p can promote the migration of EC cells.
Discussion
The miR-302 family includes miR-302a, miR-302b, miR-302c, miR-302d and miR-367. In addition, miR-302b, miR-302c and miR-302d in the miR-302 family have been identified in a variety of tumors. miR-302b inhibits the growth of hepatocellular carcinoma SMMC-7721 cells by targeting EGFR via the EGFR/AKT2/CCND1 pathway.14 miR-302b enhances the sensitivity of hepatocellular carcinoma cell lines to 5-Fu by targeting Mcl-1 and DPYD.15 Other studies have shown that miR-302b hinders epithelial ovarian cancer, inhibits ovarian cancer cell proliferation and colony formation directly through RUNX1 and induces cell cycle arrest and apoptosis in G0/G1 cells in ovarian cancer.16 miR-302c-3p inhibits the proliferation of renal cell carcinoma cells by targeting Grb2-associated binding 2 (Gab2) in renal cell carcinoma.17 In addition, studies have shown that miR-302d inhibits human glioblastoma multiformes by targeting NF-κB and FGF2.18 In the study of Yan et al,13 miR-302 inhibited the tumorigenicity of EC cells by inhibiting cyclin D1 and CDK1. However, the effect and mechanism of miR-302b-3p/302c-3p/302d-3p on the EMT process and the apoptosis of EC cells are unclear.
ZEB1 is one of the members of the zinc finger E-box-binding (ZEB) protein family. The ZEB family includes ZEB1 and ZEB2 as members, which are two important nuclear transcription factors especially for original nerve tissue, neural stem cells and neural cell subpopulations, and were first discovered in mouse embryonic tissue.19 These transcription factors are structurally similar and have highly similar N-terminal zinc finger clusters that exist between homologous domains that may be associated with protein–protein interactions.20 The most important part of the transcription factor associated with its function in the EMT function is the zinc finger structure at both ends, which specifically binds to the E-cad promoter sequence E2, thereby inhibiting the transcription of E-cadherin to induce the EMT process and thus initiating or enhancing the ability of cell metastatic invasion.21 Therefore, as repressors of E-cadherin, a class of transcriptional inhibitors, represented by ZEB family proteins, may play a key role in regulating the EMT process at the molecular level in tumor cells. Upregulation of ZEB family members in pancreatic cancer, colon cancer, renal cancer and lung cancer has been demonstrated,22–24 and their expression in EC has also been reported. ZEB1 is a transcriptional inhibitor of E-cadherin, and it has been reported that ZEB1 is abnormally expressed in highly invasive EC, such as G3 EC, uterine serous carcinoma and malignant mixed Müllerian tumors. In addition, ZEB1 is highly expressed in the tumor stroma of low-grade EC (type I). When ZEB1 is expressed abnormally in epithelial tumor cells, the expression of E-cadherin is repressed, and this negative correlation is associated with an increased tumor invasion potential.25
Bcl-2 is a proto-oncogene. The Bcl-2 family members contain one to four Bcl-2 homologous domains (BH1–4), and typically, the carboxy terminus has a transmembrane (TM) region. BH4 is domain specific for antiapoptotic proteins, and BH3 is domain associated with pro-apoptotic proteins. The Bcl-2 gene family can be divided into two categories according to function and structure: antiapoptotic proteins such as Bcl-2, Bcl-xL, Bcl-w and Mcl-1, and proapoptotic proteins such as BAX, BAK, BAD, BID and BIM and a class that contains only the BH3 structure such as BID, BAD and PUMA. The Bcl-2 family plays a crucial role in apoptosis, particularly the Bcl-2 protein, which is thought to be a promising target for the discovery of antineoplastic agents.26
In this study, we found that miR-302b-3p/302c-3p/302d-3p was downregulated in EC cells. Then, we examined the expression of miR-302b-3p/302c-3p/302d-3p in the EC cell lines Ishikawa and HEC-1A. Overexpression of miR-302b-3p/302c-3p/302d-3p can inhibit the proliferation and migration of EC cells and promote the apoptosis of EC cells. These results increase our understanding of miR-302b-3p/302c-3p/302d-3p function, suggesting that miR-302b-3p/302c-3p/302d-3p can be used as a tumor suppressor of EC.
In addition, overexpression of miR-302b-3p/302c-3p/302d-3p can reduce the expression of ZEB1, inhibit the expression of Bcl-2 and promote the expression BAX in EC at the RNA level and the protein level. Therefore, we speculate that miR-302b-3p/302c-3p/302d-3p may inhibit the progression of EMT in EC cells by inhibiting ZEB1 and may promote the apoptosis of EC cells by inhibiting Bcl-2 and facilitating BAX. However, the mechanism of how miR-302b-3p/302c-3p/302d-3p regulates ZEB1, Bcl-2 and BAX remains to be explored further.
Conclusion
miR-302b-3p/302c-3p/302d-3p is downregulated in EC cells. Overexpression of miR-302b-3p/302c-3p/302d-3p can inhibit the EMT process of EC cells, promote the apoptosis of EC cells, facilitate the proliferation and migration of EC cells and induce cell cycle arrest. These results suggest that miR-302b-3p/302c-3p/302d-3p can serve as a target for the treatment of EC and provide new ideas for the treatment of EC.
Acknowledgments
Our project was supported by the National Natural Science Foundation of China (Nos. 81272874 and 81472438), Department of Science and Technology of Liaoning Province (No. 2013225079), Shenyang City Science and Technology Bureau (No. F14-158-9-47) and Outstanding Scientific Fund of Shengjing Hospital (No. 201601).
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. | ||
Huang Q, Gumireddy K, Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10(2):202–210. | ||
Wang L, Song G, Tan W, et al. MiR-573 inhibits prostate cancer metastasis by regulating epithelial-mesenchymal transition. Oncolotarget. 2015;6(34):35978–35990. | ||
Liu T, Nie F, Yang X, et al. MicroRNA-590 is an EMT-suppressive microRNA involved in the TGFβ signaling pathway. Mol Med Rep. 2015;12(5):7403–7411. | ||
Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signaling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51(12):1638–1649. | ||
Jiang T, Guan LY, Ye YS, Liu HY, Li R. MiR-874 inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma by targeting SOX12. Am J Cancer Res. 2017;7(6):1310–1321. | ||
Cao Z, Zheng X, Cao L, Liang N. MicroRNA-539 inhibits the epithelial-mesenchymal transition of esophageal cancer cells by twist-related protein 1-mediated modulation of melanoma associated antigen A4 (MAGEA4). Oncol Res. Epub 2017 Jun 12. | ||
Feng J, Wang X, Zhu W, Chen S, Feng C. MicroRNA-630 suppresses epithelial-to-mesenchymal transition by regulating FoxM1 in gastric cancer cells. Biochemistry (Mosc). 2017;82(6):707–714. | ||
Jin C, Liang R. miR-205 promotes epithelial-mesenchymal transition by targeting AKT signaling in endometrial cancer cells. J Obstet Gynaecol Res. 2015;41(10):1653–1660. | ||
Chen S, Chen X, Sun KX, et al. MicroRNA-93 promotes epithelial– mesenchymal transition of endometrial carcinoma cells. PLoS One. 2016;11(11):e0165776. | ||
Dong P, Kaneuchi M, Watari H, et al. MicroRNA-194 inhibits epithelial to mesenchymal transition of endometrial cancer cells by targeting oncogene BMI-1. Mol Cancer. 2011;18(10):99. | ||
Yan GJ, Yu F, Wang B, et al. MicroRNA miR-302 inhibits the tumorigenicity of endometrial cancer cells by suppression of Cyclin D1 and CDK1. Cancer Lett. 2014;345(1):39–47. | ||
Wang L, Yao J, Shi X, et al. MicroRNA-302b suppresses cell proliferation by targeting EGFR in human hepatocellular carcinoma SMMC-7721 cells. BMC Cancer. 2013;13(10):448. | ||
Cai D, He K, Chang S, Tong D, Huang C. MicroRNA-302b enhances the sensitivity of hepatocellular carcinoma cell lines to 5-FU via targeting Mcl-1 and DPYD. Int J Mol Sci. 2015;16(10):23668–23682. | ||
Ge T, Yin M, Yang M, Liu T, Lou G. MicroRNA-302b suppresses human epithelial ovarian cancer cell growth by targeting RUNX1. Cell Physiol Biochem. 2014;34(6):2209–2220. | ||
Gu DH, Mao JH, Pan XD, et al. microRNA-302c-3p inhibits renal cell carcinoma cell proliferation by targeting Grb2-associated binding 2 (Gab2). Oncotarget. 2017;8(16):26334–26343. | ||
Wang F, Yang L, Sun J, et al. Tumor suppressors microRNA-302d and microRNA-16 inhibit human glioblastoma multiforme by targeting NF-κB and FGF2. Mol Biosyst. 2017;13(7):1345–1354. | ||
Darling DS, Stearman RP, Qi Y, Qiu MS, Feller JP. Expression of Zfhep/deltaEF1 protein in palate, neural progenitors, and differentiated neurons. Gene Expr Patterns. 2003;3(6):709–717. | ||
Smith GE, Darling DS. Combination of a zinc finger and homeodomain required for protein-interaction. Mol Biol Rep. 2003;30(4):199–206. | ||
Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66(5):773–787. | ||
Xiong H, Hong J, Du W, et al. Roles of stat3 and zeb1 proteins in e-cadherin down-regulation and human colorectal cancer epithelial-mesenchymal transition. J Biol Chem. 2012;287(8):5819–5832. | ||
Mazda M, Nishi K, Naito Y, Ui-Tei K. E-cadherin is transcriptionally activated via suppression of zeb1 transcriptional repressor by small RNA-mediated gene silencing. PLoS One. 2011;6(12):e28688. | ||
Bao B, Wang Z, Ali S, et al. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112(9):2296–2306. | ||
Singh M, Spoelstra NS, Jean A, et al. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21(7):912–923. | ||
Garner TP, Lopez A, Reyna DE, Spitz AZ, Gavathiotis E. Progress in targeting the BCL-2 family of proteins. Curr Opin Chem Biol. 2017;39(8):133–142. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.