Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
MicroRNA-221-3p Targets THBS1 to Promote Wound Healing in Diabetes
Authors Hu K, Liu X, Chang H, Zhang Y, Zhou H, Liu L, Zhang X, Jiao Z, Shen B , Zhang Q
Received 10 June 2023
Accepted for publication 29 August 2023
Published 11 September 2023 Volume 2023:16 Pages 2765—2777
DOI https://doi.org/10.2147/DMSO.S424847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Keyan Hu,1,2,* Xueying Liu,1,* Hongfeng Chang,1 Yi Zhang,1 Hui Zhou,3 Lei Liu,1 Xin Zhang,1 Ziying Jiao,4 Bing Shen,4 Qiu Zhang1
1Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 2Department of Endocrinology, The First Affiliated Hospital of Henan University of Science and Technology, Luoyang, Henan, 471003, People’s Republic of China; 3Institute of Brain Diseases and Cognition, School of Medicine, Xiamen University, Xiamen, Fujian, 361000, People’s Republic of China; 4School of Basic Medical Sciences, Anhui Medical University, Hefei, Anhui, 230032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiu Zhang, Department of Endocrinology, The First Affiliated Hospital of Anhui Medical University, 218 Jixi Road, Hefei, Anhui, 230022, People’s Republic of China, Tel/Fax +86-551-62923631, Email [email protected] Bing Shen, Department of Physiology, School of Basic Medical Sciences, Anhui Medical University, 81 Meishan Road, Hefei, Anhui, 230032, People’s Republic of China, Tel +86-551-65161132, Fax +86-551-65161126, Email [email protected]
Introduction: Diabetes foot ulcer (DFU) is a serious complication of diabetes characterized with chronic foot ulceration, poor wound healing (WH), and persistent inflammation. MiR-221-3p, as microRNA, has been shown to accelerate WH in previous study, but the underlying mechanisms are poorly understood.
Methods: In this study, we aimed to determine how miR-221-3p influences WH by targeting THBS1. The effect of miRNA-221-3p on wound healing of diabetes by epidermal injection of miRNA-221-3p agomir. In vitro generated human immortalized keratinocytes (HaCaT cells) were transfected with miR-mimics and negative control with high glucose treatment. The effects of miRNA-221-3p on cell apoptosis and angiogenesis using cell apoptosis assay and the tube formation assay, respectively. Direct target interaction of miR-221-3p and predicted target sites in 3’UTR of THBS1 were examined by luciferase reporter gene assay. Breeding miRNA-221 knockout mice for experimental verification.
Results: We found that miRNA-221-3p overexpression at the wound edge of normal mice and diabetes mice can promote WH. As contrast, WH of miR-221 knockout mice delayed with increased epithelial apoptosis and reduced angiogenesis in the dermis. miR-221-3p was found to inhibit apoptosis in HaCaT cells, and enhanced angiogenesis in human umbilical vein endothelial cells (HUVECs) that were co-cultured. Bioinformatics analysis as well as the dual-luciferase reporter assay revealed miR-221-3p to target 3ʹ untranslated region of THBS1.
Conclusion: Our findings suggested miR-221-3p might exert an essential impact on diabetes WH via inhibition of THBS1 and lack of miR-221-3p possibly results in impaired healing in chronic wounds of type 2 diabetes. Therefore, developing medicine such as chemically modified analogs of miR-221-3p in future could benefit patients with DFU.
Keywords: diabetes, wound healing, microRNA, keratinocyte
Introduction
Foot ulcer is the main complication of diabetes, with high morbidity and mortality, and consumes a lot of medical resources.1 Foot ulcer is caused by a variety of factors, and its treatment is challenging. Even if the treatment is successful, the risk of recurrence is extremely high, with a recurrence rate of more than 50% after 3 years.2 Therefore, diabetic foot ulcer (DFU) brings a great burden to patients, medical system, and society. The diabetic environment causes the normal wound healing (WH) process to be disrupted and prolonged, resulting in chronic non-healing wounds.3 In order to treat foot ulcers, various interventions have been studied and used in clinical practice.4 The best treatment strategies are still being developed, despite of various attempts aiming to promote WH.
Thrombospondin-1 (THBS1) is an endogenous anti-angiogenic factor, which is mainly produced by innate immune cells, vascular smooth muscle cells, endothelial cells, and epithelial cells.5 It is a key molecule in controlling skin angiogenesis and is particularly important in maintaining skin vascular homeostasis.6 In addition, more and more studies have revealed other functions of THBS1, including the regulation of cell migration, apoptosis, and adhesion.7 Previous studies have shown that high glucose (HG) environment significantly affected THBS1 expression.8,9
As the number of diabetic patients increases, more and more studies have been conducted on miRNAs as therapeutic targets for diabetic WH.10 As demonstrated by previous studies, miRNAs such as miR-132, miR-126 and miR-21 promote diabetic WH by participating in multiple links such as inflammatory response, angiogenesis, collagen recombination and re-epithelialization.11–13 Results of our previous study proved that in diabetic mice, miR-221-3p enriched in exosomes derived from endothelial progenitor cells has a potential in accelerating the healing of skin WH.14
In this study, we proved that THBS1 was miRNA-221-3p’s target gene based on bioinformatics analysis. In order to clarify the mechanism of miR-221-3p promoting diabetic WH, miRNA-221-3p overexpression, as well as addition of THBS1 exogenous protein were used to elucidate the influence of THBS1 on the function of cells. Meanwhile, miR-221 knockout (KO) mice were bred to verify Mir-221-3p’s significance in diabetic WH, elucidating mechanisms through which Mir-221-3p enhanced WH via targeting THBS1, providing a novel target for DFU treatment.
Materials and Methods
Animal Experiments
All animal experiments were approved by the local authorities and performed in accordance with the guidelines of the Animal Care and Use Committee of Anhui Medical University (LLSC20190426). The C57BL/6 wildtype (WT) mice together with Mir221 KO mice were produced and purchased by GemPharmatech (Jiangsu, China). Exon 1 of Mir221-201 transcript (ENSMUST00000083488.3) was used as the knockout region. The Mir221 gene was modified by CRISPR/Cas9 technology. Simply put, CRISPR/Cas9 system was expressed in C57BL/6JGpt mice eggs. F0 positive mice were obtained by fertilized egg transfer, and verified through PCR & sequencing. Besides, a stable model of F1 mice was established through mating the positive F0 mice with C57BL/6JGpt mice. Besides, genotype identification of KO mice based on specific primer PCR reaction (forward: GTCTAACTCTCAGAAGGATTAGGGTGC; reverse: AGAAGTGGTTAGATTCGTTGGATCA).
C57BL/6 mice (male, 8–12 weeks old) were accommodated in a controlled habitat and provided with water and rodent food. Every 5 mice were placed in a stainless steel cage lined with corncobs at a room temperature of 22°C, with a light -dark cycle of 12:12. The animals were familiarized to their habitat for 1 week and used to prepare diabetes models by intraperitoneal injection of streptozocin (STZ). The injection was measured at 50mg/kg body weight for 5 consecutive days. From the 12th day after STZ injection, the fasting blood glucose was measured twice a week by tail cutting blood sampling method for 2 weeks. When the blood glucose ≥250 mg/dl (16.7 mmol/L) twice in a row, diabetes was diagnosed.
The hair on the back of the mouse was shaved. To create excisional wounds, two full-thickness dermectomy wounds were symmetrically generated near the dorsal midline using a 6mm biopsy punch. The mice were divided into normal control group (Ctrl), diabetic control group (DM-Ctrl) and diabetic miR-221-3p agomir group (DM-miR-221-3p). The indicated amount of miR-221-3p agomir (100 nmol/kg)/miRNA mimic NC were injected subcutaneously around the wound edge every other day after injury. Before the injection of RNA analogues, the size of the wound area was photographed and measured every other day. A ruler is placed around the wound as a reference to allow for correction of the distance between the camera and the animal. The wound area was calculated in pixels with ImageJ (1.8.0) (National Institutes of Health, Bethesda, Maryland, USA). Wound closure was calculated into a percentage based on this formula: WH (%) = [(initial wound area − final wound area)/initial wound area] ×100%.
Terminal Deoxynucleotidyltransferase-Mediated Nick End Labelling Assay (TUNEL)
Apoptosis was assessed in skin tissue sections from the various groups using a TUNEL assay kit on paraffin-embedded samples (C1088, Beyotime Biotechnology, China) complying with the instructions provided by the manufacturer. In brief, mouse skin tissue samples were fixed in a 4% paraformaldehydes solution, dehydrated, and embedded in paraffin. Subsequently, the specimens were placed onto slides, and then counterstained by 4′,6-diamidino-2-phenylindole (DAPI) to facilitate visualization and localization of the nuclei. Cells were visualized using fluorescence microscopy, with apoptotic cells identified by dual staining in both green (representing fragmented DNA) and blue (representing nuclear DNA) channels. Percentage of apoptotic cells = number of TUNEL-positive cells/number of DAPI. Images were merged via the superimposition of images from different channels using a confocal laser scanning microscope (LSM 880, Leica, Germany).
Immunohistochemistry
For immunohistochemical assay, samples of skin tissue obtained from both C57BL/6 WT mice and Mir221 KO mice were excised, followed by fixation in paraformaldehyde (4%). Samples were then paraffin-embedded and sliced into sections (5 μm in thickness), which were then mounted on slides. After deparaffinization, the slides were stained with CD31 (NB100-2284, Novus, USA), followed by incubation with a secondary antibody conjugated with a horseradish peroxidase (HRP). Subsequently, slices were colored with substrate solution of 3.3’-diaminobenzidine (DAB). Images were obtained using a light microscope and further analyzed with ImageJ.
Cell Culture
HaCaT cells that purchased from Pricell (Wuhan, China) and HUVEC cells that purchased from Guandao Biological Engineering (Shanghai, China) were cultured with Roswell Park Memorial Institute (RPMI) 1640 medium (BasalMedia, Shanghai, China) containing 10% phosphate buffered saline (PBS) as well as 1% penicillin/streptomycin. After reaching 50–70% confluence, cells were put into 6-well plates, followed by exposure to 3 levels of glucose (5.5, 35 or 50 mmol/L). After 24 hours of glucose treatment, HaCaT cells were then transfected by 100 nM of miR-221-3p mimics (GenePharma, China), siTHBS1 (100 nM, Tsingke Biotechnology, China) (forward: GGAGUUCAGUACAGAAAUAdTdT; reverse: UAUUUCUGUACUGAACUCCdTdT), siRNA NC (Tsingke Biotechnology, China) via Lipofectamine 3000 (L3000015, Thermo Fisher Scientific, USA) or mimic NC (100 nM, GenePharma, China) complying with the protocol provided by the manufacturer. Cells supernatant was collected to measure cytokine content while cells were harvested, preparing for subsequent analysis.
Flow Cytometry
The apoptosis assays were run and analyzed with the help of Annexin V-FITC/PI Double Staining apoptosis detection kit (BB-4101, Bestbio, China). HaCaT cells were obtained using trypsinization 48 hours after intervention. Cells were then resuspended in binding buffer (1 × 106 cells/mL). Complying with the instructions of the manufacturer, cells were double-stained with FITC-Annexin V as well as propidium iodide (PI). Fluorescence intensity was measured using CytoFlex Analysis Flow Cytometer (Beckman Coulter) to detect early and late apoptosis of cells.
In vitro Co-Culture Experiment
A co-culture transwell chamber (0.4-μm in pore size) was adopted to evaluate the influence of supernatant of HaCaT cells after different intervention on HUVECs in vitro. HaCaT cells were seeded into the medium of RPMI 1640 with FBS (10%) at the lower chamber and interfered by HG, miRNA-221-3p mimic or siTHBS1. HUVECs were seeded in the upper compartment 24 hours after the intervention. Samples were collected after 24 h culture, preparing for subsequent experiments.
Tube Formation Assay
Harvested from co-culturing with HaCaT for a period of 24h, HUVECs were put into 96-well plates which were precoated with Matrigel Basement Membrane Matrix (354,234, BD, USA). Subsequently, they were left to solidify at 37°C for 8h. Then, the tube formation was detected using a microscope, with the results analyzed by ImageJ.
Luciferase Reporter Assay
Firefly luciferase reporter plasmids with 3′-UTR of THBS1 gene were constructed, along with an empty luciferase vector for use as a control. Corresponding to the region in the WT THBS1, sequences predicted to bind to miR-221-3p, as well as a mutated sequence that cannot bind to miR-221-3p, were cloned into different plasmids, respectively, followed by transfection into HEK-293T cells using Lipofectamine 3000. A Dual-Luciferase Reporter Gene Detection kit (E1910, Promega, USA) was adopted to detect the activity of luciferase complying with the manual provided by the manufacturer 48 hours following co-transfection. Absorbance values were measured with a microplate reader (BioTek Synergy 2, USA). Each experiment was repeated three times.
Quantitative Real-Time PCR (qRT-PCR)
Complying with the instructions provided by the manufacturer, extraction of total RNA from HaCaT with Trizol reagent was conducted (15,596–026, Invitrogen, USA), and RNA concentrations were determined with a spectrophotometer (Thermo Fisher Scientific, MA, USA). Subsequently, RNA (1 μg) was used to synthesize cDNA. To determine THBS1 (forward: TTGTCTTTGGAACCACACCA; reverse: CTGGACAGCTCATCACAGGA) mRNA levels, NovoStart SYBR qPCR kits (11202ES08, Yeasen Company) were adopted. We used β-Actin (forward: CATGTACGTTGCTATCCAGGC, reverse: CTCCTTAATGTCACGCACGAT) as an endogenous control for normalization. qRT-PCR were carried out complying with the manual based on the ABI 7500 Fast PCR system. The 2−ΔΔCt method was applied to quantify the expression levels of mRNA relative to the endogenous control genes.
Western Blot Analysis
With the application of radioimmunoprecipitation assay (RIPA) buffer (P0013B, Beyotime Biotechnology, China), extraction of total protein from cells were conducted. The obtained proteins were measured using a BCA Protein Assay Kit (P0010, Beyotime Biotechnology). Protein lysates (30μg) were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and then transferred onto membranes of polyvinylidene fluoride (Millipore, USA), which was subsequently blocked in 5% skim milk diluted using PBS with Tween-20 for 1 h, followed by incubation with primary antibodies rabbit antiTHBS1 (37879S, Cell Signaling Technology, USA) as well as rabbit anti-β-tubulin (AF7011, Affinity, China) at 4 diluted in foregoing blocking buffer. After extensively washed in Tris-buffered saline with 0.1% Tween® 20 detergent, membranes were further incubated with secondary antibodies (E-AB-1003, Elabscience, China) at room temperature for a period of 2 h. After triply washing of membranes, an enhanced chemiluminescence (CL) detection system was applied to detect the proteins on them (Bio-Rad Laboratories, Inc.). Luminescence intensity was analyzed using ImageJ, with resulting values normalized to β-tubulin densitometric values.
Elisa
To detect secretion of THBS1 protein from HaCaT in the supernatant, we plated cells in 6-well plates and subjected them to various treatments. Following HG-stimulation or transfection, supernatants were collected, and THBS1 release was measured by ELISA kits according to the manufacturer’s instructions (E-EL-H1589c, Elabscience, China). Values are presented either as concentration in ng/mL.
Statistical Analysis
GraphPad Prism (Inc. La Jolla, California) was adopted for statistical analysis. All measurable data were described with mean ± standard error of the mean (SEM). To compare data between 2 groups, unpaired Student’s t-tests were performed. In addition, to compare data in ≥3 groups, one-way/two-way analysis of variance (ANOVA) was conducted. A P value less than 0.05 was indicative of statistical significance.
Results
Role of miR-221-3p in WH Among Diabetes Mice
MiR-221-3p agomir was injected into the edges of an excisional wound in healthy mice and diabetic mice (mice injected with streptozotocin to induce a mouse model of diabetes) to elucidate the impact of miR-221-3p on WH among mice. WH was significantly delayed in diabetic mice compared to healthy mice, but accelerated in diabetic mice after application of miR-221-3p agomir compared to a miRNA mimic negative control (Ctrl) (Figure 1a and b).
Abnormal apoptosis can affect the healing process of specific wound surface. To evaluate the influence of miR-221-3p on skin epithelial layer’s apoptosis at the edge of wound induced by diabetes, we used Tunel staining to find more TUNEL+ apoptotic cells in the wound region in diabetes mice compared with healthy mice, but fewer apoptotic cells in the group administered with miR-221-3p agomir in contrast to Ctrl group both with diabetes on day 7 (Figure 2a and c). One of the reasons for the damage of WH in diabetes is the reduction of angiogenesis. Angiogenic evaluation of diabetic mouse wound skins was performed using immunohistochemistry staining for CD31. Immunohistochemical staining found blood vessel’s number decreased in diabetes mice with statistical significance in contrast to healthy mice from day 1 to day 7, and increased in the group administered with miR-221-3p agomir in contrast to the Ctrl group on days 3, and 7, but not on day 1, after treatment (Figure 2b and d).
The above findings suggested that diabetes reduced the WH rate, while miR-221-3p treatment promoted excisional WH in diabetic mice, miR-221-3p may reduce cell apoptosis in epithelial layer and promote angiogenesis in the dermis in excisional skin wounds of diabetes mice.
Impact of HG on THBS1 and Cell Function
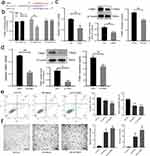
To explore the effect of HG on THBS1 in human immortalized keratinocyte cell line (HaCaT) cells, we treated these human cells for 72 h with normal glucose (NG, 5.5 mM) or HG (35 and 50mM). Results of qRT-qPCR as well as Western blot revealed that in contrast to the NG group, mRNA and protein levels in the THBS1 were upregulated in the HG group with statistical significance (Figure 3a). Furthermore, enzyme-linked immunosorbent assays (ELISAs) indicated that compared to the NG group, the protein levels that secreted by THBS1 into the culture medium were significantly increased in the HG group with statistical significance (Figure 3a).
Because THBS1 expression was increased in HG cultures, we performed an apoptosis experiment and a tube formation assay to clarify the influence of THBS1 on epithelial/endothelial cell’s function. HaCaT cells were cultured with NG medium (NG-Ctrl), NG but added with exogenous THBS1 protein (NG+THBS1) and HG medium (HG-Ctrl). The apoptotic rate was detected using flow cytometry, and results revealed that total apoptotic rate was upregulated among HaCaT cells interfered by exogenous THBS1 protein or HG compared with NG-Ctrl group with statistical significance. We further made statistics on the early apoptosis of cells (cells in Q3 quadrant), and the results were consistent with total apoptosis. (Figure 3b). The tube formation assay was conducted using human umbilical vein endothelial cells (HUVECs) co-cultured with the conditioned supernatant of HaCaT cells from the groups of NG-Ctrl, NG+THBS1 and HG-Ctrl. Results showed that compared to those from NG culture conditions, conditioned supernatant from the culture conditions of NG+THBS1 or HG blocked cell tube formation with statistical significance (Figure 3c). These findings indicated that HG significantly increase the expression of THBS1, induce the apoptosis of HaCaT cells and damage the cell tube formation of HUVEC.
Role of miR-221-3p in THBS1 as Well as Cell Function Interfered by HG
The molecular mechanisms underlying miR-221-3p’s effect on diabetic WH was further investigated. Three bioinformatics tools (TargetScan, miRDB, and Encyclopedia of RNA Interactomes [ENCORI]) were applied to search potential targets of miR-221-3p, which could regulate THBS1 and has been reported to induce apoptosis and inhibit angiogenesis. Upon sequence comparison, seed regions were identified in the 3ʹ untranslated region (3ʹ-UTR) of THBS1 mRNA, with potential base-pairing with miR-221-3p (Figure 4a). Based on a luciferase reporter assay, the functional interaction was determined. Transient transfection of THBS1 wild-type (WT) 3ʹ-UTR construct with miR-221-3p mimic into 293T cells resulted in a big reduction of the activity of luciferase in contrast to control groups with statistical significance. MiR-221-3p, on the other hand, showed no influence on the activity of luciferase of the mutated construct (Figure 4b). Further, Figure 4c reveals that THBS1 expression decreased at mRNA and protein levels by miR-221-3p over-expression among HaCaT cells with statistical significance. The above findings suggested that miR-221-3p might be potential to bind to the 3ʹ-UTR of THBS1 directly, thereby inhibiting its synthesis and expression. In addition, to facilitate the exploration of the influence of THBS1 on cell function, HaCaT cells were transfected with the THBS1-specific siRNA to suppress the expression of THBS1, the knockdown efficacy was confirmed by RT-qPCR, WB, and ELISA (Figure 4d).
To extend our results of the anti-apoptosis and pro-angiogenic impact of miR-221-3p observed in an animal model, HaCaT cells interfered with HG were transfected with a NC, miRNA-221-3p mimic (Mimic) or small interfering RNA of THBS1 (siTHBS1). Cell apoptosis assay showed that early and total apoptosis rate of cells after transfection were significantly reduced compared with HG control (Figure 4e). HUVEC co-cultured with conditioned supernatant of HaCaT cells from different conditions were collected for the tube formation assay. Results showed that tube formation of HUVECs was significantly enhanced in supernatant from HaCaT cells following the transfection of miR-221-3p mimic/siTHBS1 under HG (Figure 4f). Based on the above findings, it could be inferred that despite damage caused by HG, miR-221-3p blocked the expression of THBS1, significantly reduced HaCaT cells apoptosis and increased HUVEC cell tube formation.
Effect of miR-221 Knockout on WH in Diabetes Mice
To elucidate miR-221-3p’s impact on WH in vivo, miR-221 was knocked out in mice and constructed diabetes and skin wound models. Wound closure was delayed in miR-221 KO mice in contrast to WT mice with statistical significance (Figure 5a and b). Regarding the apoptosis of wound tissues, TUNEL+ apoptotic cells were significantly increased in the epithelial layer at the edge of wound from miR-221 KO mice compared with WT mice (Figure 5c and f). To detect the blood vessels in the newly formed skins, immunohistochemistry staining with CD31 labelling was performed. Semi-quantitative immunostaining found the number of blood vessels to be decreased among the epidermal tissues of the wound on the 11th day after the excisional wounds were created in miR-221 KO mice compared with WT mice (Figure 5d and g). In addition, THBS1 protein expression levels were also markedly increased in the epidermal tissues of the wound in miR-221 KO mice compared with WT mice (Figure 5e and h). These results suggest that miR-221 KO impaired WH, increased the apoptosis and reduced the vascularization in wounds of diabetic mice.
Discussion
DFU is a prevalent chronic complication of diabetes and a serious public health problem due to the need for hospitalization and amputation.15,16 Based on the results of our previous studies, miR-221-3p carried by EPCs exosomes promotes skin WH in mice, and this study further found that (1) HG promoted THBS1 expression at the wound edge among keratinocytes; (2) THBS1 promoted keratinocyte apoptosis and inhibited endothelial cell angiogenesis; (3) miR-221-3p targets THBS1, inhibited the synthesis and secretion of THBS1 induced by HG, inhibits epithelial cell apoptosis and promotes endothelial cell angiogenesis; (4) miR-221 knockout diabetic mice showed delayed WH, increased expression of THBS1 in the upper cortex, increased apoptosis, and decreased angiogenesis in the dermis. These key findings suggest that High glucose leads to increased THBS1 expression in wound skin, miR-221-3p may exert a pivotal impact on the regulation of the apoptosis & angiogenesis of endothelial cells by directly inhibiting THBS1 expression, promoting WH of diabetic skins and providing new ideas for the treatment of diabetic WH.
The process of WH is complex, involving multiple stages such as hemostasis, inflammation, neovascularization, re-epithelialization, and remodeling. Various cell types with different roles are involved.17 As the main epithelial cells in the skin, keratinocytes are related with multiple processes of WH to preserve the structure and function of the epidermis.18 Diabetes mellitus disrupts keratinocyte physiological function, causing a variety of cellular dysfunction, including impaired cell migration/proliferation, abnormal gap connectivity, chronic inflammation/infection, reduced angiogenesis, oxidative stress, as well as abnormal expression of matrix metalloproteinases. Research has indicated that physiological dysfunction of keratinocytes represents a significant contributing factor to the inadequate WH commonly observed in patients with chronic diabetes mellitus.19–21 As an antiangiogenic factor, THBS1 participates in different cellular functions, including angiogenesis, apoptosis and inflammation.22,23 The role of THBS1 in WH has been reported, mouse models with overexpression of THBS1 in keratinocytes showed slower WH.24 In normal human skin, THBS1 is produced by basal epidermal keratinocytes and subsequently deposited in the basal membrane region. In this study, it was found that in diabetic mice, THBS1 expression was found to be obviously elevated in the upper skin layer of the wound edge, leading to increased cell apoptosis, decreased dermal angiogenesis, as well as a marked reduction in WH rate. In vitro experiments confirmed HG’s influence on THBS1. Synthesis/secretion of THBS1 in HaCaT cells treated with HG increased significantly. This is consistent with previous findings that HG significantly affected THBS1 expression and secretion.25 Cell function experiments showed that both HG and THBS1 exogenous protein could increase HaCaT cell’s apoptotic rate and inhibit the angiogenesis of HUVECs co-cultured with HaCaT cells. Taken together, we hypothesized that hyperglucose-induced cellular dysfunction may be caused by the upregulation of THBS1 expression, which could serve as a promising therapeutic target for diabetic WH.
At present, the treatment of DFU is mainly to control blood glucose, promote lower limb blood supply and angiogenesis, improve local oxidative stress and control infection.26 As demonstrated by several studies, miRNAs are instrumental in the regulation of the complex process of WH and serve as viable targets for therapeutic intervention in cases of non-healing wounds.27 In this study, dual luciferase reporter (DLR) assay found THBS1 to be the target gene of miRNA-221-3p, and in vitro experiments verified that miRNA-221-3p overexpression significantly blocked the synthesis and secretion of THBS1 induced by HG. As previous reported, miRNA-221-3p participated in the regulation of various cellular functions, including proliferation, inflammation, apoptosis as well as angiogenesis.28–30 Results of in vivo experiments demonstrated that local miR-221-3p overexpression in diabetic mice could reduce the expression of THBS1 in the wound edge skin epithelium, reduce the apoptosis of epithelial cells, and increase the number of dermal neovascularization. Cell function assay showed that transfection of miR-221-3p or knockdown of THBS1 in HaCaT cells damaged by HG downregulated HacaT cell’s apoptotic rate and improved the tube formation ability of HUVECs co-cultured with HacaT cells. miR-221 KO mice were generated to further verify the importance of miR-221 in WH. The results showed that compared with WT diabetic mice, miR-221 KO diabetic mice had delayed WH, increased THBS1 expression and apoptosis in the epithelial layer, and reduced neovascularization in the dermis. Previous studies have shown that THBS1 induces apoptosis by activating caspase-8 and caspase-9 dependent pathways by binding to CD36 receptors,31 and THBS1 inhibits the recruitment of pro-angiogenic downstream signaling proteins by dephosphorylating VEGFR2.7 The intrinsic signaling pathway of THBS1 regulating apoptosis and angiogenesis in this study has not been addressed. The specific molecular mechanism underlying diabetic WH by miR-221-3p/THBS1 remains unclear and warrants further investigation.
Our study may suggest that the development of miR-221-3p analogs which keeps stable in vivo or topical dressings that directly cover wounds may contribute to future clinical applications. Due to the limitations of the small sample size and the large difference between mice and humans, a larger sample size and clinical experiments are still needed to verify the results of this study in the future.
Conclusions
We suggest that miR-221-3p improves diabetic skin WH by inhibiting the effects of HG on apoptosis and angiogenesis by targeting THBS1, and that miRNA-221-3p represents a candidate for novel therapies to improve diabetic WH.
Data Sharing Statement
Upon reasonable request to the corresponding author, the data involved in this study will be provided.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grants No. 81970703).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Armstrong DG, Boulton A, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–2375.
2. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366(9498):1719–1724. doi:10.1016/S0140-6736(05)67698-2
3. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi:10.1016/S0140-6736(05)67700-8
4. van Netten JJ, Price PE, Lavery LA, et al. Prevention of foot ulcers in the at-risk patient with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(1):84–98. doi:10.1002/dmrr.2701
5. Kale A, Rogers NM, Ghimire K. Thrombospondin-1 CD47 signalling: from mechanisms to medicine. Int J Mol Sci. 2021;22:
6. Yamauchi M, Imajoh-Ohmi S, Shibuya M. Novel antiangiogenic pathway of thrombospondin-1 mediated by suppression of the cell cycle. Cancer Sci. 2007;98(9):1491–1497. doi:10.1111/j.1349-7006.2007.00534.x
7. Morandi V, Petrik J, Lawler J. Endothelial cell behavior is determined by receptor clustering induced by thrombospondin-1. Front Cell Dev Biol. 2021;9:664696. doi:10.3389/fcell.2021.664696
8. Bayraktar M, Dündar S, Kirazli S, Teletar F. Platelet factor 4, beta-thromboglobulin and thrombospondin levels in type I diabetes mellitus patients. J Int Med Res. 1994;22(2):90–94. doi:10.1177/030006059402200204
9. Hong Z, Chen H, Hong H, Lin L, Wang Z. TSP-1 expression changes in diabetic rats with spinal cord injury. Neurol Res. 2009;31(8):878–882. doi:10.1179/174313209X403887
10. Ross K. MiR equal than others: microRNA enhancement for cutaneous wound healing. J Cell Physiol. 2021;236(12):8050–8059. doi:10.1002/jcp.30485
11. Li D, Wang A, Liu X, et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J Clin Invest. 2015;125(8):3008–3026. doi:10.1172/JCI79052
12. Li M, Ke QF, Tao SC, Guo SC, Rui BY, Guo YP. Fabrication of hydroxyapatite/chitosan composite hydrogels loaded with exosomes derived from miR-126-3p overexpressed synovial mesenchymal stem cells for diabetic chronic wound healing. J Mater Chem B. 2016;4(42):6830–6841. doi:10.1039/C6TB01560C
13. Wang SY, Kim H, Kwak G, et al. Development of microRNA-21 mimic nanocarriers for the treatment of cutaneous wounds. Theranostics. 2020;10(7):3240–3253. doi:10.7150/thno.39870
14. Xu J, Bai S, Cao Y, et al. miRNA-221-3p in endothelial progenitor cell-derived exosomes accelerates skin wound healing in diabetic mice. Diabetes Metab Syndr Obes. 2020;13:1259–1270. doi:10.2147/DMSO.S243549
15. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †. Ann Med. 2017;49(2):106–116. doi:10.1080/07853890.2016.1231932
16. Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51(5):747–755. doi:10.1007/s00125-008-0940-0
17. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi:10.1038/nature07039
18. Eckert RL, Efimova T, Dashti SR, et al. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J Investig Dermatol Symp Proc. 2002;7(1):36–40. doi:10.1046/j.1523-1747.2002.19634.x
19. Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31(8):817–836. doi:10.1007/s12325-014-0140-x
20. Li D, Peng H, Qu L, et al. miR-19a/b and miR-20a promote wound healing by regulating the inflammatory response of keratinocytes. J Invest Dermatol. 2021;141(3):659–671. doi:10.1016/j.jid.2020.06.037
21. Hu SC, Lan CE. High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci. 2016;84(2):121–127. doi:10.1016/j.jdermsci.2016.07.008
22. Streit M, Velasco P, Brown LF, et al. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155(2):441–452. doi:10.1016/S0002-9440(10)65140-1
23. Liu Z, Morgan S, Ren J, et al. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;117(2):129–141. doi:10.1161/CIRCRESAHA.117.305262
24. Streit M, Velasco P, Riccardi L, et al. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19(13):3272–3282. doi:10.1093/emboj/19.13.3272
25. Lan CC, Huang SM, Wu CS, Wu CH, Chen GS. High-glucose environment increased thrombospondin-1 expression in keratinocytes via DNA hypomethylation. Transl Res. 2016;169:
26. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:
27. Petkovic M, Sørensen AE, Leal EC, Carvalho E, Dalgaard LT. Mechanistic actions of microRNAs in diabetic wound healing. Cells. 2020;9:2228. doi:10.3390/cells9102228
28. Pan X, Hong X, Lai J, et al. Exosomal MicroRNA-221-3p confers adriamycin resistance in breast cancer cells by targeting PIK3R1. Front Oncol. 2020;10:441. doi:10.3389/fonc.2020.00441
29. Wei WF, Zhou CF, Wu XG, et al. MicroRNA-221-3p, a TWIST2 target, promotes cervical cancer metastasis by directly targeting THBS2. Cell Death Dis. 2017;8(12):3220. doi:10.1038/s41419-017-0077-5
30. Zhao J, Cui L, Sun J, et al. Notoginsenoside R1 alleviates oxidized low-density lipoprotein-induced apoptosis, inflammatory response, and oxidative stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis. Cell Signal. 2020;76:109781. doi:10.1016/j.cellsig.2020.109781
31. Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6(1):41–48. doi:10.1038/71517
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.