Back to Journals » Cancer Management and Research » Volume 12
microRNA-139-3p Inhibits Malignant Behaviors of Laryngeal Cancer Cells via the KDM5B/SOX2 Axis and the Wnt/β-Catenin Pathway
Received 22 June 2020
Accepted for publication 15 August 2020
Published 28 September 2020 Volume 2020:12 Pages 9197—9209
DOI https://doi.org/10.2147/CMAR.S268871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Yifei Ma,1,2,* Zili Chen,3,* Guodong Yu2
1School of Clinical Medicine, Guizhou Medical University, Guiyang 550004, Guizhou, People’s Republic of China; 2Department of Otorhinolaryngology, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou, People’s Republic of China; 3Department of Hepatobiliary Surgery, Affiliated Hospital of Guizhou Medical University, Guiyang 550004, Guizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guodong Yu Tel/Fax +86-851-86855119
Email [email protected]
Background: Laryngeal cancer (LCA) is a common head and neck cancer. Lysine demethylase 5B (KDM5B) knockdown is expected as a new target for cancer prevention. We investigated the molecular mechanism of KDM5B in LCA.
Materials and Methods: The levels of KDM5B, microRNA (miR)-139-3p and high-mobility-group box 2 (SOX2) in LCA tissues and cells, normal tissues and cells were detected. The effect of KDM5B on LCA was evaluated. The upstream miR of KDM5B and the downstream gene and pathway of KDM5B were predicted and their effects on LCA were analyzed. The Wnt/β-catenin pathway-specific activator agonist was delivered into LCA cells expressing miR-139-3p mimic to evaluate the role of the Wnt/β-catenin pathway.
Results: KDM5B was highly expressed in LCA, and inhibition of KDM5B suppressed LCA progression. miR-139-3p, downregulated in LCA tissues, was a regulatory miR of KDM5B. Overexpression of miR-139-3p significantly inhibited the malignant biological behaviors of LCA cells. KDM5B promoted SOX2 expression via histone demethylation. SOX2 was highly expressed in LCA, and overexpression of SOX2 promoted LCA progression by inducing the Wnt/β-catenin pathway. Activated Wnt/β-catenin pathway attenuated the inhibitory effect of miR-139-3p mimic on the malignant biological behaviors of LCA cells.
Conclusion: miR-139-3p overexpression inhibited LCA development via regulating the KDM5B/SOX2 axis and inhibiting the Wnt/β-catenin pathway.
Keywords: laryngeal cancer, microRNA-139-3p, lysine demethylase 5B, high-mobility-group box 2, Wnt/β-catenin pathway
Introduction
Laryngeal cancer (LCA), about 23% of head and neck squamous cell carcinoma, may occur on any mucosal surface of the larynx1 and takes major responsibility for cancer-related death in this kind of carcinoma.2 A patient-reported symptom questionnaire has noted that most LCA patients experience voice, speech and swallowing afflictions.3 Despite significant advances in therapeutic maneuvers, such as surgery and radiotherapy over the last decades, the overall survival for LCA patients is still at a low level; in addition, LCA cells are always resistant to radiotherapy, causing currently effective therapies of LCA rely mostly on surgery.4,5 In recent years, the treatment and survival trend of LCA have been highly concerned, especially when the deterioration of late LCA and the change of treatment trend are observed.6 Therefore, it is meaningful to find new ways for LCA treatment.
Histone lysine demethylases have been found to play crucial parts in gene expression, cellular differentiation, and cancer progression.7 Recently, histone lysine-specific demethylases have been evidenced to regulate human laryngeal squamous cell carcinoma (HLSCC), which is a type of head and neck cancer, promising to be new attractive targets for treating head and neck cancers.8 Lysine demethylase 5B (KDM5B), as a key regulator of histone 3 lysine 4 demethylation level, is verified to be upregulated and subsequently mediates the expression of tumor-initiating genes and cancer suppressor gene.9 KDM5B contributes to cancer initiation, invasion and metastasis with its obviously elevated expression in breast, bladder, lung and other diverse cancers.10 A previous study also indicated that overexpressed KDM5B in HLSCC is strongly related to an increased cancer risk and a reduced overall survival, with the hope of functioning as a novel biomarker for HLSCC.11 However, the mechanism of KDM5B in LCA remains unclear. In addition, it has been proposed that KDM5B is related to esophageal squamous cell carcinoma development via interactions with microRNA (miRNA).12 miRNA upregulation suppresses papillary thyroid cancer development by a KDM5B-dependent manner.13 The regulatory roles of miRNAs in gene expression in various human cancers have been adequately discussed.14 For instance, poorly expressed miR-203 leads to the increased cell invasion ability and suppressed cell apoptosis in LCA.15 Therefore, we speculate that KDM5B plays a regulatory role in LCA via the miR-mRNA system. Consequently, we performed a series of histological and molecular experiments to identify the miR-mRNA network involving KDM5B and to study the underlying molecular machinery, with the purpose to provide some novel therapies against LCA progression.
Materials and Methods
Sample Collection
Cancer tissues and adjacent normal tissues of 25 LCA patients who were operated in Affiliated Hospital of Guizhou Medical University were collected from December 2016 to February 2019. All patients were diagnosed as LCA for the first time in Affiliated Hospital of Guizhou Medical University. According to tumor, node, metastases (TNM) staging of LCA (2017 AJCC 3rd edition), there were 9 supraglottic carcinomas (Stage II: 3, Stage III: 4, Stage IV: 2), 10 glottic carcinoma (Stage I: 4, Stage II: 5, Stage III: 1), and 6 subglottic carcinoma (Stage II: 3, Stage III: 2, Stage IV: 1). All patients had complete medical data and free of other malignant tumors. A portion of fresh tissues was frozen in liquid nitrogen at −80°C, and the remaining tissues were embedded in paraffin for later experiments.
Cell Culture and Transfection
LCA cell lines HNO210, TU177 (ATCC) and the normal bronchial epithelial cell line 16HBE (Procell Life Science & Technology, Wuhan, Hubei, China) were cultured in Dulbecco’s modified eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) containing 100 unit/mL penicillin, 100 ng/mL streptomycin and 10% fetal bovine serum (FBS) in humid environment with 5% CO2 at 37°C.
Transfection plasmids miR-139-3p mimic, small hairpin (sh)-KDM5B, overexpressed (oe)-KDM5B, oe-high-mobility-group box 2 (SOX2) and their respective negative controls (NC) were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). Wnt/β-catenin pathway-specific activator agonist was purchased from MedChemExpress (HY-114321, Monmouth Junction, NJ, USA). The transfection was carried out using Lipofectamine 2000 (Life Technologies, Gaithersburg, MD, USA). Briefly, a total volume of 250 μL serum-free medium containing 2 μg transfection plasmids and a total volume of 250 μL serum-free medium containing 6 μL Lipofectamine transfectants were each incubated for 5 min and then mixed. The incubation was then continued for 5 to 6 h before medium refreshing with complete medium.
Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA (cDNA) was synthesized using PrimeScript RT Kits (Takara Bio, Shiga, Japan). PrimeScript miRNA cDNA Synthesis Kit (Takara) was used for reverse transcription. RT-qPCR was performed using SYBR Premier Ex Taq I. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for mRNAs and U6 as the control for miRNA. Relative expression was calculated by the standard 2-ΔΔCt method. The primer sequences (5ʹ-3ʹ) used are shown in Table 1.
 |
Table 1 Primer Sequence for RT-qPCR |
Immunohistochemistry
The paraffin-embedded sections were dehydrated with a series of ethanol and washed with distilled water for 2 min. Then, sections were immersed in 3% H2O2 for 20 min, followed by washes using distilled water for 2 min and using phosphate-buffered saline (PBS) for 3 min. Before immunohistochemical staining, antigen repair was performed with Tris/ethylene diamine tetraacetic acid buffer (pH = 9.0) under heating condition. Next, the sections were blocked using 10% normal goat serum for 20 min. The slides were cultured with primary antibodies against SOX2 (1:100, ab93689) or KDM5B (1:500, ab244220) at 4°C overnight. Goat anti-rabbit immunoglobulin G (IgG) H&L (horseradish peroxidase) (HRP) (1:1000, ab6721) and goat anti-mouse IgG H&L (HRP) (1:10,000, ab205719) served as the secondary antibody of SOX2 and KDM5B. After incubation with primary antibodies, sections were incubated with secondary antibodies added with HRP-conjugated streptavidin working solution (0343–10,000U; Beijing Imunbio Technologies, Co., Ltd., Beijing, China) at 37°C for 20 min. Diaminobenzidine (ST033; Whiga Biotechnology, Guangzhou, Guangdong, China) and hematoxylin (pt001; Bogoo Biotechnology, Shanghai, China) were used for visualization. After that, 1% ammonia water was added to make sections blue. Then, sections were dehydrated using gradient alcohol, cleared in xylene, and fixed with neutral gum. Sections were observed under an optical microscope and photographed.
Detection of Cell Proliferation
An EdU assay was performed using the EdU Cell Proliferation Assay Kit (RiboBio Co., Ltd., Guangzhou, Guangdong, China). In brief, the transfected LCA cells were plated in 96-well plates with 2 × 104 cells/well for 24 h, and then added with 50 µM EdU at 37°C. After 2 h, cells were fixed for 15 min in 4% paraformaldehyde and permeabilized for 20 min using 0.5% Triton X-100. After PBS washing, cells were treated with ApolloR reaction cocktail (100 μL) for 30 min and added with Hoechst 33342 (5 μg/mL) for nuclei counterstaining for 30 min. Finally, cells were imaged under a microscope (IX71, Olympus, Tokyo, Japan).
Cell Counting Kit-8 (CCK-8) assay was performed using the CCK-8 kit (Beyotime Biotechnology, Shanghai, China). The transfected cells were added into 96-well plates with 2000 cells/well together with 10 μL CCK-8 solution. The optical density (OD) value at 570 nm was detected using a microplate reader after incubation for 24 h, 48 h and 72 h.
Colony formation assay was performed as well. The transfected cells were plated into 6-well plates with 2 × 103 cells/well and cultured in DMEM containing 20% FBS for 9 days. Then, cells were fixed with 70% ethanol and stained with 0.25% crystal violet (Sigma-Aldrich, St. Louis, MO, USA). After staining, cell colony number was measured using ImageJ software.
Detection of Cell Apoptosis
Flow cytometry was performed for apoptosis detection using Annexin V-FITC cell apoptosis detection kit (Bestbio, Shanghai, China). After detachment, cells were resuspended in 1 × binding buffer at 1 × 105 cells/mL. Then, 5 μL FITC Annexin-V and 5 μL propidium iodide were put to 100 μL cell suspension for a 15-min incubation in the dark. After incubation, 400 μL 1 × binding buffer was added. Cell-Quest software (Becton Dickinson, Mountain View, CA, USA) and a FACS Calibur flow cytometer (Becton Dickinson) were used to analyze apoptosis.
Hoechst staining was performed. The transfected cells were cultured in 6-well plates at 1 × 105 cells/mL (3 mL per well) at 37°C with 5% CO2 for 24 h, and the medium was removed. Cells were fixed with paraformaldehyde, washed with PBS, and stained with Hoechst 33258 (MedChemExpress) devoid of light. After 30 min, cells were observed under an inverted microscope (Nikon, Tokyo, Japan), and five high power fields were randomly selected for each slide to evaluate the apoptosis rate.
Wound Healing Assay
The cells were cultured in 24-well plates until confluence. The cultured cells were scratched with a 10 μL sterile tip. The medium was then replaced with a fresh serum-free DMEM. The wound area was observed under the microscope with the EVOS FL cell imaging system (Life Technologies) at 0 h and 24 h after scratching. ImageJ was used to calculate wound closure.
Enzyme-Linked Immunosorbent Assay (ELISA)
Human Bax (ab199080) and Bcl-2 (ab119506) ELISA kits were utilized to measure the expression of Bax and Bcl-2 in LCA cells.
Transwell Assay
The transfected cells (5 × 104 cell) in serum-free medium were seeded in the apical chamber (Corning Life Sciences, New York, USA) coated with 0.2 mg/mL Matrigel (Becton Dickinson). Then, 600 μL DMEM supplemented with 10% FBS was paved in the basolateral chamber. After 24 h, the non-invaded cells were carefully removed using swabs. Cells were fixed with paraformaldehyde and stained with 0.25% crystal violet (Sigma-Aldrich). The invaded cells were counted in 5 randomly selected fields under the inverted microscope using ImageJ software.
Western Blot
Total protein of cells was extracted using RIPA buffer (Solarbio Co. Ltd., Beijing, China) containing proteinase inhibitor. Then, proteins were quantified using bicinchoninic acid protein assay kits (Thermo Fisher Scientific, Waltham, MA, USA), separated using SDS-PAGE, transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), and blocked with 5% skim milk. After that, membranes were probed at 4°C with primary antibodies overnight, with GAPDH as an internal reference, and then probed with the corresponding secondary antibodies for 2 h. Finally, enhanced chemiluminescence reagent (Millipore) was used for development. The antibody information was as follows: primary antibodies SOX2 (1:100, ab93689), histone 3 lysine 27 Trimethylation (H3K27me3, 1:1000, ab192985), KDM5B (1:100, ab244220), Bax (1:1000, ab32503), Bcl-2 (1:2000, ab182858), Caspase3 (1:5000, ab32351), Cleaved caspase3 (1:500, ab32042), β-catenin (1:50, ab22656), GAPDH (1:1000, #5174, Cell Signaling Technology, Danvers, MA, USA); secondary antibodies goat anti-rabbit IgG H&L (HRP) (1:10,000, ab6721), goat anti-mouse IgG H&L (HRP) (1:10000, ab205719). All antibodies were derived from Abcam (Cambridge, MA, USA).
Dual-Luciferase Reporter Gene Assay
The binding sites between miR-139-3p and KDM5B were predicted from TargetScan (http://www.targetscan.org/), amplified by PCR and cloned into pGL3 vector (Promega, Madison, WI, USA) to construct KDM5B wild-type (KDM5B-WT). KDM5B mutant (KDM5B-MT) was obtained by mutating the binding sites. These vectors were co-transfected with miR-139-3p mimic or its NC into 293T cells (ATCC) by Lipofectamine 2000. After a 48-h transfection, the luciferase activity was detected by the Luciferase Reporter Assay System (Promega).
Chromatin Immunoprecipitation (ChIP)
EZ-Magna ChIP kit (Millipore) was used for ChIP analysis of SOX2 promoter. In brief, HNO210 and TU177 cell lines were cross-linked for 10 min using 1% formaldehyde solution, which was quenched using glycine. DNA fragments were obtained by ultrasonic treatment. The supernatant was immunoprecipitated with anti-KDM5B (Abcam) or IgG antibody (Abcam). Finally, RT-qPCR was used to analyze the precipitated chromatin DNA.
RNA Immunoprecipitation (RIP) Assay
RIP lysis buffer kit (Millipore) was used for RIP assay. Briefly, LCA cells were lysed in RIP lysate buffer, and RNA was precipitated with Anti-AGO2 (Millipore) and anti-IgG (Millipore). TRIzol reagent was used to purify the immunoprecipitated RNA, and the gene expression was detected using RT-qPCR.
Statistical Analysis
SPSS 22.0 (IBM Corp., Armonk, NY, USA) was applied for data analysis. The results of three independent experiments were expressed as mean ± standard deviation. Comparisons between the two groups were analyzed with paired t test, and comparisons among multiple groups were analyzed with one-way analysis of variance (ANOVA) or two-way ANOVA. Pairwise comparisons after ANOVA were conducted by Tukey’s multiple comparisons test. The p value was obtained by a two-tailed test and p < 0.05 indicated significant difference.
Results
KDM5B Was Highly Expressed in LCA
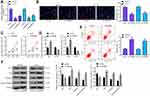
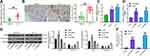
KDM5B is highly expressed in papillary thyroid cancer, a type of head and neck cancer, and promotes cancer development,13 but the mechanism of KDM5B has not been studied in LCA yet, another type of head and neck cancer. KDM5B expression in LCA tissues and adjacent normal tissues was detected by RT-qPCR. KDM5B was highly expressed in LCA tissues (Figure 1A). Meanwhile, KDM5B overexpression in LCA was also validated using immunohistochemistry (Figure 1B). KDM5B was distributed in both nucleus and cytoplasm, and the positive rate of KDM5B in cancer tissues was much higher than that in normal tissues. KDM5B mRNA and protein expression in LCA cell lines HNO210 and TU177 were much higher than that in cell line 16HBE (Figure 1C and D).
KDM5B Knockdown Inhibited LCA Cell Proliferation and Promoted Apoptosis
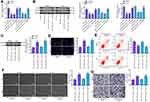
sh-KDM5B 1# and sh-KDM5B 2# were transfected into LCA cells, and the transfection efficiency was measured using RT-qPCR (Figure 2A). The sh-KDM5B 1# with better transfection efficiency was selected for next experiments. DNA replication was measured by EdU staining in LCA cells transfected with sh-KDM5B (Figure 2B). The positive rate of EdU in sh-KDM5B group reduced obviously relative to that in sh-NC group. Meanwhile, CCK-8 showed that sh-KDM5B noticeably inhibited cell proliferation (Figure 2C). The expression of Bax and Bcl-2 was measured with ELISA kits, and it was found that sh-KDM5B significantly promoted Bax expression and inhibited Bcl-2 expression (Figure 2D). Flow cytometry showed that sh-KDM5B promoted LCA cells apoptosis (Figure 2E). Finally, the Western blot results demonstrated a decline in Bcl-2 expression due to sh-KDM5B, as well as an elevated expression of Bax, Caspase3, Cleaved caspase3 (Figure 2F).
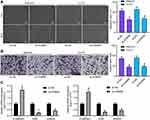
KDM5B Knockdown Inhibited LCA Cell Migration and Invasion
The migration ability of LCA cells transfected with sh-KDM5B was examined using wound healing test and Transwell assay. sh-KDM5B obviously inhibited the mobility rate of LCA cells (Figure 3A) and reduced invasive cells relative to that after sh-NC transfection (Figure 3B). The expressions of epithelial–mesenchymal transition (EMT)-related factors E-cadherin, Snail and Vimentin were measured using RT-qPCR. sh-KDM5B obviously increased E-cadherin expression, and inhibited the expression of Snail and Vimentin (Figure 3C).
miR-139-3p Targeted KDM5B
TargetScan (http://www.targetscan.org/) predicted that miR-139-3p targets KDM5B (Figure 4A). miR-139-3p expression in LCA and adjacent normal tissues were detected using RT-qPCR, and miR-139-3p was markedly downregulated in LCA tissues (Figure 4B), which was also confirmed in cancer cells (Figure 4C).
A series of experiments were designed to verify the binding relationship of miR-139-3p and KDM5B. miR-139-3p mimic was transfected into LCA cells, and RT-qPCR identified the effective transfection (Figure 4D). We found that miR-139-3p mimic notably inhibited KDM5B expression at both mRNA and protein levels (Figure 4E). Luciferase report gene assay showed that miR-139-3p mimic notably inhibited the luciferase activity of KDM5B-WT, with no dramatic effect on KDM5B-MT (Figure 4F). RIP assay indicated that Anti-AGO2 significantly enriched miR-139-3p and KDM5B (Figure 4G) compared with Anti-IgG. The above results proved that miR-139-3p targeted KDM5B in LCA cells.
miR-139-3p Overexpression Inhibited LCA Cell Development
The proliferation ability of LCA cells transfected with miR-139-3p mimic was detected using colony formation assay, which revealed that miR-139-3p overexpression prevented cell proliferation (Figure 5A). Wound healing test showed that miR-139-3p overexpression blocked cell migration ability (Figure 5B). Transwell assay proved that miR-139-3p mimic inhibited the invasion of LCA cells (Figure 5C). Hoechst staining displayed that miR-139-3p overexpression accelerated the apoptosis of LCA cells (Figure 5D).
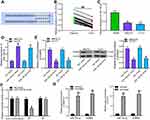
KDM5B Promoted SOX2 Expression
It is reported that histone demethylase can activate SOX2 expression through histone demethylation.16 SOX2 is considered to be an oncogene in LCA.17,18 RT-qPCR found that SOX2 was highly expressed in LCA (Figure 6A). Immunohistochemistry displayed that the positive rate of SOX2 in LCA tissues was higher than that in normal tissues, and it was mainly distributed in the nucleus (Figure 6B). The high expression of SOX2 in LCA cells was also confirmed (Figure 6C).
Afterwards, oe-KDM5B was transfected into LCA cells and the effective transfection was verified (Figure 6D). The expression of SOX2 and H3K27me3 in LCA cells transfected with sh-KDM5B and oe-KDM5B was measured (Figure 6E). It was found that oe-KDM5B significantly increased SOX2 expression and decreased H3K27me3 expression, while the reverse trends were observed following sh-KDM5B treatment. ChIP assay showed that Anti-KDM5B enriched SOX2 promoter sequence compared with Anti-IgG (Figure 6F). Our results proved that KDM5B promoted SOX2 expression by demethylation modification.
Overexpression of SOX2 Promoted the Malignant Biological Behavior of LCA Cells and Activated the Wnt/β-Catenin Pathway
oe-SOX2 was transfected into LCA cells, and the effective transfection was verified by RT-qPCR and Western blot (Figure 7A). Colony formation assay indicated that SOX2 overexpression significantly promoted LCA cell proliferation (Figure 7B). Hoechst staining showed that SOX2 overexpression obviously inhibited LCA cell apoptosis (Figure 7C). Wound healing test and Transwell assay found that SOX2 promoted LCA cell migration and invasion (Figure 7D and E). WB showed that SOX2 noticeably expedited β-catenin level (Figure 7F).
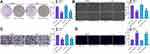
miR-139-3p Regulated the SOX2/Wnt/β-Catenin Axis via Targeting KDM5B
oe-KDM5B was transfected into LCA cells pre-transfected with miR-139-3p mimic, and RT-qPCR revealed that SOX2 mRNA expression was repressed by miR-139-3p mimic and restored by oe-KDM5B (Figure 8A). miR-139-3p mimic inhibited SOX2 and β-catenin protein expression, which could be partially reversed by oe-KDM5B (Figure 8B). The above results proved that miR-139-3p regulated SOX2 expression and Wnt/β-catenin pathway via targeting KDM5B.
To verify whether Wnt/β-catenin plays a role in LCA cells, we transfected agonist, a specific activator of Wnt/β-catenin pathway into LCA cells stably expressing miR-139-3p mimic, with DMSO as the control. By measuring β-catenin expression using Western blot, we found that agonist notably promoted β-catenin expression (Figure 8C). EdU assay showed that agonist noticeably increased EdU-positive cells and promoted DNA synthesis (Figure 8D). Flow cytometry exhibited that agonist obviously reduced the apoptosis rate increased by miR-139-3p mimic (Figure 8E). Wound healing test and Transwell assay also suggested that the activation of the Wnt/β-catenin pathway significantly weakened the inhibitory effect of miR-139-3p mimic on LCA cell migration and invasion (Figure 8F and G).
Discussion
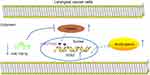
LCA remains a common head and neck cancer with over 150,000 new patients diagnosed worldwide.19 KDM5B possesses oncogenic functions and is regarded as a promising therapy target for cancer treatment.20 We identified in this study that KDM5B was upregulated in LCA, and miR-139-3p inhibited LCA cells growth and activated Wnt/β-catenin pathway via the KDM5B/SOX2 axis (Figure 9).
KDM5B is aberrantly upregulated in squamous cell carcinoma of the head and neck, and its high expression indicates short overall survival.21 This study verified that KDM5B was abnormally overexpressed in LCA. It has been evidenced that Bcl-2 is related to pro-survival and Bax is related to pro-apoptosis.22 Downregulated E-cadherin and upregulated Vimentin, Snail, and N-cadherin indicate enhanced EMT, which was related to invasion and metastasis of cells.23 Our results supported KDM5B deficiency dramatically suppressed LCA cell proliferation, migration and invasion, and induced apoptosis with upregulated Bax and E-cadherin, and downregulated Bcl-2, Vimentin and Snail level. KDM5B is abnormally elevated and is related to tumorigenesis and metastasis in HLSCC.11 Silencing of KDM5B suppresses cell proliferation and migration, and reverses EMT in breast cancer.24 KDM5B knockdown effectively promotes cell apoptosis in ovarian cancer.25 Consistently, our results in the current investigation showed that KDM5B was upregulated in LCA, and KDM5B knockdown inhibited tumor growth and promoted cancer cell apoptosis in LCA.
miR-139-3p was significantly downregulated in supraglottic LSCC patients with lymphatic metastasis relative to those free of lymphatic metastasis.26 We identified that miR-139-3p was poorly expressed in LCA, and the target relationship between miR-139-3p and KDM5B was verified in this study. miR-139-3p was found to serve as a tumor suppressor against LCA.27 Suppressed miR-139-5p is associated with H3K27me3 in modulating pancreatic cancer.28 However, there were few studies about the relationship between miR-139-3p and KDM5B, which confirmed the novelty of this study. In this study, overexpressed miR-139-3p inhibited tumor growth in LCA. hsa-miR-139-3p has been reported to contribute to suppressed proliferation and migration, increased apoptosis of human papillomavirus 16-positive cells and increased oncogenesis in head and neck cancer.29 The above results showed that miR-139-5p suppressed tumor growth and promoted cell apoptosis in LCA via targeting KDM5B.
SOX2, as a transcription factor, takes crucial parts in stem cell state maintenance and is closely related to cancer development with its ability of enhancing tumor proliferation, migration and invasion.30 SOX2 is frequently expressed in LCA and promises to play a predictive role in LCA diagnosis, functioning in promoting LCA cell ability of migration and invasion.31,32 KDM5B promoted SOX2 expression in LCA cells in the present work. KDM5B and SOX2 are closely correlated in the regulation of atypical teratoid/rhabdoid tumor.33 Moreover, we revealed that overexpressed SOX2 caused increased cancer cell growth and inhibited cell apoptosis. Overexpressed SOX2 was verified to be linked to reduced laryngeal squamous cell carcinoma apoptosis.17 In addition, SOX2 was related to the Wnt/β-catenin pathway activation. SOX2 overexpression contributes to migration, invasion, and EMT in combination with the Wnt/β-catenin pathway in LCA.34 Taken together, KDM5B upregulated SOX2 expression and activated the Wnt/β-catenin pathway to promote LCA cell malignant episodes.
SOX2 has been verified to be targeted by miRNA to sustain breast cancer cell stemness.35 Moreover, suppressed miRNA level is associated with Wnt/β-catenin pathway activation in breast cancer.36 In this study, our results suggested that miR-139-3p overexpression markedly inhibited SOX2 and Wnt/β-catenin expression, which were reversed by overexpressed KDM5B, indicating that miR-139-3p mediated KDM5B to regulate the SOX2/Wnt/β-catenin axis. In line with our findings, miR-139-3p overexpression suppressed Wnt/β-catenin signaling pathway in glioma.37 Wnt1, a factor in the Wnt/β-catenin signaling, is demonstrated as a target of miR-139-5p in C2C12 myoblasts.38 Additionally, Wnt/β-catenin pathway activation weakened the inhibition of miR-139-3p on LCA. Wnt/β-catenin downregulation is helpful to reduce LCA cell malignant episodes.39 Taken together, miR-139-3p overexpression curtails LCA cell growth and promotes cancer cell apoptosis via the KDM5B-mediated SOX2/Wnt/β-catenin axis.
Conclusion
All in all, this study supports that miR-139-3p targets KDM5B to downregulate SOX2 and to inhibit the Wnt/β-catenin pathway, thus suppressing LCA progression. Targeting the KDM5B/SOX2/Wnt/β-catenin axis might develop as a new therapeutic approach for LCA treatment. Although the current study provides a new perspective for the treatment of LCA, the clinical application effect needs to be further verified.
Data Sharing Statement
All the data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was approved and supervised by the ethics committee of Affiliated Hospital of Guizhou Medical University. All experiments follow the Declaration of Helsinki. All the subjects signed informed consents.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no potential conflicts of interest for this work.
References
1. Trotti A
2. Saito K, Inagaki K, Kamimoto T, et al. MicroRNA-196a is a putative diagnostic biomarker and therapeutic target for laryngeal cancer. PLoS One. 2013;8(8):e71480. doi:10.1371/journal.pone.0071480
3. Rinkel RN, Verdonck-de Leeuw IM, van den Brakel N, et al. Patient-reported symptom questionnaires in laryngeal cancer: voice, speech and swallowing. Oral Oncol. 2014;50(8):759–764. doi:10.1016/j.oraloncology.2014.05.009
4. Yamazaki H, Suzuki G, Nakamura S, et al. Radiotherapy for locally advanced resectable T3-T4 laryngeal cancer-does laryngeal preservation strategy compromise survival? J Radiat Res. 2018;59(1):77–90. doi:10.1093/jrr/rrx063
5. Yan SX, Luo XM, Zhou SH, et al. Effect of antisense oligodeoxynucleotides glucose transporter-1 on enhancement of radiosensitivity of laryngeal carcinoma. Int J Med Sci. 2013;10(10):1375–1386. doi:10.7150/ijms.6855
6. Misono S, Marmor S, Yueh B, Virnig BA. Treatment and survival in 10,429 patients with localized laryngeal cancer: a population-based analysis. Cancer. 2014;120(12):1810–1817.
7. Guerra-Calderas L, Gonzalez-Barrios R, Herrera LA, Cantu de Leon D, Soto-Reyes E. The role of the histone demethylase KDM4A in cancer. Cancer Genet. 2015;208(5):215–224. doi:10.1016/j.cancergen.2014.11.001
8. Bais MV. Impact of epigenetic regulation on head and neck squamous cell carcinoma. J Dent Res. 2019;98(3):268–276. doi:10.1177/0022034518816947
9. Xhabija B, Kidder BL. KDM5B is a master regulator of the H3K4-methylome in stem cells, development and cancer. Semin Cancer Biol. 2019;57:79–85. doi:10.1016/j.semcancer.2018.11.001
10. Han M, Xu W, Cheng P, Jin H, Wang X. Histone demethylase lysine demethylase 5B in development and cancer. Oncotarget. 2017;8(5):8980–8991. doi:10.18632/oncotarget.13858
11. Sun H, Jiang W, Hu J, Ma Z. Prognostic value of elevated KDM5B expression in patients with laryngeal squamous cell carcinoma. Int J Clin Exp Pathol. 2019;12(9):3500–3506.
12. Cui G, Liu D, Li W, et al. Original Research: miR-194 inhibits proliferation and invasion and promotes apoptosis by targeting KDM5B in esophageal squamous cell carcinoma cells. Exp Biol Med (Maywood). 2017;242(1):45–52. doi:10.1177/1535370216662712
13. Pu Y, Xiang J, Zhang J. KDM5B-mediated microRNA-448 up-regulation restrains papillary thyroid cancer cell progression and slows down tumor growth via TGIF1 repression. Life Sci. 2020;250:117519. doi:10.1016/j.lfs.2020.117519
14. Du J, Zhang L. Analysis of salivary microRNA expression profiles and identification of novel biomarkers in esophageal cancer. Oncol Lett. 2017;14(2):1387–1394. doi:10.3892/ol.2017.6328
15. Tan J, Han L, Jing YY, et al. [Study on the effects of microRNA-203 on the invasion and apoptosis of laryngeal cancer cells via targeting LASP1]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2019;33(2):171–175. Chinese.
16. Lu H, Xie Y, Tran L, et al. Chemotherapy-induced S100A10 recruits KDM6A to facilitate OCT4-mediated breast cancer stemness. J Clin Invest. 2020;130(9):4607–4623. doi:10.1172/JCI138577
17. Karatas OF, Yuceturk B, Suer I, et al. Role of miR-145 in human laryngeal squamous cell carcinoma. Head Neck. 2016;38(2):260–266.
18. Yang N, Wang Y, Hui L, Li X, Jiang X. Silencing SOX2 expression by RNA interference inhibits proliferation, invasion and metastasis, and induces apoptosis through MAP4K4/JNK signaling pathway in human laryngeal cancer TU212 cells. J Histochem Cytochem. 2015;63(9):721–733. doi:10.1369/0022155415590829
19. Calkovsky V, Wallenfels P, Calkovska A, Hajtman A. Laryngeal cancer: 12-year experience of a single center. Adv Exp Med Biol. 2016;911:9–16.
20. Zheng YC, Chang J, Wang LC, Ren HM, Pang JR, Liu HM. Lysine demethylase 5B (KDM5B): a potential anti-cancer drug target. Eur J Med Chem. 2019;161:131–140. doi:10.1016/j.ejmech.2018.10.040
21. Huang D, Qiu Y, Li G, et al. KDM5B overexpression predicts a poor prognosis in patients with squamous cell carcinoma of the head and neck. J Cancer. 2018;9(1):198–204. doi:10.7150/jca.22145
22. Merino D, Lok SW, Visvader JE, Lindeman GJ. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene. 2016;35(15):1877–1887.
23. Wang W, Wang L, Mizokami A, et al. Down-regulation of E-cadherin enhances prostate cancer chemoresistance via Notch signaling. Chin J Cancer. 2017;36(1):35. doi:10.1186/s40880-017-0203-x
24. Zhang ZG, Zhang HS, Sun HL, Liu HY, Liu MY, Zhou Z. KDM5B promotes breast cancer cell proliferation and migration via AMPK-mediated lipid metabolism reprogramming. Exp Cell Res. 2019;379(2):182–190. doi:10.1016/j.yexcr.2019.04.006
25. Ren R, Wu J, Zhou MY. MiR-135b-5p affected malignant behaviors of ovarian cancer cells by targeting KDM5B. Eur Rev Med Pharmacol Sci. 2020;24(7):3548–3554.
26. Sun X, Song Y, Tai X, Liu B, Ji W. MicroRNA expression and its detection in human supraglottic laryngeal squamous cell carcinoma. Biomed Rep. 2013;1(5):743–746. doi:10.3892/br.2013.143
27. Cybula M, Wieteska L, Jozefowicz-Korczynska M, Karbownik MS, Grzelczyk WL, Szemraj J. New miRNA expression abnormalities in laryngeal squamous cell carcinoma. Cancer Biomark. 2016;16(4):559–568. doi:10.3233/CBM-160598
28. Ma J, Zhang J, Weng YC, Wang JC. EZH2-mediated microRNA-139-5p regulates epithelial-mesenchymal transition and lymph node metastasis of pancreatic cancer. Mol Cells. 2018;41(9):868–880.
29. Sannigrahi MK, Sharma R, Singh V, Panda NK, Rattan V, Khullar M. Role of host miRNA Hsa-miR-139-3p in HPV-16-induced carcinomas. Clin Cancer Res. 2017;23(14):3884–3895. doi:10.1158/1078-0432.CCR-16-2936
30. Huser L, Novak D, Umansky V, Altevogt P, Utikal J. Targeting SOX2 in anticancer therapy. Expert Opin Ther Targets. 2018;22(12):983–991. doi:10.1080/14728222.2018.1538359
31. Granda-Diaz R, Menendez ST, Pedregal Mallo D, et al. The novel role of SOX2 as an early predictor of cancer risk in patients with laryngeal precancerous lesions. Cancers (Basel). 2019;11(3):286. doi:10.3390/cancers11030286
32. Yang N, Hui L, Wang Y, Yang H, Jiang X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep. 2014;31(6):2651–2659. doi:10.3892/or.2014.3120
33. Pan X, Liu W, Chai Y, Hu L, Wang J, Zhang Y. Identification of hub genes in atypical teratoid/rhabdoid tumor by bioinformatics analyses. J Mol Neurosci. 2020. doi:10.1007/s12031-020-01587-8
34. Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in laryngeal cancer Hep-2 cells. Tumour Biol. 2014;35(8):7965–7973. doi:10.1007/s13277-014-2045-3
35. Zhou L, Zhao LC, Jiang N, et al. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. Eur Rev Med Pharmacol Sci. 2017;21(1):87–94.
36. Ren L, Chen H, Song J, et al. MiR-454-3p-mediated Wnt/beta-catenin signaling antagonists suppression promotes breast cancer metastasis. Theranostics. 2019;9(2):449–465. doi:10.7150/thno.29055
37. Huo LW, Wang YF, Bai XB, Zheng HL, Wang MD. circKIF4A promotes tumorogenesis of glioma by targeting miR-139-3p to activate Wnt5a signaling. Mol Med. 2020;26(1):29. doi:10.1186/s10020-020-00159-1
38. Mi L, Li Y, Zhang Q, et al. MicroRNA-139-5p regulates C2C12 cell myogenesis through blocking Wnt/beta-catenin signaling pathway. Biochem Cell Biol. 2015;93(1):8–15. doi:10.1139/bcb-2014-0079
39. Tang X, Sun Y, Wan G, Sun J, Sun J, Pan C. Knockdown of YAP inhibits growth in Hep-2 laryngeal cancer cells via epithelial-mesenchymal transition and the Wnt/beta-catenin pathway. BMC Cancer. 2019;19(1):654. doi:10.1186/s12885-019-5832-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.