Back to Journals » Breast Cancer: Targets and Therapy » Volume 14
Micropapillary Breast Carcinoma: From Molecular Pathogenesis to Prognosis
Authors Verras GI, Tchabashvili L, Mulita F , Grypari IM, Sourouni S, Panagodimou E, Argentou MI
Received 28 October 2021
Accepted for publication 29 January 2022
Published 12 March 2022 Volume 2022:14 Pages 41—61
DOI https://doi.org/10.2147/BCTT.S346301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Georgios-Ioannis Verras,1 Levan Tchabashvili,1 Francesk Mulita,1 Ioanna Maria Grypari,2 Sofia Sourouni,3 Evangelia Panagodimou,4 Maria-Ioanna Argentou1
1Department of Surgery, Breast Unit, University Hospital of Patras, Patras, Greece; 2Department of Pathology, University Hospital of Patras, Patras, Greece; 3Department of Radiology, University Hospital of Patras, Patras, Greece; 4Department of Gynecology, University Hospital of Patras, Patras, Greece
Correspondence: Francesk Mulita, Department of Surgery, Breast Unit, University Hospital of Patras, Patras, Greece, Tel +30 6982785142, Email [email protected]
Abstract: Invasive micropapillary carcinoma (IMPC) of the breast is an infrequent type of breast cancer often discussed for its potency for lymphovascular invasion and difficulty in accurate imaging estimation. Micropapillary carcinomas are noted to be present as larger tumors, of higher histological grade and a notably higher percentage of disease-positive lymph nodes. Hormonal and HER-2 positivity in IMPC is also commoner when compared to other NST carcinomas. IMPC occurs either as a pure form or more often as a component of mixed Non-Specific Type (NST) carcinoma. The latest data suggest that despite having comparable survival rates to other histological subtypes of breast carcinoma, effective surgical treatment often requires extended surgical margins and vigilant preoperative axillary staging due to an increased incidence of lymph node invasion, and locoregional recurrence. Moreover, the presence of micropapillary in situ components within tumors also seems to alter tumor aggression and influence the nodal disease stage. In this review, we present an overview of the current literature of micropapillary carcinoma of the breast from biology to prognosis, focusing on biological differences and treatment.
Keywords: micropapillary, breast cancer, sentinel lymph node biopsy, lymphovascular invasion, mastectomy
Introduction
Invasive micropapillary carcinoma (referred to as IMPC) is a rare, distinct histological subtype of breast carcinoma. First described as an entity by Fisher et al in 1980,1 it was not until 1993 that the term and classification was introduced by Siriaunkgul et al.2 While micropapillary histological architecture is found in 2–8% of all breast cancers, pure micropapillary carcinoma is infrequent and comprises 0.9–2% of breast carcinomas.3 Mean age of diagnosis is 50–60 years, and it is predominantly found in females, with only a few cases for male IMPC reported.4–10 This review aims to provide an overview of the effect of micropapillary histology on lymph node invasion, LVI, and prognosis. Also, the effect of micropapillary component within non-pure IMPC is discussed, and any recorded differences regarding IMPC treatment compared to other histological subtypes are considered.
There is a distinct pathological morphology of IMPC, consisting of hollow cell clusters with granular or eosinophilic cytoplasm,11 arranged in a pseudopapillary manner, devoid of fibrovascular cores and laid out in an “inside-out” manner, with the luminal cellular surface being the outermost.1,12–18 This arrangement is best presented when MUC1/EMA staining is used, so much so that “reversed” staining of these markers is considered a hallmark of IMPC, shared only by mucinous histology.19–21 The distinctive histological features of pure micropapillary carcinoma can be seen in Figure 1A–D, as taken from one of our cases.161
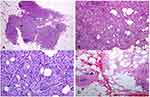 |
Figure 1 (A–D) In low magnification, through an atrophic mammary gland a neoplastic population is recognized, infiltrating the remaining ducts (A). The cells are organized in clusters, forming small-sized glandular structures and nests, arranged in a micropapillary pattern (B). Occasionally, a small proportion of them acquire a central lumina. Fibrovascular cores are absent. (C) The neoplastic cells have a moderate amount of eosinophilic cytoplasm and small round nuclei with condensed chromatin and intermediate pleomorphism. (D). In another slide of this lesion, a lymphovascular emboli is recognized (D). The morphology is highly suggestive of invasive micropapillary carcinoma, so immunohistochemical markers are performed to establish the diagnosis. Notes: Reproduced from: Verras GI, Mulita F, Tchabashvili L, et al. A rare case of invasive micropapillary carcinoma of the breast. Menopause Review/Przegląd Menopauzalny. 2022;21(1):1-8. doi:10.5114/pm.2022.113834.161 Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) License (http://creativecommons.org/licenses/by-nc-sa/4.0/). |
IMPC is emerging as an oncological and surgical challenge, due to a plethora of characteristics that constitute this histological pattern, interestingly, both elusive and aggressive. Namely, its tendency to present as a palpable mass, often of increased size and higher grade compared to the invasive ductal carcinoma (IDC), currently the most diagnosed type of breast cancer. Another especially troublesome aspect of IMPC is the comparatively increased incidence of lymphovascular invasion (LVI) characterized by both carcinomatous emboli,22,23 and clinically positive axillary lymph nodes,23 which naturally alters the surgical and adjuvant treatment regiments to more aggressive ones, with comparative prognosis still being a point of ongoing debate.5,24–28
Review Methodology
Current literature search on micropapillary carcinoma was performed using the PubMed, SCOPUS and Cochrane Library databases. Studies in the fields of Medicine, Biology, Molecular Biology and Genetics were included. Each report was screened independently for relevance, and the Mendeley referencing tool was used for duplicate detection. Keywords used included “micropapillary breast carcinoma”, “micropapillary DCIS”, “micropapillary cancer” “invasive micropapillary breast carcinoma”. The selection process (carried out under the latest PRISMA guidelines for reporting29), can be seen in Figure 2. A total of 155 reports were included in the review: 117 original articles, 9 review articles, 24 case reports, 2 meta-analyses, and 3 opinion letters/editorials.
 |
Figure 2 Report selection flowchart. |
Lymphovascular Invasion and Lymph Node Involvement
We have collected results from several published studies with variable sample sizes and characteristics. A brief summary of study findings on tumor size, lymph node involvement, and LVI presence can be seen in Table 1. One of the most studied respects of IMPC thus far is the seemingly increased frequency of lymphovascular invasion and lymph node (often clinically evident) involvement.13,15,22,30–40 A recent study by Lewis et al,41 published in 2019, used a sample of 2660 patients diagnosed with pure IMPC, one of the largest case series to date. The study demonstrated confirmed regional lymph node metastasis in 55.2% of the patients at the time of diagnosis, with other researchers such as Gokce et al reporting percentages up to 79.6%.33 Risk factors associated with nodal involvement in IMPC include tumor size, ER negativity, and advanced age.42
 |  |  |
Table 1 Data on Tumor Size, Tumor Grade, Nodal Status and LVI from Included Studies |
To put things in perspective, a comparison between IMPC and Invasive Ductal Carcinoma (IDC) is often deemed appropriate, since IDC is undoubtedly the most studied type of breast carcinoma. A comparative study by Hashimi et al15 showed that only 49.5% of the patients with IDC had any nodal involvement, and in fact N3 stage occurred in only 15.6% of the patients, as opposed to 33% in the IMPC group. Lymphovascular involvement has also been found to be more common among IMPC patients, as shown in a study by Tang et al,13 with 14.7% versus only 0.1% in the IDC group, and a staggering 94.7% being reported by Gokce et al.33 Both points are of great surgical significance, since radiologically, clinically or biopsy-proven positive lymph nodes have been an indication for more extensive surgery and axillary dissection.13,32 It is indicative that Tang et al reported selection of partial mastectomy in 7.4% of the IDC group, as opposed to 3.0% of the IMPC group.13 A previous study by Paterakos et al43 showcased not only lymphovascular involvement in 95% of the patients but also a relation with higher-grade tumors at presentation and higher scores on the mitotic index.
Tumor size at diagnosis has also been a much-discussed issue regarding IMPC. Hao et al compared the percentage of tumors larger than 5cm at the time of diagnosis, reporting 4.3% in IMPC and 3% in IDC.44 Ye et al demonstrated that IMPC presented at a higher stage tumor at diagnosis also attributed to a larger size, in a meta-analysis.45 It is worth noting that the reported difference in mean tumor size can be attributed to the rapid growth patterns of IMPC, as well as its insidious presentation, leading to larger tumors being diagnosed more often.38,46 However, more basic research on the underlying molecular biology of IMPC is needed. Another point of concern is the lack of specific guidelines regarding the percentage of micropapillary element required to report a tumor as partially or purely micropapillary. This leads to a lack of systematic sample classification and comparison.13,32,47,48
Pathology – HR and HER2
Molecular testing has provided an insight on the correlations of the hormonal status and clinical presentation, treatment, and prognosis of IMPC patients. Authors report higher percentages of estrogen receptor (ER) and progesterone receptor (PgR) positive tumors when comparing IMPC with IDC.1,3,14,49–52 Collected data on the hormonal status of IMPC tumors, and relevant comparisons from included studies can be found in Table 2. Positive ER staining has been commented upon as positively associated with survival duration in a large series of IMPC patients.49,52,53 A large study by Cui et al14 reported 88% ER positivity and 64% PgR positivity when studying IMPC specimens. A study conducted by Lewis et al, including 865 cases, has reported that the IMPC tumors are characterized as Luminal A in 75.3% of the instances, Luminal B in 14.8%, HER2-enriched in 4.7%, and Triple Negative in 5.2%.41 However, most studies have found that micropapillary carcinomas tend to be in the Luminal B category when genomic sequencing is used instead of staining alone.54–56 While the incidence of the triple-negative classification seems to be lower in IMPC, it is associated with higher-grade tumors, higher disease stage at diagnosis, and an increase in total mastectomies performed.7,15,44,57–59
 |  |  |
Table 2 Data on Hormone Receptor and HER-2 Status of IMPC Patients from Included Studies |
Overall, in terms of surveillance, hormonal positivity and HER2-positive staining are reported to be higher in IMPC than IBC.55,60 However, no difference in survival rates is reported between HER2-positive and HER2-negative groups. According to the authors,14,28,41,49,61,62 this is largely attributed to the latest HER2 targeting biological therapeutic regimens added to systemic therapy. A noteworthy study, run by Perron et al, provided insight into the expression of HER2 in IMPC. In particular, it is suggested that due to the tumor’s peculiar histological arrays, the interpretation of HER2 staining in IMPC should be updated from the previously known ASCO/CAP recommendations.62 The authors mention that HER2 expression in IMPC by immunohistochemistry (IHC) ranges from 12.5% to 95%, possibly a result of scoring variability before the 2007/2013 guidelines.54 Furthermore, they analysed 1684 IMPC cases by IHC alone and found 11.6% to be positive (3+) and 29.4% to be equivocal (2+). Analysis of further 1272 IMPC cases by in situ hybridization (ISH) alone showed 20.4% of the cases were HER2-amplified and 7.4% were equivocal. Upon dual analysis of 411 cases by both IHC and ISH, 4.4% of the cases were found to be positive (3+) by IHC and of these, 83.3% were HER2-amplified. Interestingly, they showed that 43% of IMPCs with a HER2 staining score of 1+ were found to be HER2-amplified by ISH.54 They also claim that the morphology of the tumor seems to exclude the luminal side of the cells from staining. Therefore, they suggest lowering the “1+” categorization to tumor staining described as “weak to moderate but incomplete”. In fact, further testing of equivocal staining seemed to yield HER2 positivity in 35% of the specimens, indicating that a more inclusive definition would benefit many IMPC patients by encompassing them in HER2 targeted treatment, a finding also reported by more research groups.54
Lymphovascular Tropism
With the emergence of readily available methods of genomic and molecular analysis, a pathogenetic mechanism to explain the increased incidence of vascular, lymphovascular, and lymph node involvement has been proposed. As discussed earlier, IMPC cases appear with higher percentages of nodal involvement15 and lymphovascular involvement was detected in 14.7% to as high as 94.7% of the IMPC cases, compared to IDC cases.13,33
Recent studies have shown an overexpression of metalloproteinases and adherence molecules,6,15,46,50,63–65 as well as several cytotropic molecules, namely TNF-α, TNFreceptor II, E-cadherin, kindlin-2, integrinβ1, plakoglobin and β-catenin overexpression, occurring within pure IMPC cancer cells.50,51,66–70 Interleukin 1-β is associated with high microvascular density in IMPC tumors, as well as nodal metastases.71 N-cadherin, an adhesive protein, was also upregulated in IMPC cells when compared to non-IMPC cells.72 Well-known tumor chemotaxis factors SDF-1/CXCR4 also facilitate nodal invasion in IMPC.73 The findings mentioned above are indicative of the tumor cell’s ability to separate from neighboring cells, and invade the vascular and lymphatic systems, exhibiting a certain tropism towards lymphatic metastasis.15,50,74,75
The upregulation of glucose transporters has also been observed in a small number of patients, with significant differences in genomic expression when compared to non-IMPC tumors.76 The authors hypothesized that the apparent increase in GLUT-1 transporters with the simultaneous expression of hypoxia-inducible transcription factors is another process that enables IMPC cells to adapt, survive, and metastasize more than their non-IMPC counterparts.77
Another molecular-based study target that can give additional insights in the lymphovascular tropism of the tumors has been the observed predominance of CD44-positive and CD24-negative phenotype on IMPC cells. Alterations in the expression of these two molecules are partially responsible for certain stem cell properties that tumor cells exhibit (self-renewal, survivability, proliferation, lack of apoptosis). Among them, CD24 loss was associated with tumor spread and invasion.78,79 Indeed, a study by Li et al demonstrated a higher percentile presence of such cells, in comparison to IDC tumors, namely 48.5% versus 31.9%.78 CD44 loss was also found to be significantly higher in IMPC tumors when compared to NST tumors and was also associated with lymph node metastasis in IMPC patients as well.69,80 CD146 expression is also positively correlated with high microvascular density and was found to be more significant in IMPC rather than NST tumors.81 These findings serve as a plausible explanation of the IMPC invasive lymphotropic properties. A recent study by Kramer et al showed that IMPC tumor cells were in a highly epithelial state and did not use the EMT pathway, but rather form cell clusters during invasion and metastasis.82
The utilization of deep mRNA sequencing has also demonstrated at least 45 different miRNAs thought to be involved in IMPC development,83 and karyotype studies have also shown certain reproducible aberrations, such as gain of chromosomes 1q,8q,17q,20q and loss of chromosomes 1p,8p,13q,16q,20q, involved in the depolarization of IMPC cells.3,84–86 Among them, alterations in chromosome 8 seem to affect known malignancy-associated genes and could be one of the causes for the tumor’s invasive behavior.87 Other common genetic variations encountered specifically in IMPC include ESR1, KDR, ARID1B, ATR genes.88,89 Loss of LTZS1 expression is associated with IMPC development and nodal infiltration.90
The Role of Micropapillary Element or Micropapillary DCIS
A much-discussed topic in the study of IMPC is the significance and impact of micropapillary DCIS, or micropapillary foci, encountered within breast cancer tumors. Presence of micropapillary DCIS was associated with significantly larger tumor size and higher grade,91,92 as well as lymphatic invasion with nodal metastases.93,94 Recurrence rates, when micropapillary DCIS alone is present, also seem to be elevated,91 with a study reporting 29% versus 8% when compared to patients with non-micropapillary DCIS histology.91 All this is thought to be the result of higher histological grade tumors having a distinctly aggressive comedo necrosis96 and micro-invasion profile, thus explaining the local and locoregional recurrence of disease despite treatment.43,91,97 Another characteristic of micropapillary DCIS is the presentation as a large, multifocal, and often under-diagnosed breast tumor, as reported by a study from MD Anderson Cancer Centre.98 Literature indicates unfavorable recurrence profiles whenever such DCIS histology was present. In fact, even incomplete “inside-out” histological patterns, even without being characterized as micropapillary, are associated with LVI, nodal invasion, poorer survival, and larger tumor size when found in NST carcinomas.99,100
Micropapillary DCIS within NST tumors also differs when compared to non-otherwise specified DCIS within NST tumors. Higher incidence of vascular invasion, increased stage at diagnosis, high recurrence rates and increased lymph node infiltration are all well documented.101
A relatively common histological combination is that of mucinous breast carcinoma with micropapillary DCIS.102–107 Approximately 20% of all mucinous carcinomas are classified as “Mucinous Carcinoma with Micropapillary Features (MPMC)”.20,108,109 MPMC demonstrates higher percentages of lymphovascular invasion and lymph node invasion than mucinous breast carcinoma, likely explained by the higher instances of metastasis-associated mutations in genes associated with the PI3K-Akt, mTOR, AMPK signaling pathways,20,110 such as in GATA3 (20%), TP53 (20%) and SF3B1 (20%).20 Comparison with pure mucinous carcinomas has demonstrated lower frequency of HER2-positivity (20% for IMPC versus none of the mucinous carcinoma of breast111) and PR-negativity, lower nuclear grade and overall more aggressive biological behavior,111–115 as well as worse prognosis.105–107 Micropapillary mucinous carcinoma also shows evidence of being from the same lineage as pure IMPC, a finding that would explain their much-observed combination.116
IMPC Imaging
The mammographic appearance of IMPC is thought to be often nonspecific, and most lesions are an irregular, spiculated high-density mass, with scattered microcalcification in about 66.7% of the cases, often resembling IDC or DCIS.117–121 Micropapillary DCIS imaging in simple mammography often has a segmental or scattered microcalcification pattern.98,122,123 In fact, microcalcification patterns in mammography have been associated with worse prognosis in IMPC.124 Mammographic evaluation has a clear trend to underestimate the true disease size when IMPC is concerned.97,98,122 False-negative rates in mammography evaluation have been reported as high as 12% for IMPC patients,125 whereas patients with Invasive Lobular Carcinoma have false-negative rates higher than 14%,126 and up to 19%.127
When utilizing the ultrasound (U/S), the lesions are mainly hypoechoic, and it has been reported that the use of U/S often misses the true depth of the IMPC tumor invasion.26,119 A single hypoechoic lesion with irregular margins is the most encountered finding in U/S evaluation.117,122–129 In one study, micropapillary DCIS evaluation with U/S yielded a false-negative rate of 47%, and in those that were identified, the true extent was underestimated in 81% of the cases.122 Addition of shear wave elastography has been reported as helpful in better estimating IMPC tumors.130,131 Axillary evaluation of IMPC patients often yields suspicious lymph nodes with cortical thickening, and authors report positivity rates of suspicious nodes in 69% of the patients.128
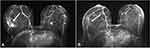
MRI study is the most helpful at IMPC distinction, with the lesions presenting as spiculated, irregular masses with characteristic rapid enhancement and delayed washout patterns.118,132 Patterns of single or multiple irregular mass with rapid washout waveforms are the most well-recognized patterns of IMPC presentation in MRI.125,128 Mass and non-mass enhancement have also been previously described, while not as frequently as a solitary enhancing mass presentation.91,119,122 The probability of a non-mass enhancement of the lesion being found in MRI ranges from 16.7% to 38.9%.133 The non-mass enhancement is attributed to local lymphovascular infiltration, a finding attributed to the lesion pathology. In literature, non-mass enhancement of IMPC has also been attributed to the presence of DCIS within the lesion, an observation that needs larger case series for validation.128,133 Multifocal IMPC lesions are also better diagnosed and more accurately staged with MRI, compared to any other modality.43,91,98,122,125,128,133 While MRI may be the best imaging modality for IMPC, there is still a percentage of lesions that will be missed, especially diffuse multifocal lesions with extensive DCIS or residual disease after PST.117,134 An example of pre- and post-PST MRI imaging of micropapillary carcinoma can be seen in Figure 3.
PET-CT scans are also utilized, showing FDG (fluorodeoxyglucose) uptake of the primary tumor, with high (FDG) uptake being a prognostic factor for worse outcomes regarding breast cancer.119,135 As discussed earlier, IMPCs are characterized as Luminal A in 75.3% of the cases.41 Recently, Akin et al investigated how accurately PET-CT scan and MRI could detect breast cancer subtypes in 55 tumors.136 They found that although the SUVmax value from PET-CT scan was high for the Luminal A subtype, it was lower than the SUVmax value of the other breast cancer subtypes. PET-CT scan was better at identifying the molecular subtype of the breast cancer; however, MRI was superior at determining the tumor size, thus better for staging.
Treatment Options
Treatment of IMPC remains controversial, especially among breast surgeons. To begin with, there is a lack of guidelines regarding the impact of micropapillary element being present in several histological subtypes, as well as for the pure IMPC subtype itself. The well-known potency for lymphatic spread did influence surgical approaches in the past, since many authors report high percentages of axillary lymph node dissection (ALND) during surgery32,47 without any current evidence showing a need for more radical axillary approaches.137 While surgeons must strive for breast conserving therapy where possible,137,138 the majority of IMPC case reports were treated with modified radical mastectomy, as shown in a 2017 study by Yu et al, with 99% of the IMPC patients undergoing modified radical, or total mastectomy. Until recently, authors suggested a more radical approach towards locoregional management, with some adding larger surgical margin recommendations,28 and even locoregional radiation therapy to avoid extranodal recurrence. Indications for adjuvant and neoadjuvant treatment administration do not seem to be altered in IMPC, except for more cases being HER2 positive, and therefore candidates for biologically targeted treatment.62 Mercogliano et al demonstrated a possible resistance to HER2-directed therapy in IMPC tumors by investigating the mucin 4 (MUC4) molecule.61 Their study showed that MUC4 was overexpressed in IMPC tumors and had the ability to conceal the target epitope of trastuzumab, leading to treatment resistance and lower survival for IMPC patients (hazard ratio = 2.6, P = 0.0340). It is recommended that physicians have a high degree of suspicion, to avoid underdiagnosis, and to be vigilant in the axillary evaluation of such patients.44,139 To the best of our knowledge, the effect of adjuvant chemotherapy on survival or complete pathological response (CPR), or the role of the endocrine reaction in IMPCs has not been studied.
Newer developments in diagnostic markers and cancer therapy are currently being investigated for use in IMPC. One study evaluated the molecular profile of IMPC for potential response in immune-checkpoint inhibition treatments but showcased unfavorable status of the target ligands.140
Regarding the post-operative radiotherapy treatments (PORT), an informative study was published by Wu et al, studying 881 IMPC patients. The study uses a multivariate analysis of several patient factors and determined that both the surgical approach (mastectomy or breast conserving surgery) and the election to undergo PORT or not, did not alter the 5-year BCSS (breast cancer–specific survival) or OS (overall survival), which remained favorable for patients with IMPC. These results are also in line with older, smaller studies.141
Prognosis of IMPC
The comparative prognosis of IMPC has been a long-standing debate among scientists. A summary of studies evaluating the prognosis of IMPC can be seen in Table 3. However, recent studies and meta-analyses seem to suggest that there is no tangible difference in disease-free survival, recurrence-free survival, or overall prognosis.23,58,142–145 One such meta-analysis, that utilizes a great number of previous prognostic comparative studies, is the one by Hao et al.44 After a meticulous process of balancing key characteristics of the two populations (age, lymph nodes, grade, stage), the analysis demonstrated no statistically significant difference in overall survival and disease-free survival between patients with IMPC and those with IDC. Additionally, they demonstrated that the micropapillary subtype did not carry any gravity as an independent prognostic factor. Favorable prognostic factors for patients with IMPC include receipt of radiation treatment, estrogen receptor positivity, age <65 years and <4 positive lymph nodes.147,148 Lymphovascular invasion and negative ER status are among the most recognized negative predictors for IMPC.53 Lymphatic vessel density and VEGF-C expression are associated with lymph node infiltration in IMPC.149 It is worth mentioning that there are several older or with fewer patients comparative analyses,32,37,47,95,150–153 such as the one of Wu et al,7 or Yu et al28 that demonstrated worse recurrence-free survival, despite being in accordance with similar disease-free survival rates. This was attributed to a higher incidence of lymph node recurrence in the IMPC group of patients.7,28 Therefore, a question arose as to whether locoregional recurrence truly influenced the long-term overall survival of patients with IMPC. A study by Chen et al, also notes that it might be useful to compare overall survival in patient groups with similar nodal involvement and it demonstrated better breast cancer–specific survival as well as overall survival rates in the IMPC group of patients when compared to IDC patients.15,23,142 A recently published nomogram predicting the individual risk for locoregional recurrence, specific for micropapillary breast carcinoma, could be of use in risk-stratifying these patients.154
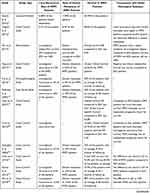 |  |  |
Table 3 Data on Local Recurrence, Distant Metastasis, and Survival from the Included Studies |
Several prognostic indicators are being studied for IMPC. In a recent study, sialyl LewisX (sLex) and mucin 1 (MUC1) expression in tissue specimens were found to be significantly different in IMPC cells when compared to NOS carcinoma cells. Furthermore, high levels of sLex expression, when combined with low levels of MUC1 expression, were also found to be a reliable prognostic factor for IMPC, making these two molecules potential specialized markers or therapeutic targets.155,156 Absence of caveolin-1 expression in stromal fibroblasts of IMPC is a candidate predictor for advanced axillary staging at diagnosis, as well as shortened progression-free survival.157 GATA3 is another IMPC-specific marker that seems to be expressed in tumors with better prognosis lacking however large confirmatory studies121,158 P63 expression was also found to be significantly associated with high Ki-67 index in IMPC cases, indicating another possible aggression marker that needs further study.159 Loss of ARID1A function was also noted to negatively correlate with disease-free survival (DFS) and 10-year overall survival (OS), especially in luminal B IMPC tumors.160
Conclusion
In the past few years, the previously unknown effect of the presence of micropapillary histological elements or pure IMPC on breast cancer has been explored. Due to its rarity as an entity, and the resulting difficulty in patient accumulation, there are not many studies that have produced tangible and statistically significant conclusions regarding all aspects of IMPC.
Micropapillary carcinomas of the breast have a well-recognized lymphovascular tropism that leads to more patients presenting with clinically disease-positive lymph nodes. In fact, the underlying biology of micropapillary histological patterns is detrimental in the lymphatic tropism of tumors, even when they present as a percentage of the malignancy’s histology or as foci of micropapillary DCIS. Basic research has revealed that there is a multitude of adherence molecules and chemotactic factors involved in the histology’s tendency for lymphatic invasion. Future, translational research, perspectives of such findings could include the utilization of said molecules as treatment targets or prognostic predictors for IMPC patients.
This review highlights the importance of approaching a breast cancer patient in accordance with the personalized medicine principles and making prompt therapeutic decisions in an individualized fashion based on the current literature and taking into consideration all aspects of a patient’s ailment. While no specific guidelines exist yet, it is made clear that micropapillary histology has an effect on treatment choices, and breast surgeons should be aware of the possible wider margin excision needed for this type of breast cancer. Further research is needed to confirm the role of chemotherapy and hormone agents, as well as resistance to trastuzumab. Imaging identification of micropapillary breast cancer is often underestimated regarding tumor invasion and size, and among the available options, breast MRI is the best one to perform. Recent research suggests that the – once thought – worse survival prognosis does not hold true; however, the alarming frequency of lymphovascular involvement and disease recurrence makes a more radical surgical approach more appropriate, for both the axillary and breast tumor burden.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Fisher ER, Gregorio R, Redmond C, Dekker A, Fisher B. Pathologic findings from the national surgical adjuvant breast project (protocol no. 4). II. The significance of regional node histology other than sinus histiocytosis in invasive mammary cancer. Am J Clin Pathol. 1976;65:21–30. doi:10.1093/ajcp/65.1.21
2. Invasive micropapillary carcinoma of the breast - PubMed. Available from: https://pubmed.ncbi.nlm.nih.gov/8302807/.
3. Yang Y-L, Liu -B-B, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med. 2016;140:799–805. doi:10.5858/arpa.2016-0040-RA
4. Stranix JT, Kwa MJ, Shapiro RL, Speyer JL. Invasive micropapillary carcinoma of the male breast: case report and review of the literature. Cancer Treat Commun. 2015;3:44–49. doi:10.1016/j.ctrc.2014.12.001
5. Tanaka Y, Morishima I, Kikuchi K. Invasive micropapillary carcinomas arising 42 years after augmentation mammoplasty: a case report and literature review. World J Surg Oncol. 2008;6:1–5. doi:10.1186/1477-7819-6-33
6. Vingiani A, Maisonneuve P, Dell’Orto P, et al. The clinical relevance of micropapillary carcinoma of the breast: a case-control study. Histopathology. 2013;63:217–224. doi:10.1111/his.12147
7. Wu Y, Zhang N, Yang Q. The prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in the breast: a meta-analysis. BMC Cancer. 2017;17:1–9. doi:10.1186/s12885-017-3855-7
8. Coyle EA, Taj H, Comba I, Vasquez J, Zayat V. Invasive micropapillary carcinoma: a rare case of male breast cancer. Cureus. 2020;12:10–13. doi:10.7759/cureus.10571
9. Tsushimi T, Mori H, Harada T, Ikeda Y, Ohnishi H. Invasive micropapillary carcinoma of the breast in a male patient: report of a case. Int J Surg Case Rep. 2013;4:988–991. doi:10.1016/j.ijscr.2013.09.001
10. Dong C-G, Yang Y-P, Zhu Y-L. Invasive micropapillary carcinoma of male breast with neuroendocrine differentiation: report of a case. Chin J Pathol. 2011;40:704–706. doi:10.3760/cma.j.issn.0529-5807.2011.10.016
11. Marchiò C, Pietribiasi F, Castiglione R, Fusco N, Sapino A. “Giants in a microcosm”: multinucleated giant cells populating an invasive micropapillary carcinoma of the breast. Int J Surg Pathol. 2015;23:654–655. doi:10.1177/1066896915605616
12. Lui PCW, Lau PPL, Tse GMK, et al. Fine needle aspiration cytology of invasive micropapillary carcinoma of the breast. Pathology. 2007;39:401–405. doi:10.1080/00313020701444499
13. Tang S-L, Yang J-Q, Du Z-G, et al. Clinicopathologic study of invasive micropapillary carcinoma of the breast. Oncotarget. 2017;8:42455–42465. doi:10.18632/oncotarget.16405
14. Cui Z-Q, Feng J-H, Zhao Y-J. Clinicopathological features of invasive micropapillary carcinoma of the breast. Oncol Lett. 2015;9:1163–1166. doi:10.3892/ol.2014.2806
15. Hashmi AA, Aijaz S, Mahboob R, et al. Clinicopathologic features of invasive metaplastic and micropapillary breast carcinoma: comparison with invasive ductal carcinoma of breast. BMC Res Notes. 2018;11:1–7. doi:10.1186/s13104-018-3623-z
16. Pettinato G, Pambuccian SE, Di Prisco B, Manivel JC. Fine needle aspiration cytology of invasive micropapillary (pseudopapillary) carcinoma of the breast: report of 11 cases with clinicopathologic findings. Acta Cytol. 2002;46:1088–1094. doi:10.1159/000327112
17. Ongürü O, Deveci S, Günhan O, Öngürü Ö, Deveci S, Günhan Ö. Cytological findings of invasive micropapillary carcinoma of the breast: a report of two cases. Cytopathology. 2002;13:160–163. doi:10.1046/j.1365-2303.2002.00390.x
18. Madakshira MG, Saikia UN. Neutrophilic emperipolesis in micropapillary carcinoma breast. Breast J. 2020;26:539–540. doi:10.1111/tbj.13563
19. Troxell ML. Reversed MUC1/EMA polarity in both mucinous and micropapillary breast carcinoma. Hum Pathol. 2014;45:432–434. doi:10.1016/j.humpath.2013.08.026
20. Sun P, Zhong Z, Lu Q, et al. Mucinous carcinoma with micropapillary features is morphologically, clinically and genetically distinct from pure mucinous carcinoma of breast. Mod Pathol. 2020;33:1945–1960. doi:10.1038/s41379-020-0554-8
21. Li YS, Kaneko M, Sakamoto DG, Takeshima Y, Inai K. The reversed apical pattern of MUC1 expression is characteristics of invasive micropapillary carcinoma of the breast. Breast Cancer. 2006;13:58–63. doi:10.2325/jbcs.13.58
22. Akiyoshi T, Nagaie T, Tokunaga M, et al. Invasive micropapillary carcinoma of the breast with minimal regional lymph node metastasis regardless of the huge size: report of a case. Breast Cancer. 2003;10:356–360. doi:10.1007/BF02967657
23. Chen H, Wu K, Wang M, Wang F, Zhang M, Zhang P. Invasive micropapillary carcinoma of the breast has a better long-term survival than invasive ductal carcinoma of the breast in spite of its aggressive clinical presentations: a comparison based on large population database and case–control analysis. Cancer Med. 2017;6:2775–2786. doi:10.1002/cam4.1227
24. Mayer AP, Greenberg ML. FNB diagnosis of breast carcinoma associated with HIV infection: a case report and review of HIV associated malignancy. Pathology. 1996;28:90–95. doi:10.1080/00313029600169623
25. Lai PC, Chiu TH, Huang YT. Overexpression of BDNF and TrkB in human. Anticancer Res. 2010;31:1265–1270.
26. Kamitani K, Kamitani T, Ono M, Toyoshima S, Mitsuyama S. Ultrasonographic findings of invasive micropapillary carcinoma of the breast: correlation between internal echogenicity and histological findings. Breast Cancer. 2012;19:349–352. doi:10.1007/s12282-011-0293-2
27. Fowler AM, Andersen J, Conway PD. Local recurrence of invasive micropapillary breast cancer after mammosite brachytherapy: a case report and literature review. Clin Breast Cancer. 2009;9:253–257. doi:10.3816/CBC.2009.n.043
28. Yu JI, Choi DH, Huh SJ, et al. Differences in prognostic factors and failure patterns between invasive micropapillary carcinoma and carcinoma with micropapillary component versus invasive Ductal carcinoma of the breast: retrospective multicenter case-control study (KROG 13-06). Clin Breast Cancer. 2015;15:353–361.e2. doi:10.1016/j.clbc.2015.01.008
29. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. doi:10.1136/BMJ.N71
30. Zekioglu O, Erhan Y, Çiris M, Bayramoglu H, Özdemir N. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004;44:18–23. doi:10.1111/j.1365-2559.2004.01757.x
31. Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297–303. doi:10.1097/01.pap.0000138142.26882.fe
32. Chen AC, Paulino AC, Schwartz MR, et al. Population-based comparison of prognostic factors in invasive micropapillary and invasive ductal carcinoma of the breast. Br J Cancer. 2014;111:619–622. doi:10.1038/bjc.2014.301
33. Gokce H, Durak MG, Akin MM, et al. Invasive micropapillary carcinoma of the breast: a clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J. 2013;19:374–381. doi:10.1111/tbj.12128
34. Taketani K, Tokunaga E, Yamashita N, et al. A case of invasive micropapillary carcinoma of the breast involving extensive lymph node metastasis. World J Surg Oncol. 2014;12:1–6. doi:10.1186/1477-7819-12-84
35. Lezid Á, Rodríguez P. Carcinoma micropapilarinvasor, una varianteagresiva de carcinoma de glándulamamaria. Revisión a propósito de 12 casos [Invasive micropapillary carcinoma, an aggressive variant of mammary gland carcinoma. Review of 12 cases]. Patol Rev Latinoam. 2008;46(3):215–221. Spanish.
36. Middleton LP, Tressera F, Sobel ME, et al. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999;12:499–504.
37. Moorman AM, Vink R, Rutgers EJT, Kouwenhoven EA. Incidence, clinical features, and outcomes of special types in breast cancer in a single institution population. Breast J. 2020;26:2163–2169. doi:10.1111/tbj.14069
38. Akdeniz N, Kaplan MA, Küçüköner M, et al. Rare breast cancer types: a study about characteristics, outcomes, and peculiarities. J Oncol Sci. 2020;6:164–172. doi:10.37047/jos.2020-78231
39. Kaygusuz EI, Cetiner H, Yavuz H. Clinico-pathological significance of extra-nodal spread in special types of breast cancer. Cancer Biol Med. 2014;11:116–122. doi:10.7497/j.issn.2095-3941.2014.02.006
40. Zheng L, Liu J-T, Wei L-J. Clinicopathological analysis of 104 cases of invasive micropapillary breast carcinoma. J Pract Oncol. 2010;25:184–187.
41. Lewis GD, Xing Y, Haque W, et al. The impact of molecular status on survival outcomes for invasive micropapillary carcinoma of the breast. Breast J. 2019;25:1171–1176. doi:10.1111/tbj.13432
42. Ye F-G, Xia C, Ma D, Lin P-Y, Hu X, Shao Z-M. Nomogram for predicting preoperative lymph node involvement in patients with invasive micropapillary carcinoma of breast: a SEER population-based study. BMC Cancer. 2018;18. doi:10.1186/s12885-018-4982-5
43. Paterakos M, Watkin WG, Edgerton SM, et al. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol. 1999;30:1459–1463. doi:10.1016/S0046-8177(99)90168-5
44. Hao S, Zhao Y, Peng J, et al. Invasive micropapillary carcinoma of the breast had no difference in prognosis compared with invasive ductal carcinoma: a propensity-matched analysis. Sci Rep. 2019;9:1–8. doi:10.1038/s41598-018-36362-8
45. Ye F, Yu P, Li N, et al. Prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in breast: a meta-analysis of PSM studies. Breast. 2020;51:11–20. doi:10.1016/j.breast.2020.01.041
46. De La Cruz C, Moriya T, Endoh M, et al. Invasive micropapillary carcinoma of the breast: clinicopathological and immunohistochemical study. Pathol Int. 2004;54:90–96. doi:10.1111/j.1440-1827.2004.01590.x
47. Chen AC, Paulino AC, Schwartz MR, et al. Prognostic markers for invasive micropapillary carcinoma of the breast: a population-based analysis. Clin Breast Cancer. 2013;13:133–139. doi:10.1016/j.clbc.2012.10.001
48. Kaya C, Uçak R, Bozkurt E, et al. The impact of micropapillary component ratio on the prognosis of patients with invasive micropapillary breast carcinoma. J Investig Surg. 2020;33:31–39. doi:10.1080/08941939.2018.1474302
49. Luna-Moré S, Casquero S, Pérez-Mellado A, Rius F, Weil B, Gornemann I. Importance of estrogen receptors for the behavior of invasive micropapillary carcinoma of the breast. Review of 68 cases with follow-up of 45. Pathol Res Pract. 2000;196:35–39. doi:10.1016/S0344-0338(00)80019-9
50. Mahe E, Farag M, Boutross-Tadross O. Invasive micropapillary breast carcinoma: a retrospective study of classification by pathological parameters. Malays J Pathol. 2013;35:133–138.
51. Kuroda H, Sakamoto G, Ohnisi K, Itoyama S. Overexpression of her2/neu, estrogen and progesterone receptors in invasive micropapillary carcinoma of the breast. Breast Cancer. 2004;11:301–305. doi:10.1007/BF02984553
52. Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001;32:583–589. doi:10.1053/hupa.2001.24988
53. Li W, Han Y, Wang C, et al. Precise pathologic diagnosis and individualized treatment improve the outcomes of invasive micropapillary carcinoma of the breast: a 12-year prospective clinical study. Mod Pathol. 2018;31:956–964. doi:10.1038/s41379-018-0024-8
54. Stewart RL, Caron JE, Gulbahce EH, Factor RE, Geiersbach KB, Downs-Kelly E. HER2 immunohistochemical and fluorescence in situ hybridization discordances in invasive breast carcinoma with micropapillary features. Mod Pathol. 2017;30:1561–1566. doi:10.1038/modpathol.2017.65
55. Bandyopadhyay S, Bluth MH, Ali-Fehmi R. Breast carcinoma: updates in molecular profiling 2018. Clin Lab Med. 2018;38:401–420. doi:10.1016/j.cll.2018.02.006
56. Min SY, Jung E-J, Seol H, Park IA. Cancer subtypes of breast carcinoma with micropapillary and mucinous component based on immunohistochemical profile. Korean J Pathol. 2011;45:125–131. doi:10.4132/KoreanJPathol.2011.45.2.125
57. Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14:836–841. doi:10.1038/modpathol.3880399
58. Kim J, Kim JY, Lee H-B, et al. Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: comparative analysis to invasive carcinoma of no special type. Breast Cancer Res Treat. 2020;184:527–542. doi:10.1007/s10549-020-05861-6
59. Aggarwal G, Reid MD, Sharma S. Metaplastic variant of invasive micropapillary breast carcinoma: a unique triple negative phenotype. Int J Surg Pathol. 2012;20:488–493. doi:10.1177/1066896912436552
60. Varga Z, Zhao J, Öhlschlegel C, Odermatt B, Heitz PU. Preferential HER-2/neu overexpression and/or amplification in aggressive histological subtypes of invasive breast cancer. Histopathology. 2004;44:332–338. doi:10.1111/j.1365-2559.2004.01843.x
61. Mercogliano MF, Inurrigarro G, De Martino M, et al. Invasive micropapillary carcinoma of the breast overexpresses MUC4 and is associated with poor outcome to adjuvant trastuzumab in HER2-positive breast cancer. BMC Cancer. 2017;17:1–8. doi:10.1186/s12885-017-3897-x
62. Perron M, Wen HY, Hanna MG, Brogi E, Ross DS. HER2 immunohistochemistry in invasive micropapillary breast carcinoma: complete assessment of an incomplete pattern. Arch Pathol Lab Med. 2020. doi:10.5858/arpa.2020-0288-oa
63. Zhou S, Yang F, Bai Q, et al. Intense basolateral membrane staining indicates HER2 positivity in invasive micropapillary breast carcinoma. Mod Pathol. 2020;33:1275–1286. doi:10.1038/s41379-020-0461-z
64. Zouine S, Orfi Z, Kojok K, et al. Immunohistochemical and genetic exploration of incompatible A blood group antigen expression in invasive micropapillary breast carcinoma: a case report. Curr Res Transl Med. 2017;65:71–76. doi:10.1016/j.retram.2017.05.002
65. Lin Y, Duan Q, Yang Y, Zhu Y, Zhang J, Dong C. Immunohistochemistry of phosphatase and tensin homolog and metalloproteinase-9 in breast invasive micropapillary carcinoma. Eur J Gynaecol Oncol. 2019;40:380–383. doi:10.12892/ejgo4735.2019
66. Lü F, Zhang Y-Q, Guo X-J, Qian X-L, Li Y-Q, Fu L. Expression of integrin β1 and Kindlin-2 in invasive micropapillary carcinoma of the breast. Chin J Cancer Prev Treat. 2015;22:929–935.
67. Gong Y, Sun X, Wiley EL, Rao MS. Expression of cell adhesion molecules, CD44s and E-cadherin, in infiltrating micropapillary versus tubular carcinomas of the breast. Breast Cancer Res Treat. 2001;69:295.
68. Liu B, Zheng X, Meng F, et al. Overexpression of β1 integrin contributes to polarity reversal and a poor prognosis of breast invasive micropapillary carcinoma. Oncotarget. 2018;9:4338–4353. doi:10.18632/oncotarget.22774
69. Badyal RK, Bal A, Das A, Singh G. Invasive micropapillary carcinoma of the breast: immunophenotypic analysis and role of cell adhesion molecules (CD44 and E-Cadherin) in nodal metastasis. Appl Immunohistochem Mol Morphol. 2016;24:151–158. doi:10.1097/PAI.0000000000000167
70. Huang L, Ji H, Yin L, et al. High expression of plakoglobin promotes metastasis in invasive micropapillary carcinoma of the breast via tumor cluster formation. J Cancer. 2019;10:2800–2810. doi:10.7150/jca.31411
71. Cui L-F, Guo X-J, Wei J, et al. Significance of interleukin-1β expression and microvascular density in invasive micropapillary carcinoma of breast. Chin J Pathol. 2008;37:599–603.
72. Nagi C, Guttman M, Jaffer S, et al. N-cadherin expression in breast cancer: correlation with an aggressive histologic variant - Invasive micropapillary carcinoma. Breast Cancer Res Treat. 2005;94:225–235. doi:10.1007/s10549-005-7727-5
73. Liu F, Lang R, Wei J, et al. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology. 2009;54:741–750. doi:10.1111/j.1365-2559.2009.03289.x
74. Sun X, Gong Y, Wiley EL, Rao MS. Microvessel density is higher in invasive micropapillary carcinoma than in tubular carcinoma of the breast. Breast Cancer Res Treat. 2001;69:254.
75. Fan Y, Lang RG, Wang Y, Sun BC, Fu L. Relationship between expression of cell adhesion molecules and metastatic potential in invasive micropapillary carcinoma of breast. Zhonghua Bing Li Xue Za Zhi. 2004;33:308–311.
76. Nosaka K, Makishima K, Sakabe T, et al. Upregulation of glucose and amino acid transporters in micropapillary carcinoma. Histol Histopathol. 2019;34:1009–1014. doi:10.14670/HH-18-099
77. Doublier S, Belisario DC, Polimeni M, et al. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12. doi:10.1186/1471-2407-12-4
78. Li W, Liu F, Lei T, et al. The clinicopathological significance of CD44+/CD24-/low and CD24+ tumor cells in invasive micropapillary carcinoma of the breast. Pathol Res Pract. 2010;206:828–834. doi:10.1016/j.prp.2010.09.008
79. Simonetti S, Terracciano L, Zlobec I, et al. Immunophenotyping analysis in invasive micropapillary carcinoma of the breast: role of CD24 and CD44 isoforms expression. Breast. 2012;21:165–170. doi:10.1016/j.breast.2011.09.004
80. Umeda T, Ishida M, Murata S, et al. Immunohistochemical analyses of CD44 variant isoforms in invasive micropapillary carcinoma of the breast: comparison with a concurrent conventional invasive carcinoma of no special type component. Breast Cancer. 2016;23:869–875. doi:10.1007/s12282-015-0653-4
81. Li W, Yang D, Wang S, et al. Increased expression of CD146 and microvessel density (MVD) in invasive micropapillary carcinoma of the breast: comparative study with invasive ductal carcinoma-not otherwise specified. Pathol Res Pract. 2011;207:739–746. doi:10.1016/j.prp.2011.09.009
82. Kramer Z, Kenessey I, Gángó A, Lendvai G, Kulka J, Tőkés AM. Cell polarity and cell adhesion associated gene expression differences between invasive micropapillary and no special type breast carcinomas and their prognostic significance. Sci Rep. 2021;11(1):18484. doi:10.1038/s41598-021-97347-8
83. Li S, Yang C, Zhai L, et al. Deep sequencing reveals small RNA characterization of invasive micropapillary carcinomas of the breast. Breast Cancer Res Treat. 2012;136:77–87. doi:10.1007/s10549-012-2166-6
84. Le zhang YW, Zhang L, Xing H, et al. Invasive micropapillary carcinoma with cep17 monosomy of the bilateral breast: a rare case report and review of the literature. Onco Targets Ther. 2020;13:6425–6432. doi:10.2147/OTT.S251934
85. Gruel N, Benhamo V, Bhalshankar J, et al. Polarity gene alterations in pure invasive micropapillary carcinomas of the breast. Breast Cancer Res. 2014;16. doi:10.1186/bcr3653
86. Denisov EV, Skryabin NA, Vasilyev SA, et al. Relationship between morphological and cytogenetic heterogeneity in invasive micropapillary carcinoma of the breast: a report of one case. J Clin Pathol. 2015;68:758–762. doi:10.1136/jclinpath-2015-203009
87. Thor AD, Eng C, Devries S, et al. Invasive micropapillary carcinoma of the breast is associated with chromosome 8 abnormalities detected by comparative genomic hybridization. Hum Pathol. 2002;33:628–631. doi:10.1053/hupa.2002.124034
88. Pareja F, Ferrando L, Lee SSK, et al. The genomic landscape of metastatic histologic special types of invasive breast cancer. Npj Breast Cancer. 2020;6. doi:10.1038/s41523-020-00195-4
89. Marchiò C, Iravani M, Natrajan R, et al. Genomic and immunophenotypical characterization of pure micropapillary carcinomas of the breast. J Pathol. 2008;215:398–410. doi:10.1002/path.2368
90. Wang -X-X, Liu -B-B, Wu X, Su D, Zhu Z, Fu L. Loss of Leucine Zipper Putative Tumor Suppressor 1 (LZTS1) expression contributes to lymph node metastasis of breast invasive micropapillary carcinoma. Pathol Oncol Res. 2015;21:1021–1026. doi:10.1007/s12253-015-9923-x
91. Castellano I, Marchiò C, Tomatis M, et al. Micropapillary ductal carcinoma in situ of the breast: an inter-institutional study. Mod Pathol. 2010;23:260–269. doi:10.1038/modpathol.2009.169
92. Guerrieri C, Hudacko R. Tubulopapillary carcinoma: an aggressive variant of invasive breast carcinoma with a micropapillary DCIS-like morphology. Int J Surg Pathol. 2020;28:536–540. doi:10.1177/1066896919892699
93. Ide Y, Horii R, Osako T, et al. Clinicopathological significance of invasive micropapillary carcinoma component in invasive breast carcinoma. Pathol Int. 2011;61:731–736. doi:10.1111/j.1440-1827.2011.02735.x
94. Chen L, Fan Y, Lang R-G, Guo X-J, Sun Y-L, Fu L. Diagnosis and prognosis study of breast carcinoma with micropapillary component. Chin J Pathol. 2007;36:228–232.
95. Bomeisl PE, Thompson CL, Harris LN, Gilmore HL. Comparison of oncotype DX recurrence score by histologic types of breast carcinoma. Arch Pathol Lab Med. 2015;139:1546–1549. doi:10.5858/arpa.2014-0557-OA
96. Perez AA, Balabram D, Salles MA, Gobbi H. Ductal carcinoma in situ of the breast: correlation between histopathological features and age of patients. Diagn Pathol. 2014;9:227. doi:10.1186/s13000-014-0227-3
97. Evers K. Significance of finding micropapillary DCIS on core needle biopsy. Acad Radiol. 2011;18:795–796. doi:10.1016/j.acra.2011.05.001
98. Lee YS, Mathew J, Dogan BE, Resetkova E, Huo L, Yang WT. Imaging features of micropapillary DCIS: correlation with clinical and histopathological findings. Acad Radiol. 2011;18:797–803. doi:10.1016/j.acra.2011.01.022
99. Kuba S, Ohtani H, Yamaguchi J, et al. Incomplete inside-out growth pattern in invasive breast carcinoma: association with lymph vessel invasion and recurrence-free survival. Virchows Arch. 2011;458:159–169. doi:10.1007/s00428-010-1033-2
100. Guo X, Chen L, Lang R, Fan Y, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: association of pathologic features with lymph node metastasis. Am J Clin Pathol. 2006;126:740–746. doi:10.1309/AXYY4AJTMNW6FRMW
101. Guan X, Xu G, Shi A, et al. Comparison of clinicopathological characteristics and prognosis among patients with pure invasive ductal carcinoma, invasive ductal carcinoma coexisted with invasive micropapillary carcinoma, and invasive ductal carcinoma coexisted with ductal carcinoma. Medicine (Baltimore). 2020;99:e23487. doi:10.1097/MD.0000000000023487
102. Kim M-J, Gong G, Joo HJ, Ahn S-H, Ro JY. Immunohistochemical and clinicopathologic characteristics of invasive ductal carcinoma of breast with micropapillary carcinoma component. Arch Pathol Lab Med. 2005;129:1277–1282. doi:10.5858/2005-129-1277-IACCOI
103. Ranade AC, Batra R, Sandhu G, Chitale RA, Balderacchi J. Clinicopathological evaluation of 100 cases of mucinous carcinoma of breast with emphasis on axillary staging and special reference to a micropapillary pattern. J Clin Pathol. 2010;63:1043–1047. doi:10.1136/jcp.2010.082495
104. Lim GH, Yan Z, Gudi M. Diagnostic dilemma of micropapillary variant of mucinous breast cancer. BMJ Case Rep. 2018;2018. doi:10.1136/bcr-2018-225775
105. Shet T, Chinoy R. Presence of a micropapillary pattern in mucinous carcinomas of the breast and its impact on the clinical behavior. Breast J. 2008;14:412–420. doi:10.1111/j.1524-4741.2008.00616.x
106. Bal A, Joshi K, Sharma SC, Das A, Verma A, Wig JD. Prognostic significance of micropapillary pattern in pure mucinous carcinoma of the breast. Int J Surg Pathol. 2008;16:251–256. doi:10.1177/1066896908314784
107. Barbashina V, Corben AD, Akram M, Vallejo C, Tan LK. Mucinous micropapillary carcinoma of the breast: an aggressive counterpart to conventional pure mucinous tumors. Hum Pathol. 2013;44:1577–1585. doi:10.1016/j.humpath.2013.01.003
108. Collins K, Ricci A. Micropapillary variant of mucinous breast carcinoma: a distinct subtype. Breast J. 2018;24:339–342. doi:10.1111/tbj.12935
109. Asano Y, Kashiwagi S, Nagamori M, et al. Pure Mucinous Breast Carcinoma with Micropapillary Pattern (MUMPC): a case report. Case Rep Oncol. 2019;12:554–559. doi:10.1159/000501766
110. Kim H-J, Park K, Kim JY, Kang G, Gwak G, Park I. Prognostic significance of a micropapillary pattern in pure mucinous carcinoma of the breast: comparative analysis with micropapillary carcinoma. J Pathol Transl Med. 2017;51:403–409. doi:10.4132/jptm.2017.03.18
111. Doval DC, Tripathi R, Pasricha S, Goyal P, Agrawal C, Mehta A. HER2 positive mucinous carcinoma of breast with micropapillary features: report of a case and review of literature. Hum Pathol Case Rep. 2021;25:200531. doi:10.1016/j.ehpc.2021.200531
112. Pareja F, Selenica P, Brown DN, et al. Micropapillary variant of mucinous carcinoma of the breast shows genetic alterations intermediate between those of mucinous carcinoma and micropapillary carcinoma. Histopathology. 2019;75:139–145. doi:10.1111/his.13853
113. Lin H-Y, Gao L-X, Jin M-L, Ding H-Y. Clinicopathologic features of micropapillary variant of pure mucinous carcinoma of breast. Chin J Pathol. 2012;41:613–617. doi:10.3760/cma.j.issn.0529-5807.2012.09.009
114. Jiménez-Ayala M. Micropapillary carcinoma and mucinous carcinoma with a micropapillary pattern. Acta Cytol. 2007;51:1–2. doi:10.1159/000325673
115. Xu X, Bi R, Shui R, et al. Micropapillary pattern in pure mucinous carcinoma of the breast – does it matter or not? Histopathology. 2019;74:248–255. doi:10.1111/his.13722
116. Xu M, Ye M-N, Wang C, Ye H. Clinicopathological observation of breast micropapillary pure mucinous carcinoma combined with invasive micropapillary carcinoma. J Shanghai Jiaotong Univ. 2015;35:549–553.
117. Günhan-Bilgen I, Zekioglu O, Üstün EE, Memis A, Erhan Y. Invasive micropapillary carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. Am J Roentgenol. 2002;179:927–931. doi:10.2214/ajr.179.4.1790927
118. Adrada B, Arribas E, Gilcrease M, Yang WT. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and MRI features. Am J Roentgenol. 2009;193:58–63. doi:10.2214/AJR.08.1537
119. Yun SU, Choi BB, Shu KS, et al. Imaging findings of invasive micropapillary carcinoma of the breast. J Breast Cancer. 2012;15:57–64. doi:10.4048/jbc.2012.15.1.57
120. Kubota K, Ogawa Y, Nishioka A, et al. Radiological imaging features of invasive micropapillary carcinoma of the breast and axillary lymph nodes. Oncol Rep. 2008;20:1143–1147. doi:10.3892/or_00000122
121. Bandyopadhyay S, Ali-Fehmi R. Breast carcinoma. molecular profiling and updates. Clin Lab Med. 2013;33:891–909. doi:10.1016/j.cll.2013.08.009
122. Alsharif S, Daghistani R, Kamberoǧlu EA, Omeroglu A, Meterissian S, Mesurolle B. Mammographic, sonographic and MR imaging features of invasive micropapillary breast cancer. Eur J Radiol. 2014;83:1375–1380. doi:10.1016/j.ejrad.2014.05.003
123. Romero C, Carreira C, Urbasos M, Martín J, Lombardía J, García E. Carcinoma intraductal micropapilaren un varón con microcalcificaciones como único hallazgo radiológico [Intraductal micropapillary carcinoma in a male patient exhibiting microcalcification as sole radiological finding]. Radiologia. 2003;45:273–275. doi:10.1016/s0033-8338(03)77920-x
124. Yoon GY, Cha JH, Kim HH, Shin HJ, Chae EY, Choi WJ. Comparison of invasive micropapillary and invasive ductal carcinoma of the breast: a matched cohort study. Acta Radiol. 2019;60:1405–1413. doi:10.1177/0284185119834689
125. Rhee SJ, Han B-K, Ko EY, Shin JH. Invasive micropapillary carcinoma of the breast: mammographic, sonographic and MR imaging findings. J Korean Soc Magn Reson Med. 2012;16(3):205–216. doi:10.13104/jksmrm.2012.16.3.205
126. Michael M, Garzoli E, Reiner CS. Mammography, sonography and MRI for detection and characterization of invasive lobular carcinoma of the breast. Breast Dis. 2008;30:21–30. doi:10.3233/BD-2009-0279
127. Kim SH, Cha ES, Park CS, et al. Imaging features of invasive lobular carcinoma: comparison with invasive ductal carcinoma. Jpn J Radiol. 2011;29(7):475–482. doi:10.1007/s11604-011-0584-8
128. Jones KN, Guimaraes LS, Reynolds CA, Ghosh K, Degnim AC, Glazebrook KN. Invasive micropapillary carcinoma of the breast: imaging features with clinical and pathologic correlation. Am J Roentgenol. 2013;200:689–695. doi:10.2214/AJR.12.8512
129. Mizushima Y, Yamaguchi R, Yokoyama T, Ogo E, Nakashima O. Recurrence of invasive micropapillary carcinoma of the breast with different ultrasound features according to lesion site: case report. Kurume Med J. 2011;58:81–85. doi:10.2739/kurumemedj.58.81
130. Choi JS, Han B-K, Ko EY, Ko ES, Shin JH, Kim GR. Additional diagnostic value of shear-wave elastography and color Doppler US for evaluation of breast non-mass lesions detected at B-mode US. Eur Radiol. 2016;26:3542–3549. doi:10.1007/s00330-015-4201-6
131. Pan LJ, Xiao Y. Ultrasonic elastography diagnosis of special type breast cancers. Chin J Med Imaging Technol. 2010;26:683–685.
132. Lim HS, Kuzmiak CM, Jeong SI, et al. Invasive micropapillary carcinoma of the breast: MR imaging findings. Korean J Radiol. 2013;14:551–558. doi:10.3348/kjr.2013.14.4.551
133. Han CH, Yao WG, He J, Gao ZB, Hu HJ. Invasive micropapillary carcinoma of the breast: MR imaging findings. Oncol Lett. 2020;20:2811–2819. doi:10.3892/ol.2020.11848
134. Gandhi A, Coles C, Makris A, et al. Axillary surgery following neoadjuvant chemotherapy – Multidisciplinary Guidance From the Association of Breast Surgery, Faculty of Clinical Oncology of the Royal College of Radiologists, UK Breast Cancer Group, National Coordinating Committee for Breast. Clin Oncol. 2019;31:664–668. doi:10.1016/j.clon.2019.05.021
135. Dong A, Wang Y, Lu J, Zuo C. Spectrum of the breast lesions with increased 18F-FDG uptake on PET/CT. Clin Nucl Med. 2016;41:543–557. doi:10.1097/RLU.0000000000001203
136. Akin M, Orguc S, Aras F, Kandiloglu AR. Molecular subtypes of invasive breast cancer: correlation between PET/computed tomography and MRI findings. Nucl Med Commun. 2020;41(8):810–816. doi:10.1097/MNM.0000000000001220
137. Terando AM, Agnese DM, Holmes DR. Treatment and prognosis of rare breast cancers. Ann Surg Oncol. 2015;22:3225–3229. doi:10.1245/s10434-015-4748-0
138. Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021:
139. Kim SH, Hur SM, Lee SK, et al. Characteristics of invasive micropapillary carcinoma of the breast: in comparison with invasive ductal carcinoma. J Breast Cancer. 2010;13:174–179. doi:10.4048/jbc.2010.13.2.174
140. Simonetti S, Dominguez N, Elguezabal A, et al. Analysis of programmed death-ligand 1 expression, stromal tumor-infiltrating lymphocytes, and mismatch repair deficiency in invasive micropapillary carcinoma of the breast. J Immunother Precis Oncol. 2019;2:130–136. doi:10.4103/JIPO.JIPO_17_19
141. Wu SG, ZhangWW, Sun JY, Li FY, Chen YX, Zhen-Yu ZY. Postoperative radiotherapy for invasive micropapillary carcinoma of the breast: an analysis of surveillance, epidemiology, and end results database. Cancer Manag Res. 2017;9:453–459. doi:10.2147/CMAR.S141338
142. Chen H, Liang H-L, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast. 2015;24:723–731. doi:10.1016/j.breast.2015.09.001
143. Li D, Zhong C, Cheng YY, et al. A competing nomogram to predict survival outcomes in invasive micropapillary breast cancer. J Cancer. 2019;10:6801–6812. doi:10.7150/jca.27955
144. Deman F, Punie K, Laenen A, et al. Assessment of stromal tumor infiltrating lymphocytes and immunohistochemical features in invasive micropapillary breast carcinoma with long-term outcomes. Breast Cancer Res Treat. 2020;184:985–998. doi:10.1007/s10549-020-05913-x
145. Han Y, Wang J, Xu B. Clinicopathological characteristics and prognosis of breast cancer with special histological types: a surveillance, epidemiology, and end results database analysis. Breast. 2020;54:114–120. doi:10.1016/j.breast.2020.09.006
146. Li G, Yang S, Yao J, et al. Invasive micropapillary carcinoma of the breast had poor clinical characteristics but showed no difference in prognosis compared with invasive ductal carcinoma. World J Surg Oncol. 2016;14. doi:10.1186/s12957-016-0960-z
147. Lewis GD, Xing Y, Haque W, et al. Prognosis of lymphotropic invasive micropapillary breast carcinoma analyzed by using data from the National Cancer Database. Cancer Commun. 2019;39:1–9. doi:10.1186/s40880-019-0406-4
148. Liu Y, Huang X, Bi R, Yang W, Shao Z. Similar prognoses for invasive micropapillary breast carcinoma and pure invasive ductal carcinoma: a retrospectively matched cohort study in China. PLoS One. 2014;9. doi:10.1371/journal.pone.0106564
149. Guo X-J, Chen L, Lang R-G, Fan Y, Fu L. Relationship between lymph node metastasis and pathologic features of invasive micropapillary carcinoma of breast. Chin J Pathol. 2006;35:8–12.
150. Liu F, Yang MM, Li Z, et al. Invasive micropapillary mucinous carcinoma of the breast is associated with poor prognosis. Breast Cancer Res Treat. 2015;151:443–451. doi:10.1007/s10549-015-3413-4
151. Wilson PC, Chagpar AB, Cicek AF, et al. Breast cancer histopathology is predictive of low-risk Oncotype Dx recurrence score. Breast J. 2018;24:976–980. doi:10.1111/tbj.13117
152. Kuroda H, Sakamoto G, Ohnisi K, Itoyama S. Clinical and pathologic features of invasive micropapillary carcinoma. Breast Cancer. 2004;11:169–174. doi:10.1007/BF02968297
153. Shi W-B, Yang L-J, Hu X, Zhou J, Zhang Q, Shao Z-M. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: a population-based study from China. PLoS One. 2014;9. doi:10.1371/journal.pone.0101390
154. Meng X, Ma H, Yin HH, et al. Nomogram predicting the risk of locoregional recurrence after mastectomy for invasive micropapillary carcinoma of the breast. Clin Breast Cancer. 2021;21(4):e368–e376. doi:10.1016/j.clbc.2020.12.003
155. Song Y, Sun H, Wu K, et al. sLex expression in invasive micropapillary breast carcinoma is associated with poor prognosis and can be combined with MUC1/EMA as a supplementary diagnostic indicator. Cancer Biol Med. 2021;18:477–489. doi:10.20892/j.issn.2095-3941.2020.0422
156. Nassar H, Pansare V, Zhang H, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045–1050. doi:10.1038/modpathol.3800166
157. Ren M, Liu F, Zhu Y, et al. Absence of caveolin-1 expression in carcinoma-associated fibroblasts of invasive micropapillary carcinoma of the breast predicts poor patient outcome. Virchows Arch. 2014;465:291–298. doi:10.1007/s00428-014-1614-6
158. Wendroth SM, Mentrikoski MJ, Wick MR. GATA3 expression in morphologic subtypes of breast carcinoma: a comparison with gross cystic disease fluid protein 15 and mammaglobin. Ann Diagn Pathol. 2015;19:6–9. doi:10.1016/j.anndiagpath.2014.12.001
159. Yamaguchi R, Tanaka M, Kondo K, et al. Characteristic morphology of invasive micropapillary carcinoma of the breast: an immunohistochemical analysis. Jpn J Clin Oncol. 2010;40:781–787. doi:10.1093/jjco/hyq056
160. Onder S, Fayda M, Karanlık H, et al. Loss of ARID1A expression is associated with poor prognosis in invasive micropapillary carcinomas of the breast: a clinicopathologic and immunohistochemical study with long-term survival analysis. Breast J. 2017;23:638–646. doi:10.1111/tbj.12823
161. Verras GI, Mulita F, Tchabashvili L, et al. A rare case of invasive micropapillary carcinoma of the breast. Menopause Review/Przegląd Menopauzalny. 2022;21(1):1-8. doi:10.5114/pm.2022.113834
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.