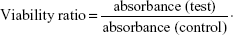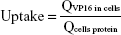Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Microemulsion-based synergistic dual-drug codelivery system for enhanced apoptosis of tumor cells
Authors Qu D , Ma Y, Sun W, Chen Y , Zhou J, Liu C, Huang M
Received 31 October 2014
Accepted for publication 16 December 2014
Published 5 February 2015 Volume 2015:10(1) Pages 1173—1187
DOI https://doi.org/10.2147/IJN.S76742
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Lei Yang
Ding Qu,1 Yihua Ma,1 Wenjie Sun,1,2 Yan Chen,1 Jing Zhou,1 Congyan Liu,1 Mengmeng Huang1
1Key Laboratory of New Drug Delivery System of Chinese Materia Medica, Jiangsu Provincial Academy of Chinese Medicine, 2Department of Pharmaceutics, School of Pharmacy, Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China
Abstract: A microemulsion-based synergistic dual-drug codelivery system was developed for enhanced cell apoptosis by transporting coix seed oil and etoposide into A549 (human lung carcinoma) cells simultaneously. Results obtained by dynamic light scattering showed that an etoposide (VP16)-loaded coix seed oil microemulsion (EC-ME) delivery system had a small size around 35 nm, a narrow polydispersity index, and a slightly negative surface charge. The encapsulating efficiency and total drug loading rate were 97.01% and 45.48%, respectively, by high-performance liquid chromatography. The release profiles at various pH values showed an obvious pH-responsive difference, with the accumulated amount of VP16 released at pH 4.5 (and pH 5.5) being 2.7-fold higher relative to that at pH 7.4. Morphologic alteration (particle swelling) associated with a mildly acidic pH environment was found on transmission electron microscopy. In the cell study, the EC-ME system showed a significantly greater antiproliferative effect toward A549 cells in comparison with free VP16 and the mixture of VP16 and coix seed oil. The half-maximal inhibitory concentration of the EC-ME system was 3.9-fold and 10.4-fold lower relative to that of free VP16 and a mixture of VP16 and coix seed oil, respectively. Moreover, fluorescein isothiocyanate and VP16 (the green fluorescent probe and entrapped drug, respectively) were efficiently internalized into the cells by means of coix seed oil microemulsion through intuitive observation and quantitative measurement. Importantly, an EC-ME system containing 20 µg/mL of VP16 showed a 3.3-fold and 3.5-fold improvement in induction of cell apoptosis compared with the VP-16-loaded microemulsion and free VP16, respectively. The EC-ME combination strategy holds promise as an efficient drug delivery system for induction of apoptosis and treatment of lung cancer.
Keywords: microemulsion, synergistic effect, dual-drug codelivery, coix seed oil, apoptosis induction
Introduction
Non-small cell lung carcinoma (NSCLC), accounting for 80% of all lung cancers, is a lethal lung disorder because of uncontrollable tumor cell growth and consequent metastasis, invasion of adjacent tissue, and loss of lung function.1–3 According to a recent report, NSCLC is expected to cause more than 30% of all cancer-related deaths throughout the world in the near future.4 The conventional therapeutic regimen for NSCLC is combined application of surgery and chemotherapy.5 Although the majority of solid tumors are able to be removed surgically, chemotherapy is still of great importance for the destruction of residual tumor cells in the body. A myriad of classical chemotherapeutic drugs, including etoposide (VP16), paclitaxel, doxorubicin, carboplatin, and cisplatin, are used as cytotoxic agents to induce cell apoptosis and cell death by interfering with DNA synthesis or stabilizing microtubules in cancer cells.1,6–8 However, the severe unwanted effects resulting from chemotherapeutic agents cannot be ignored, especially immunosuppression and gastrointestinal responses.9,10
Recent studies have explored several useful approaches to alleviating side effects and enhancing the extent of tumor cell killing by moderating the rate of drug exposure during treatment. Among these strategies, nanosized drug delivery systems are becoming an increasing focus because of their potential effects of reducing toxicity and enhancing the efficacy of chemotherapy.11,12 For example, when doxorubicin was assembled into a nanogel delivery system, cardiotoxicity was markedly reduced due to smart release of the drug.13 Paclitaxel-loaded micelles also displayed less cytotoxicity against Caco-2 cells than free paclitaxel after oral administration, mainly because of avoidance of a direct reaction between the chemotherapeutic agent and intestinal cells.14 To enhance anticancer efficacy in a similar manner, we developed a triterpene-loaded microemulsion system with a stronger tumor cell killing ability and less severe side effects in normal tissues.15
Nevertheless, conventional microemulsion-based chemotherapeutic delivery systems mainly utilize improved drug internalization into targeted cells to increase their therapeutic effect, instead of changing the intrinsic mechanism of antitumor action. Recently, to further enhance the performance of anticancer drugs, a synergistic drug combination approach has achieved a markedly improved apoptosis rate by altering signaling pathways.16 Inspired by this strategy, screening of two synergistic drugs and encapsulating them into a microemulsion system appeared to be able to render cancer cells more susceptible to apoptosis than administration of the two drugs separately. However, limited by an excessive amount of excipient and low drug loading efficiency of the microemulsion, embedding two drugs into a microemulsion simultaneously has been very challenging, albeit highly desirable.
Coix seed oil has been approved as an ancillary drug for various chemotherapeutics in the treatment of NSCLC, and often improved undesirable immunologic suppression and presented obvious sensitization effect on anticancer drugs in the combination treatment. Importantly, coix seed oil can replace a conventional oil excipient when creating a traditional Chinese medicine-loaded microemulsion, thereby markedly improving drug loading efficiency and addressing the issue of creating a dual-drug microemulsion in a smart manner. Herein, we report on a dual-drug microemulsion delivery system using a combination strategy of VP16 and coix seed oil. This study focused on whether incorporation of VP16 and coix seed oil enhances apoptosis of tumor cells. In addition, the advantages of the dual-drug microemulsion over its conventional counterparts were also evaluated with regard to drug loading efficiency, cellular uptake, cytotoxicity, and apoptosis.
Materials and methods
Materials
Coix seed oil was obtained by supercritical CO2 extraction technology (purity >85%, determined by ultraviolet spectroscopy). Labrafil® M 1944CS was received as a gift from Gattefossé Co Ltd (Nanterre Cedex, France). Cremophor® RH40 was a generous gift from BASF Co Ltd (Ludwigshafen, Germany). PEG400 was purchased from Sigma-Aldrich Co Ltd (Poole, UK). VP16 was sourced from Sinopharma Group Co Ltd (Shanghai, People’s Republic of China). Sodium dodecyl sulfate cell lysis buffer and a bicinchoninic acid protein assay kit were provided by Beyotime Institute of Biotechnology (Jiangsu, People’s Republic of China). Roswell Park Memorial Institute 1640 medium, fetal bovine serum, penicillin-streptomycin solution, and phosphate-buffered saline were purchased from Thermo Fisher Scientific Inc. (HyClone®, Waltham, MA, USA). The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was sourced from Amresco (Solon, OH, USA). Water in this study was obtained using a Milli Q-water purification system (Merck-Millipore, Billerica, MA, USA). All other chemicals and solvents were of analytical grade.
Preparation of etoposide-loaded microemulsions
VP16-loaded microemulsions were synthesized by aqueous titration as described in our previous reports but with some modifications.15 In this study, two types of VP16-loaded microemulsions were prepared: VP16-loaded coix seed oil microemulsion (EC-ME) and VP16-loaded conventional microemulsion (E-ME, with Labrafil M 1944CS as oil phase), in which adlay seed oil (or Labrafil M 1944CS), Cremophor® RH40, and PEG400 were used as the oil, surfactant, and cosurfactant, respectively. In brief, 1–4 mg of VP16 was dissolved in 400 mg of adlay seed oil with stirring at 600 rpm using an 85-2 homoiothermal magnetic stirrer (Youyi Instruments Co Ltd, Jiangsu, People’s Republic of China) for 2 hours. Next, 300 mg of Cremophor RH40 and 100 mg of PEG400 were added to the above mixture, with further vigorous magnetic stirring at room temperature until the mixture was completely homogeneous. Next, 2.0 mL of deionized water was added to the resulting mixture drop by drop. E-ME was prepared using a similar method after replacement of coix seed oil by Labrafil M 1944 CS.
Characterization of microemulsions
The average particle size and zeta potential of the various microemulsions were measured by dynamic light scattering (Nano ZS; Malvern Instruments Ltd, Malvern, UK). All measurements were carried out in triplicate. The morphology of the various samples at different pH values was observed by transmission electron microscopy (JEOL Ltd, Tokyo, Japan). Each sample was prepared by depositing a drop of ten-fold diluted microemulsion solution onto a film-coated copper grid, staining with a drop of 1.5% aqueous solution of phosphotungstic acid prior to the experiment, and allowing it to dry at room temperature.
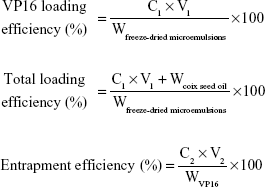
The drug encapsulation efficiency and drug loading efficiency were analyzed using high-performance liquid chromatography (HPLC, 1260 Infinity system; Agilent Technologies, Santa Clara, CA, USA) with a reverse Diamond C18-column (4.6 mm ×150 mm, 5 μm particles, 1.0 mL/min). VP16 was quantified by ultraviolet detection at 285 nm, and the mobile phase was a mixture of methanol and water (55/45, v/v). The column temperature was maintained at 30°C and the sample volume injected was 10 μL. The VP16 loading efficiency and encapsulation efficiency in the microemulsions were calculated by the following equations:
|
where C1, C2, V1, V2, Wfreeze-dried microemulsions, WVP16, and Wcoix seed oil represented the VP16 concentration of freeze-dried micelles reconstituted in water, the VP16 concentration of the microemulsions, the volume of freeze-dried microemulsions reconstituted in water, the volume of the microemulsions, the weight of freeze-dried microemulsions, the weight of VP16, and the weight of added coix seed oil, respectively.
In vitro release of EC-ME
In vitro release of EC-ME was carried out using a modified dialysis method. Briefly, 5 mL of EC-ME containing 4 mg of VP16 was added to a dialysis bag (molecular weight cutoff 10 kDa), followed by immersion in 500 mL of release medium at 37°C with stirring at 60 rpm. The release medium was phosphate buffer (pH 7.4, 6.5, and 5.5, see Table S1) or acetate buffer (pH 4.5, see Table S2) with 1% (w/v) Tween 80 in accordance with a sink condition. At predetermined time intervals (0.5, 1, 2, 4, 6, 8, 12, 24, 48 hours), 1 mL of sample was withdrawn, replaced with 1 mL of 37°C fresh buffer solution with the same pH value, and filtered through a polycarbonate membrane filter (0.22 μm pore size). The amount of VP16 released was assayed by HPLC.
Cell culture
A549 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). The cells were cultured in Roswell Park Memorial Institute 1640 medium with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin in an incubator at 37°C in an atmosphere of 5% CO2 and 90% relative humidity. The cells were subcultivated every 4–5 days (at 80% confluence) using trypsin at a split ratio of 1:4.
Cytotoxicity studies
The cells were seeded at a density of 1×104 cells per well in 96-well plates (Costar, Corning, NY, USA) for 24 hours. The culture medium was replaced with 200 μL of EC-ME and the mixture of VP16 and coix seed oil containing various concentrations of VP16. After further cell culture for 24 hours, 20 μL of 5 mg/mL MTT phosphate-buffered saline solution was added into each well, and the cells were then stained at 37°C for 4 hours. The medium was then removed, and the resulting formazan crystal was dissolved in 150 μL of dimethyl sulfoxide. Absorbance was measured at 570 nm by an enzyme-linked immunosorbent assay (Thermo Fisher Scientific Inc., Rockford, IL, USA). The relative cell viability ratio was calculated as follows:
|
Apoptosis studies
Apoptosis of the A549 cells at prearranged time interval was determined using an Annexin V-PE apoptosis detection kit (Merck-Millipore) according to the manufacturer’s protocol. Briefly, the cells were trypsinized and then terminated rapidly by phosphate-buffered saline containing 10% fetal bovine serum. Next, 50 μL of the above solution was mixed with 50 μL Annexin V-PE and incubated for 15 minutes. The cells were analyzed immediately by flow cytometry (Guava 6HT; Merck-Millipore).
Cell uptake studies
In order to evaluate the enhancement of A549 cellular uptake of VP16 by the microemulsion system, the following investigations were performed by HPLC and flow cytometry.
Accumulation of VP16 in A549 cells as evaluated by HPLC
The cells were seeded into 24-well plates at an appropriate density. After 24 hours of incubation (at 80% confluence), the medium was removed. The cells were treated with 400 μL of EC-ME, the mixture of VP16 and coix seed oil, E-ME, and VP16 suspension containing various concentrations of VP16, respectively, and then coincubated at 37°C for 4 hours. The cells were then washed in phosphate-buffered saline at 4°C three times, and cultured with 200 μL of 0.1% sodium dodecyl sulfate cell lysis buffer for 3 minutes. The VP16 content in the A549 cells was tested by HPLC and cell protein was detected using a bicinchoninic acid protein assay kit. Uptake was calculated using the following equation:
|
where QVP16 in cells and Qcells protein represent the amounts of VP16 and cell protein in A549 cells, respectively.
A549 cellular uptake evaluated by flow cytometry
Fluorescein isothiocyanate (FITC) is a fluorescence probe that is commonly used to visualize cellular uptake experiments. In this experiment, various FITC formulations including the mixture of FITC and coix seed oil, FITC-loaded coix seed oil microemulsion (FITC-CME) and conventional FITC-loaded microemulsion (FITC-ME) were prepared by a method similar to that used to prepare the corresponding VP16 microemulsion. Free FITC solution acted as a control. The cells were seeded in six-well plates at a density of 1×105 cells per well. After reaching 80% confluence, the cells were washed in phosphate-buffered saline three times, and cultured with 1 mL of the various FITC test solutions at 37°C for 4 hours. At the end of incubation, the cells were washed in ice-cold phosphate-buffered saline three times. The cell suspension was then collected through ethylenediaminetetraacetic acid (EDTA)-free pancreatin-mediated trypsinization. The cells were washed three times with phosphate-buffered saline to remove extracellular pancreatin, harvested in 0.2 mL of phosphate-buffered saline, and then analyzed by flow cytometry (Guava 6HT). The fluorescence intensity of 3,000 cells (the prearranged cell count) tested by flow cytometry represented the cellular uptake. In the flow cytometry results, right shift of the curve indicated enhanced FITC accumulation in A549 cells.
Endocytosis pathways
To investigate the endocytosis pathways for EC-ME, E-ME, and the mixture of coix seed oil and VP16, A549 cells were treated with various endocytosis inhibitors for 1 hour at 37°C as follows: 154 mg/mL of sucrose (inhibitor of clathrin-mediated endocytosis), 54 μg/mL of genistein (inhibitor of caveolae-mediated endocytosis), and 133 μg/mL of amiloride (inhibitor of macropinocytosis). After coincubation, the cells were further treated with various freshly prepared VP16 formulations in the presence of the corresponding inhibitor for 2 hours. The cells were then rinsed with phosphate-buffered saline at 4°C three times. The amounts of VP16 in the cells were assayed by HPLC and the protein in the cells was detected using the bicinchoninic acid protein assay kit.
Statistical analysis
The data are shown as the mean ± standard deviation. Statistical significance was tested by the two-tailed Student’s t-test. Statistical significance was set at P<0.05, and extreme statistical significance was set at P<0.01.
Results and discussion
Preparation and characterization of EC-ME
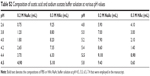
Various EC-ME samples with different mass ratios of VP16 to coix seed oil were prepared by a one-step aqueous titration method at room temperature. To demonstrate the compatibility of VP16 with the microemulsion system, we measured surface properties by dynamic light scattering and evaluated pharmaceutical characteristics by HPLC. As shown in Table 1, various feeding ratios of the drug to oil had no influence on the particle size and zeta potential of EC-ME. Each test sample displayed a small size around 35 nm, which was speculated to have the potential for deep tumor penetration.17,18 The zeta potential of the various EC-ME formulations was distributed between −15 mV and −16 mV, ie, a moderately negative charge, suggesting good stability and high cellular uptake.19 As shown in Figure 1, the presence and concentration of VP16 did not change blank MEs’ particle size or zeta potential solubilization mechanism of VP16 might be miscibility with coix seed oil instead of entrapment in the hydrophobic core.20,21 In addition, a narrow polydispersity index indicated an acceptable preparation technology. More importantly, both of coix seed oil and VP16 had their certain dosage regimen in the clinical application. Therefore, it is essential to find the optimal mass ratio of encapsulated VP16 to coix seed oil. According to the drug encapsulation efficiency shown in Table 1, almost all of the VP16 we fed in was encapsulated into the microemulsion. Although the drug loading efficiency of VP16 seemed to be very low, the total drug loading efficiency was extremely high due to a large proportion of coix seed oil in this microemulsion system. Referring to the clinical usage of coix seed oil and VP16, the EC-ME formulation with a feeding ratio of VP16 to coix seed oil of 1/100 (w/w) was selected for further study.
  | Figure 1 The influence on size and zeta potential after introduction of VP16 into microemulsions (n=4). |
In vitro release behavior of EC-ME
To evaluate the release behavior of EC-ME in various physiological environments and to investigate in a preliminary manner whether VP16 undergoes endocytosis in the form of intact microemulsion, we measured the release profiles of the EC-ME formulations at different pH values. In this experiment, the pH values of 7.4, 6.5, 5.5, and 4.5 simulated that of the physiological environment, tumor microenvironment, and endosomal and lysosomal compartments, respectively.22 As shown in Figure 2, the EC-ME formulation released VP16 in both a time-dependent and pH-dependent manner. When the EC-ME formulation was incubated in phosphate-buffered saline at pH 7.4, the cumulative amount of VP16 released was less than 10% 12 hours-cumulative amount of VP16 released was less than 10%, indicating low drug leakage while circulating in blood. However, when pH decreased to 6.5, the release rate of VP16 showed a two-fold increase for 12 hours. In sharp contrast, a significant increase in free VP16 (around 45%) was detected at pH 5.5–4.5 for 48 hours. This difference in release seen at various pH values was attributed to the stability of coix seed oil under conditions of mild acidity. The main ingredient of coix seed oil is glyceride, which is susceptible to hydrolysis in the presence of Lewis acid at room temperature. Once the chemical structure of oil phase is destroyed, the permeability of the microemulsion would change in a corresponding manner. However, no significant difference in the release rate was found at pH 4.5 and 5.5. This might be attributed to the similar hydrolysis rate of coix seed oil at a corresponding pH environment. Based on this, EC-MEs had an acid-sensitive release potential, which was beneficial to free enough drug at tumor extracellular and endolysosomal site and achieve optimal anticancer efficacy.
  | Figure 2 Release profile of EC-MEs at various pH environments (n=3). **P<0.01 vs pH 7.4. |
Morphology of EC-MEs
To further explore the relationship between release profile and change in morphology at different pH values, we contrasted the transmission electron microscopic images of the EC-ME formulations before and after 48 hours of coincubation with phosphate-buffered saline (at pH 4.5, 5.5, 6.5, and 7.4). As shown in Figure 3, all untreated EC-ME formulations (A1, B1, C1, and D1) had a well defined spherical shape with a small size around 30–40 nm. Of note, obvious erosion (A2 and B2) and size increase were found on the microemulsion surface at pH 5.5 and 4.5 during such treatment for 48 hours. This pH-triggered microemulsion swelling might result from the partial coix oil hydrolysis, which attracted multiple protons to the inside of microemulsion and thus led to an increase of osmotic pressure.23 In comparison, only a little change (C2 and D2) was observed in the morphology of EC-MEs at pH 6.5 and 7.4 after incubation for 48 hours. Such trends in morphologic alteration in different pH environments were reasonable and consistent with the release profiles. All the above-mentioned results suggest that the EC-MEs could circulate steadily in a normal physiological environment and release their therapy contents rapidly at an acidic tumor site.
Antiproliferative effects in vitro
The cytotoxicity of the EC-ME formulations against A549 cells in vitro was evaluated using the MTT assay. VP16 and coix seed oil were dissolved in 1% DMSO solution, respectively. The two solutions were used as positive controls in this work. To demonstrate the advantage of a dual-drug codelivery system in terms of enhancing cytotoxicity, a mixture of coix seed oil and VP16 was also used as the other control group. Of note, the mixture of coix seed oil and VP16 also contained 5% RH40 (but without cosurfactant) and was prepared by vigorous magnetic stirring. It was a classical emulsion formulation observing from sample F in Figure S1. As shown in Figure 4A, dose-dependent antiproliferative activity was found for both the EC-ME and mixture formulations. With an increase in VP16 concentration up to 2 μg/mL, the EC-ME formulations showed an enhanced effect relative to that of the mixture formulations for 24 hours. The half-maximal inhibitory concentrations (IC50) of the EC-ME and mixture formulations against A549 cells were calculated to be 3.16 μg/mL and 12.47 μg/mL, respectively. By comparison, the IC50 of EC-ME was 3.9-fold and 10.4-fold lower relative to that of the mixture formulation and free VP16, respectively. The significant difference in antiproliferative activity between the EC-ME and mixture formulation suggested that codelivery of coix seed oil and VP16 was critical to exert a synergistic antitumor effect. The mixture of coix seed oil and VP16 was not able to guarantee that two components entered the cells at the same time, due to their respective differences in physicochemical properties.24 In addition, Figure 4B shows that only a concentration of coix seed oil higher than 500 μg/mL achieved strong cytotoxicity. This experiment confirms the advantage of a dual-drug codelivery system from the perspective of an anticancer effect in vitro.
Cellular uptake assay of EC-MEs
In order to validate the advantage of our dual-drug codelivery system, we intuitively observed and quantitatively measured the cellular uptake by flow cytometry and HPLC, respectively.
In the flow cytometry study, FITC (with green fluorescence) was used as a marker for the entrapped drug. The fluorescence images of A549 cells after incubation with 5 μM of free FITC solution, a mixture of FITC and coix seed oil, conventional FITC microemulsion, and FITC-loaded coix seed oil microemulsions (FITC-CME, 5 μM and 10 μM) for 4 hours are shown in Figure 5A and B. In comparison with free FITC, both the conventional FITC microemulsion and the FITC-CME formulations showed an increased amount of internalized FITC, indicating a significant improvement in endocytosis for the microemulsion. Interestingly, the presence or absence of coix seed oil did influence the cellular uptake of FITC by comparison with free FITC and the mixture of FITC and coix seed oil. The probable cause of coix seed oil-mediated uptake enhancement was the intermiscibility of coix seed oil and FITC and consequently alteration of the endocytosis pathway. Of note, this effect was only found for the mixture of coix seed oil and FITC. Moreover, FITC-CME at two concentrations of FITC did not exhibit obvious difference in fluorescence intensity, suggesting saturated absorption by A549 cells at a concentration of 5 μM for 4 hours. Similar results were also observed in the fluorescent images of EC-ME internalization (see Figure 5C).
We further evaluated the cellular uptake of free VP16, the mixture of VP16 and coix seed oil, E-ME, and EC-ME by HPLC. Firstly, the concentration of VP16 (100, 200, and 300 μg/mL) and treatment time (4 hours) were decided by a cellular uptake kinetics study, but no obvious cytotoxicity and size alteration were found within this time interval (see Figure S2). As seen in Figure 5D, the various formulations, containing the same amount of VP16, showed a noticeable difference in internalization after incubation with A549 cells for 4 hours. The uptake of the microemulsions was greatly improved compared with that of free VP16, indicating an inherent superiority of the nanosized delivery system in cellular uptake. Considering the poor absorption of free VP16 and FITC by A549 cells, solubility was not the only critical factor in crossing the cell membrane. The degree of lipophilicity, surface charge, and particle size were also very important factors. Although the EC-ME formulations had a slightly negative charge, their small particle size and amphiphilicity provided superiority in some specific protein-mediated endocytosis, such as the clathrin-mediated and caveolae-mediated pathways, which are commonly found in endocytosis of nanosized drug delivery systems.7 No significant difference in cellular uptake was found between the E-ME and EC-ME formulations at various concentrations, suggesting that microemulsion formulation was the primary reason to the internalization enhancement.
Endocytosis pathways
To explore the potential mechanisms involved in the cellular uptake of the test formulations, sucrose, genistein, and amiloride were used as specific agents for clathrin-mediated, caveolae-mediated, and macropinocytosis, respectively, to investigate each type of cellular uptake mechanism. In this study, cellular uptake of EC-ME containing 200 mg/mL of VP16 by A549 cells without any endocytosis inhibitors was defined as a control. As shown in Figure 6, different endocytosis inhibitors had different effects on internalization. Incubation of A549 cells with the EC-ME and E-ME formulations brought about a significant (P<0.05) decrease in cellular uptake of VP16 in the presence of sucrose, indicating that clathrin was involved in the internalization of these systems. However, after culture of the coix seed oil and VP16 mixture (emulsion) with A549 cells for 4 hours, the cellular uptake of VP16 was significantly (P<0.05) inhibited in the presence of sucrose and amiloride, suggesting that the emulsion formulation could enter cells through clathrin-mediated endocytosis and by the macropinocytosis pathway. The different physicochemical properties and differences in appearance between the EC-ME and the mixture of coix seed oil and VP16 shown in Table 1 and Figure S1 indicate that the particle size of the various VP16 formulations played an important role in the mechanism of internalization.14,25
Induction of apoptosis
According to the design strategy of EC-MEs, combination of coix seed oil and VP16 was able to induce cell apoptosis. To validate our idea, we investigated the induction of apoptosis by the free VP16, E-ME, and EC-ME formulations using the Annexin V-PE apoptosis detection kit. The apoptosis results were showed through quadrant analysis. Events in each of the four quadrants were as follows: lower-left, lower-right, upper-right and upper-left quadrant represented viable cells, early to mid stages of apoptosis, late stages of apoptosis and dead cells, respectively. As shown in Figure 7A, when the concentration of VP16 was set at 2 μg/mL, there was obvious induction of apoptosis with free VP16 when compared with the negative control after treatment for 5 hours; however, the E-ME formulation did not show any advantage over the free drug. It was speculated that the E-ME formulation needed time to release enough drug to induce apoptosis, since the concentration of VP16 was too low. More interestingly, owing to the synergistic effect of coix seed oil and VP16, the EC-ME formulation showed significantly enhanced apoptosis relative to E-ME. Our assumption was further validated when the concentration of VP16 was increased up to 10–20 μg/mL (see Figure 7B and C). According to Figure 7D, the 20 μg/mL EC-ME formulation displayed a 3.3-fold and 3.5-fold increase in induction of cell apoptosis compared with the E-ME formulation and free VP16, respectively. This synergistic effect was not found when the cells were treated with the mixture of coix seed oil and VP16 at the same concentration (data not shown), suggesting that the combination of components, incubation time, release profile, and surface properties of the particles were of great importance in induction of apoptosis. In addition, the trend of promotion of apoptosis was more obvious using early apoptosis rate as an evaluation index (see Figure S3).
Conclusion
In summary, we developed a microemulsion-based synergistic dual-drug codelivery system with a small size, mildly negative charge, and high drug-loading and drug-encapsulating efficiency. Both release profile and morphology were associated with the extracellular and intracellular environment. Incorporation of coix seed oil and VP16 into one drug delivery system enhanced cytotoxicity against A549 cells. In the study of cellular uptake, the microemulsion system promoted significant internalization of VP16 through the clathrin-mediated endocytosis pathway. Our findings also indicate that the EC-ME formulation has a noticeable advantage in induction of cell apoptosis. Further work on the mechanism of intracellular transportation, subcellular localization, and anticancer activity in vivo is being undertaken by our group.
Acknowledgments
This work was supported financially by Jiangsu Provincial Natural Science Foundation (BK20141038), the Fundamental Research Funds for the Central Public Welfare Research Institutes (ZZ08080015), the National Natural Science Foundation (81373979), and the Jiangsu Provincial Chinese Medicine Leading Talent Project (LJ200913).
Disclosure
The authors report no conflicts of interest in this work.
References
Jinturkar KA, Anish C, Kumar MK, Bagchi T, Panda AK. Liposomal formulations of etoposide and docetaxel for p53 mediated enhanced cytotoxicity in lung cancer cell line. Biomaterials. 2012;33(8):2492–2507. | ||
Everaert H, Flamen P, Franken PR, Verhaeghe W, Bossuyt A. Sigma-receptor imaging by means of I123-IDAB scintigraphy: clinical application in melanoma and non-small cell lung cancer. Anticancer Res. 1997;17(3B):1577–1582. | ||
Wu Y, Crawford M, Yu B, et al. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol Pharm. 2011;8(4):1381–1389. | ||
Yu CJ, Wang CL, Wang CI, et al. comprehensive proteome analysis of malignant pleural effusion for lung cancer biomarker discovery by using multidimensional protein identification technology. J Proteome Res. 2011;10(10):4671–4682. | ||
Ma N, Xu H, An L, et al. Radiation-sensitive diselenide block co-polymer micellar aggregates: toward the combination of radiotherapy and chemotherapy. Langmuir. 2011;27(10):5874–5878. | ||
Mo R, Xiao Y, Sun M, Zhang C, Ping Q. Enhancing effect of N-octyl-O-sulfate chitosan onetoposide absorption. Int J Pharm. 2011;409(1–2):38–45. | ||
Qu D, Lin H, Zhang N, Xue J, Zhang C. In vitro evaluation on novel modified chitosan for targeted antitumor drug delivery. Carbohydr Polym. 2013;92(1):545–554. | ||
Marques C, Ferreira JM, Andronescu E, Ficai D, Sonmez M, Ficai A. Multifunctional materials for bone cancer treatment. Int J Nanomedicine. 2014;9:2713–2725. | ||
Park S, Yoon J, Bae S, et al. Therapeutic use of H2O2-responsive anti-oxidant polymer nanoparticles for doxorubicin-induced cardiomyopathy. Biomaterials. 2014;35(22):5944–5953. | ||
Bae S, Ma K, Kim TH, et al. Doxorubicin-loaded human serum albumin nanoparticles surface-modified with TNF-related apoptosis-inducing ligand and transferrin for targeting multiple tumor types. Biomaterials. 2012;33(5):1536–1546. | ||
Tang BC, Fu J, Watkins DN, Hanes J. Enhanced efficacy of local etoposide delivery by poly(ether-anhydride) particles against small cell lung cancer in vivo. Biomaterials. 2010;31(2):339–344. | ||
Yu J, Deng H, Xie F, Chen W, Zhu B, Xu Q. The potential of pH-responsive PEG-hyperbranched polyacylhydrazone micelles for cancer therapy. Biomaterials. 2014;35(9):3132–3144. | ||
Ju C, Mo R, Xue J, et al. Sequential intra-intercellular nanoparticle delivery system for deep tumor penetration. Angew Chem Int Ed Engl. 2014;53(24):6253–6258. | ||
Mo R, Jin X, Li N, et al. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials. 2011;32(20):4609–4620. | ||
Qu D, He J, Liu C, Zhou J, Chen Y. Triterpene-loaded microemulsion using Coix lacryma-jobi seed extract as oil phase for enhanced antitumor efficacy: preparation and in vivo evaluation. Int J Nanomedicine. 2014;9:109–119. | ||
Morton SW, Lee MJ, et al. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal. 2014;7(325):ra44. | ||
Li L, Sun J, He Z. Deep penetration of nanoparticulate drug delivery systems into tumors: challenges and solutions. Curr Med Chem. 2013;20(23):2881–2891. | ||
Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–2431. | ||
Chen JX, Liu W, Zhang M, Chen JH. Heparosan based negatively charged nanocarrier for rapid intracellular drug delivery. Int J Pharm. 2014;473(1–2):493–500. | ||
Szczepanowicz K, Bazylińska U, Pietkiewicz J, Szyk-Warszyńska L, Wilk KA, Warszyński P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: from controlling physical state and stability to biological impact. Adv Colloid Interface Sci. October 23, 2014. [Epub ahead of print]. | ||
Bardhan S, Kundu K, Saha SK, Paul BK. Physicochemical investigation of mixed surfactant microemulsions: water solubilization, thermodynamic properties, microstructure, and dynamics. J Colloid Interface Sci. 2013;411:152–161. | ||
Mo R, Sun Q, Xue J, et al. Multistage pH-responsive liposomes for mitochondrial-targeted anticancer drug delivery. Adv Mater. 2012;24(27):3659–3665. | ||
Hu J, Liu G, Wang C, et al. Spatiotemporal monitoring endocytic and cytosolic pH gradients with endosomal escaping pH-responsive micellar nanocarriers. Biomacromolecules. 2014;15(11):4293–4301. | ||
Ke PC, Lamm MH. A biophysical perspective of understanding nanoparticles at large. Phys Chem Chem Phys. 2011;13(16):7273–7283. | ||
Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. |
Supplementary materials
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.