Back to Journals » Infection and Drug Resistance » Volume 15
Microbiology and Drug Susceptibility Pattern of Bacterial Isolates from Patients with Chronic Suppurative Otitis Media at a Tertiary Care Hospital in Somalia
Authors Mohamed Ali I, Duman C, Bozdağ İ, Artan Abdi A, Nor Abdi M , Karakurt SE, Yiğit Ö
Received 14 October 2022
Accepted for publication 20 December 2022
Published 28 December 2022 Volume 2022:15 Pages 7733—7739
DOI https://doi.org/10.2147/IDR.S390886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Ismail Mohamed Ali,1 Cihan Duman,1 İlkay Bozdağ,2 Abdihakim Artan Abdi,1 Mohamed Nor Abdi,1 Süleyman Emre Karakurt,1 Özgür Yiğit3
1Department of Otorhinolaryngology, Head and Neck Surgery, University of Health Sciences, Somalia Turkey Recep Tayyip Erdogan Education and Research Hospital, Mogadishu, Somalia; 2Department of Microbiology, University of Health Sciences, Somalia Turkey Recep Tayyip Erdogan Education and Research Hospital, Mogadishu, Somalia; 3Head Department of Otorhinolaryngology, Head and Neck Surgery, University of Health Sciences, Istanbul Training and Research Hospital, Istanbul, Turkey
Correspondence: Ismail Mohamed Ali, Department of Otorhinolaryngology, Head and Neck Surgery, University of Health Sciences, Somalia Turkey Recep Tayyip Erdogan Education and Research Hospital, Mogadishu, Somalia, Tel +252615304261, Email [email protected]
Background: This study aimed to determine the microbiological profile and antibiotic susceptibility pattern of bacterial isolates obtained from patients with chronic suppurative otitis media (CSOM) presenting to the otorhinolaryngology clinic of a tertiary care hospital in Mogadishu, Somalia.
Methods: A total of 225 patients diagnosed with chronic suppurative otitis media were included in the study. Samples of middle ear discharge were collected from each patient and cultured using standard microbiological techniques, and bacterial identification was performed. Drug susceptibility pattern was assessed according to the Clinical and Laboratory Standards Institute criteria.
Results: The study sample (n=225) comprised 122 females and 103 males. Among 225 samples tested, bacterial growth was present in 200 (88.9%) and absent in 25 (11.1%) samples. Of 200 samples with bacterial growth, monomicrobial growth was detected in 176 (88%). Gram-positive bacteria were observed in 40 (22.7%) and Gram-negative bacteria in 136 (77.3%) samples. The bacteriology of the samples with monomicrobial growth consisted of (in decreasing frequency) Pseudomonas spp, Staphylococcus aureus, Escherichia coli, Coagulase-negative staphylococci, Proteus mirabilis, and Klebsiella sp. A high rate of resistance was detected against penicillin antibiotics, erythromycin, tetracycline, and co-trimoxazole. Resistance to cephalosporins, clindamycin, vancomycin, linezolid, daptomycin, quinupristin/dalfopristin, levofloxacin, meropenem, and ertapenem was low.
Conclusion: While the frequencies of isolated bacterial species were consistent with other reports from the region, differences were observed in the antibiotic resistance of bacterial isolates when examined individually for each antibiotic. Further studies are warranted in the same region and different parts of Somalia, coupled with ongoing assessment of antibiotic susceptibility patterns in CSOM.
Keywords: drug susceptibility, otitis media, microbial sensitivity tests, Somalia
Introduction
In its classical definition, chronic suppurative otitis media (CSOM) is characterized by recurrent ear discharge through a perforated tympanic membrane due to chronic inflammation of the middle ear and mastoid cells.1 Worldwide, it is estimated that 65–300 million people are affected by CSOM, with hearing impairment reported in about 60%of them.2,3 The incidence of CSOM is higher in developing countries.4 In addition to low socioeconomic status, frequent episodes of acute otitis media and other upper respiratory tract infections are major risk factors for CSOM.5,6
The most common cause of otitis media is a bacterial infection of the tympanomastoid cavity. Pseudomonas aeruginosa, Staphylococcus aureus, Proteus species (spp.), and Klebsiellaspp. are the most frequently isolated aerobic pathogens.7–9 Since CSOM is a preventable cause of hearing loss, effective treatment of this condition is essential. As with other bacterial infections, antibiotic resistance in CSOM is a growing concern. Drug resistance caused by inappropriate antibiotic use has resulted in CSOM complication rates returning to pre-antibiotic era levels. Resistance to antibiotics, the mainstay of treatment, may vary across geographic regions.10 In this respect, identifying antibiotic resistance at the national level is of paramount importance. To the best of our knowledge, no study is available in the literature evaluating antibiotic susceptibility patterns in patients with CSOM in Somalia. This study aimed to investigate the species, frequency, and drug resistance of bacteria isolated from the cultures of patients with CSOM presenting to a tertiary care hospital in Somalia.
Materials and Methods
This prospective, cross-sectional study was conducted at a tertiary care hospital between August 2021 and June 2022. This study was approved by the Clinical Research Ethics Committee of the Mogadishu Somali Turkish Training and Research Hospital (Reference number: MSTH/7072). In addition, all study participants and a parent of participants under 18 years of age previously consented to use their medical and surgical data in this study. This study was carried out following the Helsinki Declaration contents.
A total of 225 patients diagnosed with CSOM were recruited for the study. The patients were grouped into seven age categories: <5y, 5–14 y, 15–24 y, 25–34 y, 35–44, 45–54, and >54 y.
Patients with tympanic membrane perforation and ear discharge for more than three months were included. Patients with intact tympanic membrane and ear discharge were excluded from the study, as were those who had received antibiotic therapy within seven days of sample collection. After cleaning the external ear canal, samples of middle ear discharge were aseptically collected from each patient by the ENT specialist using a sterile cotton swab (Letsswab, Turkey) and sent to the microbiology laboratory of the hospital. Ear discharge samples were inoculated on Blood agar, Chocolate agar, and EMB (Eosin-Methylene Blue) agar plates (Laborlar, Turkey). Then, the plates were incubated at 37°C for 48 hours in aerobic conditions. Bacterial isolates were characterized by colony morphology, Gram staining, catalase, and coagulase test. Bacterial species were identified according to a standard microbiological procedure.11
Antibiotic susceptibility testing was performed on Mueller-Hinton agar (Laborlar-Turkey) using the disk diffusion technique.12 Antimicrobial agents tested were as follows: Penicillin G (10 μg), Ampicillin (10 μg), Cefoxitin (30 μg), Erythromycin (15 μg), Ciprofloxacin (5 μg), Clindamycin (2 μg), Co-trimoxazole (25 μg), Vancomycin (30 μg), Linezolid (30 μg), Daptomycin (30 μg), Quinupristin/Dalfopristin (15 μg), Cefuroxime (30 μg), Meropenem (10 μg), Levofloxacin (5 μg), Amikacin (30 μg), Ertapenem (10μg), Cefoperazone/ sulbactam (75/30 µg), Piperacillin/tazobactam (100/10 μg), Tetracycline (30 μg) and Ceftazidime (30 μg) (Bioanalyse, Turkey). Drug susceptibility pattern was assessed according to the Clinical and Laboratory Standards Institute (CLSI) criteria 13. Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 2592 reference strains were used for quality control in antibiotic susceptibility testing.13
The study data were analyzed using SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY). Data were presented in a tabulated form as number (n) and percentage.
Results
The study sample (n=225) consisted of 122 females and 103 males. The mean age of the participants was 19.13±16.5 (1–83) years. The age and sex distribution of the participants are shown in Table 1. Among 225 samples tested, bacterial growth was present in 200 (88.9%) and absent in 25 (11.1%) samples. Of 200 samples showing bacterial growth, monomicrobial growth was detected in 176 (88%) and polymicrobial growth in 24 (12%) samples. Among 176 samples with monomicrobial growth, Gram-positive bacteria were observed in 40 (22.7%) and Gram-negative bacteria in 136 (77.3%) samples. The bacteriology of the samples with monomicrobial growth consisted of (in decreasing frequency) Pseudomonas spp. (50%), Staphylococcus aureus (15.9%), Escherichia coli (15.3%), Coagulase-negative staphylococci (6.8%), Proteus mirabilis (6.8%) and Klebsiellaspp. (5.1%) (Table 2).
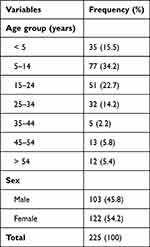 |
Table 1 Age and Sex Distribution of the Participants |
 |
Table 2 Frequency of Bacteria Isolated from Ear Discharge Cultures |
Table 3 shows the drug susceptibility pattern of Gram-positive bacteria. The overall resistance rate of the Gram-positive bacteria was 32.2%, with the resistance rates ranging between 3.6% and 100%. Very high resistance was detected against Penicillin G (93.8%) and Ampicillin (100%). 66.7% of the Gram-positive bacteria were resistant to Cefoxitin. 50% or higher resistance rates were observed against erythromycin, ciprofloxacin, and co-trimoxazole (73.3%, 55.6%, and 50%, respectively). A lower resistance was found against clindamycin, vancomycin, linezolid, daptomycin, and quinupristin/dalfopristin (21.7%, 0%, 3.6%, 0%, and 3.9%, respectively).
 |
Table 3 Drug Susceptibility Pattern of Gram-Positive Bacterial Isolates |
Table 4 summarizes the drug susceptibility pattern of Gram-negative bacteria. The overall resistance rate of Gram-negative bacteria was 22.4%, varying from 3.1% and 86.7%. A very high resistance rate (86.7%) to cefuroxime was observed. 62.5% of the Gram-negative bacteria were resistant to tetracycline. Resistance to levofloxacin, erythromycin, and ceftazidime was below 59% (27.9%, 40.6% and 25.5%, respectively). Low resistance rates were found for meropenem, amikacin, piperacillin/tazobactam, and cefoperazone/sulbactam (3.1%, 8%, 7.8% and 4.5%, respectively).
 |
Table 4 Drug Susceptibility Pattern of Gram-Negative Bacterial Isolates |
Discussion
Chronic suppurative otitis media (CSOM) remains a major public health concern in developing countries. Low socioeconomic status is a risk factor for this disease associated with reduced quality of life. From this standpoint, appropriate treatment of CSOM is crucial. Antibiotics are the mainstay of treatment for CSOM, and the development of antimicrobial resistance represents a significant barrier to effectively managing the condition. Differences in populations, antibiotic prescribing practices, and frequency of resistant bacterial strains across geographic regions account for regional variability in antimicrobial-resistant patterns.10 This mandates the identification of antibiotic resistance in CSOM by each country and all regions within a country individually. As a result of these efforts, studies have been conducted in developing countries.14–16 While a female or male predilection was reported in those studies, there was no significant gender predominance for CSOM. Also, the mean age of the patients studied ranged between 17 and 18 years.14–16 In line with previous reports, although our study had a slight female predilection, we did not observe a significant gender predominance. The mean age of our study sample was also consistent with those reported in former studies. 15.5% of our study population was under the age of 5, and 34.2% were under the age of 15.
There are some cases where no bacterial growth occurs in an ear discharge culture. While this may cause challenges in choosing the appropriate antibiotic, most cases show positive cultures, which facilitates the clinician’s approach to managing this disease. Two studies from African countries reported high culture positivity rates. Similarly, the overall culture positivity rate was 90% in our study.14,17,18 Monomicrobial growth was detected in 88% of the positive cultures, which is consistent with previous reports from the countries in the African continent.14,17,18 Gram-negative bacterial growth was observed in 77.3% of the samples, similar to the rates reported in the literature.19–22
Looking at the studies from African countries, Proteus species were the most common isolates, followed by Staphylococcus aureus.14 In a study from Nigeria, Pseudomonas aeruginosa was isolated predominantly, followed in decreasing frequency by Staphylococcus aureus, Klebsiellaspp. and Proteusspp.18 A systematic review and meta-analysis involving Sub-Saharan African countries, Staphylococcus aureus, Pseudomonas aeruginosa, and Proteus spp. were reported as the primary bacterial pathogens accounting for CSOM.23 Likewise, Pseudomonasspp. were the most prevalent species, followed by S. aureus and Escherichia coli in the current study. Variations in the rates of bacterial isolates across studies from different African countries may be explained by climatic and geographic differences.
Getaneh et al reported a high resistance rate against tetracycline, penicillin, co-trimoxazole, chloramphenicol, and erythromycin.15 However, low resistance rates to fluoroquinolones, aminoglycosides, and cephalosporins were reported in the same study.15 High resistance rates to penicillin, tetracycline, and macrolides were found in studies from different regions of Sub-Saharan Africa.24–26 The current study detected a high resistance rate against penicillin-class antibiotics, erythromycin, tetracycline, and co-trimoxazole. Resistance to cephalosporins, clindamycin, vancomycin, linezolid, daptomycin, quinupristin/dalfopristin, levofloxacin, meropenem, and ertapenem was low. Our findings closely match those reported in studies from Ethiopia.24–26
Different studies have reported variable rates of antibiotic resistance in bacteria isolated from ear discharge cultures. In a Nigerian study, Pseudomonas aeruginosa and Staphylococcus aureus were resistant to almost all antibiotics tested. The only resistance that may be interpreted as high was against ofloxacin, over 50% 18. The same study observed low resistance rates in Escherichia Coli and Klebsiella spp. against all antibiotics tested 18. In a study from Ethiopia, Pseudomonas sp. and Escherichia coli showed high resistance to tetracycline 14. The same study detected a high resistance of Staphylococcus aureus to penicillin antibiotics, whereas resistance to erythromycin and ciprofloxacin was low14. In our study, Staphylococcus aureus showed high resistance to penicillin antibiotics, cephalosporins, ciprofloxacin, and erythromycin but low resistance to clindamycin, co-trimoxazole, and vancomycin. When the antibiotic resistance of Pseudomonas spp. (one of the three dominant species detected in this study) was examined, and high resistance to tetracycline and low resistance to cephalosporins, levofloxacin, and amikacin were found. Escherichia coli showed resistance to levofloxacin and cefuroxime and susceptibility to amikacin, erythromycin, cefoperazone/sulbactam, ceftazidime, and meropenem. Drug susceptibility patterns reported by studies from the countries in the region show some similarities as well as differences. Regional differences in antibiotic susceptibility patterns may be related to regional differences in antimicrobial prescribing practices and the presence of resistant bacterial strains. It is noteworthy that the antibiotic resistance of Staphylococcus aureus was higher in the current study compared to previous reports from the region.
The main limitation of our study is that it did not provide any data on anaerobic bacteria and fungi.
Conclusion
While the frequencies of isolated bacterial species were consistent with other reports from the region, differences were observed in the antibiotic resistance of bacterial isolates when examined individually for each antibiotic. This may be explained by geographic and climatic differences, differences in antibiotic prescribing patterns, and the prevalence of resistant bacterial strains. Since this is the first study to report on antibiotic susceptibility patterns in Somalia, we hope our study inspires further studies from different parts of Somalia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Acuin J. Global Burden of Disease Due to Chronic Suppurative Otitis Media: Disease, Deafness, Deaths and DALYs Chronic Suppurative Otitis Media–Burden of Illness and Management Options. Geneva: World Health Organisation; 2004:
2. Woodfield G, Dugdale A. Evidence behind the WHO guidelines: hospital care for children: what is the most effective antibiotic regime for chronic suppurative otitis media in children? J Tropical Pediatric. 2008;54(3):151–156. doi:10.1093/tropej/fmn042
3. Kombade SP, Kaur N, Patro SK, Nag VL. Clinico-bacteriological and antibiotic drug resistance profile of chronic suppurative otitis media at a tertiary care hospital in Western Rajasthan. J Family Med Prim Care. 2021;10:2572–2579. doi:10.4103/jfmpc.jfmpc_2480_20
4. Khatun MR, Alam KMF, Naznin M, Salam MA. Microbiology of chronic suppurative otitis media: an update from a tertiary care hospital in Bangladesh. Pak J Med Sci. 2021;37(3):821–826. doi:10.12669/pjms.37.3.3942
5. Kumar D, Agrawal A, Kumar S, Gupta N. A study of the microbiological profile of CSOM in A tertiary care centre of North India. IOSR J Dent Med Sci. 2019;18:20–24.
6. Wang J, Chen B, Xu M, et al. Etiological factors associated with chronic suppurative otitis media in a population of Han adults in China. Acta Otolaryngol. 2016;136(10):1024–1028. doi:10.1080/00016489.2016.1183818
7. Sattar A, Alamgir A, Hussain Z, Sarfraz S, Nasir J. Bacterial spectrum and their sensitivity pattern in patients of chronic suppurative otitis media. J Coll Physicians Surg Pak. 2012;22:128–129.
8. Aduda DS, Macharia IM, Mugwe P, et al. Bacteriology of chronic suppurative otitis media (CSOM) in children in Garissa district, Kenya: a point prevalence study. Int J Pediatr Otorhinolaryngol. 2013;77:1107–1111. doi:10.1016/j.ijporl.2013.04.011
9. Prakash R, Juyal D, Negi V, et al. Microbiology of chronic suppurative otitis media in a tertiary care setup of Uttarakhand state, India. N Am J Med Sci. 2013;5:282–287. doi:10.4103/1947-2714.110436
10. Noh KT, Kim CS. The changing pattern of otitis media in Korea. Int J Pediatrician Otorhinolaryngol. 1985;9:77–87. doi:10.1016/S0165-5876(85)80006-9
11. Cheesbourgh M. Medical Laboratory Manual for Tropical Countries.
12. Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by standard single disc method. Am J Clin Pathol. 1966;45:493–496. doi:10.1093/ajcp/45.4_ts.493
13. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; seventeenth information supplement. In: CLSI Document M100-S17. Clinical and Laboratory Standards Institute Wayne Pennsylvania; 2006.
14. Muluye D, Wondimeneh Y, Ferede G, Moges F, Nega T. Bacterial isolates and drug susceptibility patterns of ear discharge from patients with ear infection at Gondar University Hospital, Northwest Ethiopia. BMC Ear Nose Throat Disord. 2013;13(1):10. doi:10.1186/1472-6815-13-10
15. Getaneh A, Ayalew G, Belete D, Jemal M, Biset S. Bacterial etiologies of ear infection and their antimicrobial susceptibility pattern at the university of Gondar comprehensive specialized hospital, Gondar, Northwest Ethiopia: a six-year retrospective study. Infect Drug Resist. 2021;14:4313–4322. doi:10.2147/IDR.S332348
16. Afolabi OA, Salaudeen AG, Ologe FE, Nwabuisi C, Nwawolo CC. Pattern of bacterial isolates in the middle ear discharge of patients with chronic suppurative otitis media in a tertiary hospital in North central Nigeria. Afr Health Sci. 2012;12(3):362–367. doi:10.4314/ahs.v12i3.18
17. Abera B, Kibret M. Bacteriology and antimicrobial susceptibility of otitis media at Dessie regional health research laboratory, Ethiopia. Ethiopian J Health Develop. 2011;25(2):161–167.
18. Osazuwa F, Osazuwa E, Osime C, et al. Aetiologic agents of otitis media in Benin city, Nigeria. North Am J Med Sci. 2011;3:95–98. doi:10.4297/najms.2011.395
19. Akter S, Shamsuzzaman SM, Nehar N, Siddique I, Ferdush J, Islam S. Bacterial isolates and drug susceptibility patterns of ear discharge from patients with ear infection at Shaheed Monsur Ali Medical College. Bangladesh J Med Microbiol. 2015;9(2):20–23. doi:10.3329/bjmm.v9i2.31422
20. Asima B, Samhitha V, Karthik S. Spectrum and antibiogram of bacteria in chronic suppurative otitis media and biofilm formation. J Stem Cell Res Ther. 2017;2(4):115–118.
21. Sahu MC, Swain SK. Surveillance of antibiotic sensitivity pattern in chronic suppurative otitis media of an Indian teaching hospital. World J Otorhinolaryngol Head Neck Surg. 2019;5(2):88–94. doi:10.1016/j.wjorl.2018.05.008
22. Khan JA, Paul SK, Chowdhury CS, et al. Bacteriology of Chronic Suppurative Otitis Media (CSOM) at a tertiary care hospital, Mymensingh. Mymensingh Med J. 2020;29(3):545–552.
23. Tesfa T, Mitiku H, Sisay M, et al. Bacterial otitis media in sub-Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):225. doi:10.1186/s12879-020-4950-y
24. Tadesse B, Shimelis T, Worku M. Bacterial profile and antibacterial susceptibility of otitis media among pediatric patients in Hawassa, Southern Ethiopia: cross-sectional study. BMC Pediatr. 2019;19(1):398. doi:10.1186/s12887-019-1781-3
25. Hailu D, Mekonnen D, Derbie A, Mulu W, Abera B. Pathogenic bacteria profile and antimicrobial susceptibility patterns of ear infection at Bahir Dar Regional Health Research Laboratory Center, Ethiopia. Springerplus. 2016;5(1):466. doi:10.1186/s40064-016-2123-7
26. Argaw-Denboba A, Abejew AA, Mekonnen AG. Antibiotic-resistant bacteria are major threats of otitis media in Wollo Area, Northeastern Ethiopia: a ten-year retrospective analysis. Int J Microbiol. 2016;2016:8724671. doi:10.1155/2016/8724671
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
