Back to Journals » Drug Design, Development and Therapy » Volume 17
Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes
Authors Dutta S , Shah RB , Singhal S, Dutta SB, Bansal S, Sinha S , Haque M
Received 20 February 2023
Accepted for publication 10 June 2023
Published 26 June 2023 Volume 2023:17 Pages 1907—1932
DOI https://doi.org/10.2147/DDDT.S409373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Siddhartha Dutta,1 Rima B Shah,1 Shubha Singhal,1 Sudeshna Banerjee Dutta,2 Sumit Bansal,3 Susmita Sinha,4 Mainul Haque5
1Department of Pharmacology, All India Institute of Medical Sciences, Rajkot, Gujarat, India; 2Department of Medical Surgical Nursing, Shri Anand Institute of Nursing, Rajkot, Gujarat, 360005, India; 3Department of Anaesthesiology, All India Institute of Medical Sciences, Rajkot, Gujarat, India; 4Department of Physiology, Khulna City Medical College and Hospital, Khulna, Bangladesh; 5Unit of Pharmacology, Faculty of Medicine and Defence Health, Universiti Pertahanan Nasional Malaysia (National Defence University of Malaysia), Kuala Lumpur, 57000, Malaysia
Correspondence: Shubha Singhal, Department of Pharmacology, All India Institute of Medical Sciences-Rajkot, Ayush Block, Khanderi, Para Pipaliya, Rajkot, Gujarat, India, Tel +91 9560372056, Email [email protected] Mainul Haque, Unit of Pharmacology, Faculty of Medicine and Defence Health Sciences, Universiti Pertahanan Nasional Malaysia, National Defence University of Malaysia- Kem, Perdana, Sungai Besi, Kuala Lumpur, 57000, Malaysia, Tel +60 109265543, Email [email protected]
Abstract: Metformin has been designated as one of the most crucial first-line therapeutic agents in the management of type 2 diabetes mellitus. Primarily being an antihyperglycemic agent, metformin also has a plethora of pleiotropic effects on various systems and processes. It acts majorly by activating AMPK (Adenosine Monophosphate-Activated Protein Kinase) in the cells and reducing glucose output from the liver. It also decreases advanced glycation end products and reactive oxygen species production in the endothelium apart from regulating the glucose and lipid metabolism in the cardiomyocytes, hence minimizing the cardiovascular risks. Its anticancer, antiproliferative and apoptosis-inducing effects on malignant cells might prove instrumental in the malignancy of organs like the breast, kidney, brain, ovary, lung, and endometrium. Preclinical studies have also shown some evidence of metformin’s neuroprotective role in Parkinson’s disease, Alzheimer’s disease, multiple sclerosis and Huntington’s disease. Metformin exerts its pleiotropic effects through varied pathways of intracellular signalling and exact mechanism in the majority of them remains yet to be clearly defined. This article has extensively reviewed the therapeutic benefits of metformin and the details of its mechanism for a molecule of boon in various conditions like diabetes, prediabetes, obesity, polycystic ovarian disease, metabolic derangement in HIV, various cancers and aging.
Keywords: metformin, diabetes, pleotropic effect, cancer, cardiovascular, neuroprotection, Prediabetes
Introduction
Metformin, as a drug, was suggested as an initial treatment for type 2 diabetes. Metformin has been used as a first-line therapy in diabetes but current guidelines suggest its use in people with a BMI between 25 and 59 kg/m2, higher fasting glucose (>110 mg/dL), higher A1C >6.0%, and patients with a propensity to cause gestational diabetes.1 Currently, as per the evidence, an SGLT2 (“sodium-glucose cotransporter 2”) inhibitor and/or GLP-1(“glucagon-like peptide 1”) receptor agonist with proven CV (cardiovascular) benefit should be used in people with type 2 diabetes with established ASCVD (“atherosclerotic cardiovascular disease”) or indicators of high CV risk, established renal disease, or heart failure.1 Metformin traces its origins to the traditional medicinal plant Galega officinalis, which was used to treat diabetes symptoms in Europe in the 18th century. It was rich in guanidine; later, many mono-guanidines (galegine) and diguanidines (synthalin) derivatives were developed.2 The toxicity profile of these medicines prevented them from achieving widespread recognition as antidiabetic medications, even though they considerably lowered the blood glucose level in animals.3 The three important members of the biguanide family were phenformin, buphormin, and metformin.3,4
In the 1950s, all three biguanides were researched in humans and approved for diabetic treatment in Europe.3,5 Due to the risk of lactic acidosis, phenformin and buphormin were removed from the market nearly 20 years after their introduction. Metformin users had a substantially reduced incidence of lactic acidosis. It occurred only in patients with compromised renal function, which itself is a contraindication for metformin use. Metformin was authorized by United States FDA (Food and Drug Administration) to treat diabetes in 1994, and it became commercially available in the following year.3 In 1998, the UKPDS (UK Prospective Diabetes Study) found that long-term metformin therapy is related to a lower risk of hypoglycemia, a reduction in cardiovascular events, and an improved survival rate.6 Metformin got included to the WHO’s essential medicines list in 2011.3,4 Currently, metformin enjoys the status of the most often utilized anti-diabetic drug, either alone or in combination, in patients with type 2 diabetes mellitus (T2D).7
Metformin is an oral antihyperglycemic agent that lowers both basal and post-prandial plasma glucose in T2D. In addition to increasing insulin sensitivity, it acts by decreasing hepatic glucose synthesis and intestinal glucose absorption. It differs from other groups of oral hypoglycemic agents as it does not result in hypoglycemia or hyperinsulinemia.8 In addition to diabetes, metformin is being investigated for use in weight loss, PCOS (polycystic ovary syndrome), malignancies, HIV, and even in COVID-19.9
It takes 1.5 hours for metformin to start working after being absorbed. The plasma half-life of metformin is 2–3 hours, and the active duration is about 6–10hrs. The typical metformin dose ranges from 250 to 2550 mg/day, with plasma levels varying between 5 μM and 20 μM in the therapeutic range for the treatment of T2D.10–13 Both half-life and duration of action depend on the type of formulations (immediate release or extended release) and vary accordingly. It is rapidly distributed and does not bind with plasma protein.9,14 Metformin clearance depends on renal function and declines in the presence of renal impairment. Metformin is not recommended if the serum creatinine level is ≥1.4 mg/dl in females and ≥1.5 mg/dl in males due to the rare but fatal lactic acidosis risk. The non-serious side impacts of metformin include nausea, metallic taste, anorexia, flatulence, and diarrhoea. Mostly such adverse events are temporary and wane off if dose is titrated gradually, or the drug is taken along with meal.14,15 Metformin use for an extended period is linked to a deficiency of vitamin B12. The cause of vitamin B12 insufficiency is complex and poorly understood, involving the altered vitamin B12’s enterohepatic circulation, decreased IF (“Intrinsic Factor”) secretion, bacterial overgrowth, and interference with the calcium-dependent coupling of IF-vitamin B12 complex to the “cubilin receptor”.16 The pharmacokinetic properties of metformin are summarized in Table 1.
 |
Table 1 Summary of the Pharmacokinetics Characteristics of Metformin |
Mechanism of Action- Based on its Potential Uses
Being a drug that can act on various organ system and have varied effects, the mechanism of action of metformin tends to be diverse and are based on the indication of the drug. This section elaborates on the mechanism of action of metformin under various potential uses (Figure 1).
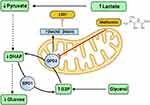 |
Figure 1 Actions of Metformin. LDH- Lactate dehydrogenase; DHAP- Dihydroxyacetone phosphate; NADH- Nicotinamide adenine dinucleotide hydrogen; NAD+- Nicotinamide adenine dinucleotide; GPD2- Glycerol 3 phosphate dehydrogenase 2; GPD1- Glycerol 3 phosphate dehydrogenase 1; G3P- Glycerol 3 phosphate. This figure was created using the premium version of BioRender (https://biorender.com/) with License No.: UJ25D0W3WI. Created with BioRender.com. |
Glucose Metabolism
Metformin is primarily recognized as a glucose-lowering agent and is established as a monotherapy and combination therapy with other “anti-diabetic” drugs as a first-line therapy conventionally.5,17 Metformin being a hydrophilic molecule has also been observed to have metal-binding properties, principally with copper.18 Metformin’s hydrophilic nature makes it arduous to cross the cell membrane and hence it depends on the membrane transporters like hENT4(”human equilibrative nucleoside transporter 4”), MATE (‘Multidrug and Toxin Extrusion Protein’), and SLC22A (Solute Carrier Family 22 members) for its uptake into the cell and secretion.19 SLC22A gene family is further subdivided into several subgroups and OCTs (‘Organic Cation Transporters’) like OCT1, OCT2, and OCT3 are also a part of it and are coded by genes namely SLC22A1, SLC22A2, and SLC22A3, respectively, which are clustered on chromosome 6q26-q27 and share a structure with 11 coding exons and 10 introns.12,20–23 These OCT transporters are responsible for the transport of drugs and other molecules across the drug absorption, metabolism, and excretion in the small intestine, liver, and kidney. Metformin has been found to be a substrate of OCT3 present in the brush border membrane of the enterocytes.24
Conventionally, it is quite established that metformin lowers blood glucose primarily by its action on the liver with a major effect by minimizing hepatic gluconeogenesis leading to curtailment in endogenous glucose production by the liver without a concomitant increase in plasma insulin concentrations.25,26 Literature reveals that gluconeogenesis is responsible for about 28–97% of total hepatic glucose output which in turn further depends on the feeding status in the case of normal persons and can be higher in patients with chronic T2D.27 In cases of T2D, which is a chronic condition, this mechanism can be crucial as enhanced and unregulated hepatic glucose release is a prominent pathophysiological mechanism, and controlling it can improve the prognosis of the disease to a large extent.10
The high expression of SLC22A1 in the liver and high concentration of metformin in portal circulation as compared to other parts of the body aids in its enhanced action on liver gluconeogenesis pathways.28 Evidence shows that metformin enhances the activity of IRS-2 (“Insulin Receptor Substrate 2”) in the cells and translocates the glucose transporters like Glucose transporter (GLUT)-1 to boost glucose uptake by the cells.29
At the cellular level, metformin was thought to primarily act on the mitochondrion and has an ephemeral inhibitory action on the complex I of the electron transport chain of mitochondria culminating to a dip in the energy level of the cell.30–34 Though recent evidence does not favor metformin’s effect on complex I because the concentration needed to inhibit it is way higher than what is observed in the clinical use of this drug.13,35 In support of this, a complex I inhibitor piericidin was pumped into rat livers via an indwelling portal venous catheter, and the procedure showed that piericidin does not suppress hepatic gluconeogenesis.36 This decline in the energy level which is directly correlated with diminished synthesis of ATP (“Adenosine Triphosphate”) and enhanced AMP (“Adenosine monophosphate”) levels could be the driving force for crucial processes in the liver such as blocking cAMP (“cyclic AMP”) generation induced by glucagon and the activating 5’- AMPK (“Adenosine Monophosphate-Activated Protein Kinase”).32,34 The same concept is also evident from the preclinical studies on isolated hepatocytes of rats where metformin was observed to reduce ATP concentration in the mitochondria. ATP is known to allosterically block the enzyme pyruvate kinase and hence a reduction in ATP concentration leads to enhanced pyruvate kinase activity and decreased glucose output.37,38
Metformin was also found to activate AMPK intracellularly which acts as a sensor of energy in the cell and it is observed that on activation of AMPK, the catabolic processes of the cells get triggered and lead to the generation of ATP whereas disabling the anabolic pathways using ATP for synthetic processes.34,39,40 Evidence shows AMPK is a crucial facilitator for complex intracellular molecular signaling pathways of metabolism and growth which act in an integrated manner to ensure a balance of cellular energy (Figure 2).34,40
 |
Figure 2 Beneficial effects of metformin. AMPK- adenosine monophosphate-activated protein kinase; SREBP-1C: sterol regulatory element-binding protein 1; PEPCK- Phosphoenolpyruvate carboxy kinase; G6Pase- glucose 6-phosphatase; GLUT4- Glucose transporter 4. This figure was created using the premium version of BioRender (https://biorender.com/) with License No.: MT250DY0DR. Created with BioRender.com. |
The decline in energy levels by diminished synthesis of ATP and resultant enhanced levels of AMP by increased activity of enzyme adenylate kinase is observed.33 Evidence shows that metformin can even elevate AMP levels by blocking the activity of an enzyme named AMP deaminase that breaks down AMP.40,41 Metformin, by elevating levels of intracellular AMP levels, might block adenylate cyclase, which facilitates the conversion of ATP to cAMP, hence reducing intracellular cAMP levels and ultimately reducing the signalling of glucagon, leading to reduced glucose levels.32,42–44 The above effect of reduced glucose output by gluconeogenesis through activation of AMPK is also supported by the fact that 5- AICAR (“aminoimidazole-4-carboxamide riboside”), which is an AMPK activating molecule has also shown the evident effect of diminished enzymes expression involved in gluconeogenesis, hence leading to decreased glucose output as seen in preclinical trials.45–47 The effect of metformin is also supported by a study done by Shaw et al on mice given a high-fat diet, where deletion of gene LKB1 (“Liver Kinase B1”), which is a crucial enzyme phosphorylating the catalytic α AMPK domain led to the deactivation of AMPK leading to elevated glucose levels, hence nullifying the glucose reducing metformin effect.48
These mechanisms have been thought to explain metformin’s action, but recent evidence reveals that the action of metformin can be otherwise. Schäfer et al reported that the binding affinity of metformin for mitochondrial membranes is modest and Wilcock et al observed that the distribution of metformin is majorly in the cytosol and only less than 10% enters mitochondria.13,49,50 Meng et al, in their study on mouse-isolated hepatocytes, reported that a low concentration of metformin (25–100 μM) activated AMPK, whereas a higher concentration (500 μM) inhibited it.51 Ravera et al studied the concentration-dependent metabolic effect of metformin and reported that low concentration (15–150 μM), which represents the clinical levels of the drug triggered oxidative phosphorylation, the oxidative stress response, and the AMPK/Sirt1 pathway, whereas 1.5 mM was hazardous.52 Metformin’s antihyperglycemic impact via the reduction of hepatic gluconeogenesis was first revealed by Zhou et al and they observed that it activated AMPK in rat hepatocytes at doses of 10 μM and 20 μM and provided a cellular basis for the drug’s blood glucose lowering action.34 Cao et al demonstrated that modest dosages of metformin decreases glucose synthesis in primary hepatocytes without altering levels of ATP or the AMP/ATP ratio.53 A study by Miller et al on hepatocytes demonstrated that a cessation of glucose production in primary hepatocytes was achieved at 125 μM, and it needed a higher concentration of 250 μM to considerably reduced cellular cAMP levels and much lower plasma levels are also efficient at reducing the synthesis of glucose by hepatocytes.32,53,54
To sum up, metformin has been found to diminish blood glucose by pathways relying on AMPK or independent of it.48,55–57 The AMPK-dependent pathways help in inhibiting gluconeogenesis by blocking the expression of genes of crucial enzymes like phosphoenolpyruvate carboxykinase, pyruvate carboxylase, and glucose 6 phosphatases that are responsible for gluconeogenesis.58,59 This activation of AMPK can lead to subduing of mTORC1 (“Mammalian Target of Rapamycin Complex I”), which too can block the gluconeogenetic pathways.60 The metformin-induced decreased gluconeogenesis from the liver by AMPK-independent processes including dampening the activity of glucagon, blocking crucial enzymes of gluconeogenesis like fructose-1,6-bisphosphatase-1 and glycerol-3-phosphate dehydrogenase and enhancing glucose uptake by increased GLUT1 activity and IRS2 hence leading to reduction in plasma glucose levels.29,61,62 Metformin also acts by varied peripheral mechanisms like enhanced peripheral glucose uptake in skeletal muscles by the rising activity of GLUT4, modulating glucose absorption from intestines by delaying the absorption and also stimulating the activity of glucagon-like-peptide-1.62–66 Evidence also shows that there is a disruption in intestinal microbiota in the patients of T2D and metformin was found to modulate the microbiota texture by decreasing the count of Bacteroides fragilis.67 This leads to elevated glycoursodexoycholic acid levels, which in turn depress the activity of intestinal farnesoid X receptor culminating in improved glucose tolerance.68
With a plethora of research for the mechanism of action of metformin, currently, the exact mechanism still stands controversial as the previous research studies conducted on preclinical models, in vitro and cell line models used a very high concentration (~milli-Moles) of metformin as compared to the therapeutic concentration (~micro-Moles) used in humans. The used concentration in the preclinical research was almost 10–100 times the threshold concentration achieved in patients with T2D.13,54 It is postulated that, at higher concentrations, metformin acts through the mitochondrial chain and may conceal the AMPK-dependent mechanism. The enzyme glycerol 3-phosphate dehydrogenase 2, which functions similarly to the mitochondrial enzyme in mammals, is also phosphorylated and negatively regulated by AMPK. This indicates that metformin’s direct impact on AMPK and a route that regulates the function of the mitochondrial glycerol 3-phosphate dehydrogenase may play a crucial role in the reduction of blood glucose when given in therapeutic concentration.54,69
Cardiovascular Benefits
Cardiovascular disorders are one of the prime causes of mortality and morbidity across the world and the probability enhances with the addition of risk factors like sedentary lifestyle diabetes, obesity, hyperlipidaemia, and hypertension.70 Increased blood sugar can lead to oxidative stress, dyslipidaemia, and endothelial dysfunction culminating in enhanced cardiovascular risk.62 Among the antidiabetic medications, metformin was observed to minimize the incidence of cardiovascular diseases and associated mortality.71–74 A recent systematic study and meta-analysis conducted on 701,843 participants of T2D with metformin and 1,160,254 controls observed a decreased risk of mortality [“OR=0.44 (0.34–0.57)”] or adverse cardiovascular events [“OR=0.73 (0.59–0.90)”] with metformin.75 Similar results were also reported by a meta analysis where metformin reduced all-cause and cardiovascular death in patients having coronary artery illnesses.76 The “MetCool ACS” trial has been initiated in patients without diabetes, to evaluate the effectiveness of metformin in acute coronary syndrome. The trial is ongoing and expected to be completed in 2025 and hence there is no concluding evidence regarding the protective effect of metformin in cardiovascular diseases.77
Metformin decreases the synthesis of AGE (“Advanced Glycation End”) product formation and hyperglycaemic-induced ROS (“Reactive Oxygen Species”) production in the endothelium and other places in the body, hence improving the cardiovascular prognosis.78 These AGE products modify the HDL residues, which leads to the formation of dysfunctional HDL unable to perform cholesterol efflux consequently increasing the cardiovascular risk.79 In diabetes, the sugar metabolite methylglyoxal could be responsible for increased oxidative stress, synthesis of AGE product with a simultaneous enhancement of inflammation, and a decline in NO (nitric oxide) availability in the endothelium.80 The anti-inflammatory, anti-oxidative, and protective activity against homocysteine-thiolactone are a few characteristics of HDL that can be attributed to the PON-1(paraoxonase-1) enzyme in HDL.79,81,82 Any modification in the apolipoprotein can lead to dysfunctional PON-1 activity, which can hamper the protective effects of HDL and can add up to complications in various diseases. There is evidence that metformin can instate endothelial functionality, minimize oxidative stress and improve glycation.78 Decrease NO availability in endothelium has been associated with cardiovascular risk. Few in-vitro studies of metformin have shown to increase NO production, but in vivo studies failed to confirm this finding.78,83 With the available evidence from the preclinical studies, the effect of metformin on NO release remains a grey area requiring further exploration in clinical studies in humans.83–85 Metformin by activating AMPK blocks the alpha-dicarbonyl-mediated alteration of the apolipoprotein component leading to the mitigation of HDL dysfunction and minimizing low-density lipoprotein-induced damage.86 Studies in in vitro settings have reported that metformin can modulate the interaction of HDL with efflux receptors and reduce the AGE formation leading to minimizing the effect on impaired cholesterol efflux capacity of HDL in cases of lipoprotein modification in oxidative stress or increased AGE products.87,88
There is also evidence that metformin can be beneficial in cases of heart failure by modulating the status of myocardial energy metabolic through its activation of AMPK and controlled regulation of lipid and glucose metabolism in cardiomyocytes.89 The exact mechanism is unknown but with the existing evidence, it is reported that metformin acts by elevating NO availability, restricting interstitial fibrosis and deposition of AGE products, and restraining apoptosis of cardiomyocytes leading to diminished cardiac remodeling, and these effects conserve the systolic/diastolic functions of left ventricle hence minimizing the propensity to cause heart failure.89–93 The benefit of metformin in heart failure has also been proven in randomized controlled trials and now might also be considered safe for its usage in stable chronic heart failure with ongoing monitoring of renal and cardiac function.15,92,94
Metformin and its Anticancer Effects
The disorder, recognized as cancer, results when certain body cells proliferate out of control as well as spread to other body parts. Multiple signals such as growth factors, nutrient availability, and the presence of intracellular ATP regulate cell growth and proliferation. In the presence of nutrients, the IGF1 (Insulin-like Growth Factor-1) signalling pathway gets activated which promotes glycogen, protein, and lipid synthesis in mammals.95 IGF1R (IGF1 receptor) contributes to neutrophil physiology and glucose metabolism and is related to the development and occurrence of cardiovascular illness, inflammation, and diabetes. Whereas when cells are starved for energy, the AMPK pathway gets activated, which is responsible for inhibiting various biosynthetic pathways such as fatty acid and protein synthesis, gluconeogenesis, cholesterol biosynthesis, catabolic processes promotion like fatty acid beta-oxidation and glycolysis.96,97 Evidence shows that patients with diabetes have been related to an enhanced risk of cancer.98 A cohort analysis in northern Italy reported that in patients with at least 2 years of diabetes, the overall occurrence of cancer was greater in T2D than in non-T2D (IRR = 1.22, 95% CI 1.15–1.29).99 Literature shows that mostly T2D has been found to be linked with elevated risk for various cancers like the pancreas, liver, breast, colorectal, endometrium, and bladder.100 Similar results have been reported by Bjornsdottir et al, who carried out a national observational study on individuals with diabetes between 1998 and 2014 and observed that patients with T2D have a greater risk for various cancers, HR = 1.10 (95% CI 1.09–1.12).101 This rise in the risk of cancer, however, cannot be entirely attributed to T2D but could also be possible owing to other risk factors like weight, diet, physical activity, and smoking/alcohol intake.100 Obesity itself might be a solitary risk factor for cancer and evidence reveals that the adipose tissue in our body also releases molecules like IL-6 (“Interleukin-6”), tumor necrosis factor-α, leptin, adiponectin, monocyte chemoattractant protein, and PAI-1 (“Plasminogen Activator Inhibitor-1”).102 IL-6 can activate the STAT (“Signal Transducer and Activator of Transcription”) protein signalling pathway and can lead to raised survival, invasion, and cell proliferation in carcinoma cells while also guarding them against host immunity.103
There is growing evidence that metformin has anti-cancer effects based on clinical and preclinical studies. A meta-analysis conducted on 17 studies observed a significant reduction in cancer risk with the use of metformin in patients with diabetes or without diabetes as compared to patients, not on metformin.104 A similar study published in 24 articles also reported metformin use can decrease the risk of cancer as compared to other anti-diabetic medications.105 An analysis done on eight cohort studies concluded that metformin was observed to be related to reduced cancer incidence as compared to sulfonylurea monotherapy in T2D patients.106 A meta-analysis of lung cancer studies also observed similar results which reported that metformin use might decrease the risk of lung cancer within T2D patients as compared to other conventional agents.107 Evidence from the preclinical studies shows that metformin was presented to cell’s restrain proliferation and lead to partial cell cycle arrest in cell line culture of cancer cells.108–110 Several studies on cancer cell lines have observed that metformin treatment leads to inhibition of development and proliferation and induces apoptosis of the cancer cells of organs like the breast, kidney, brain, ovary, lung, and endometrium.108–116
The mechanism behind the anticancer or growth-prohibiting effect of metformin in cancer cells can be ascribed to AMPK activation leading to different downstream impacts that work together to restrain the growth of tumors.114,117 The AMPK-mediated downstream pathways for its anticancer activity majorly act through mTOR (“Mammalian Target of Rapamycin”) inhibition and modulation of other molecules like cyclin D1, p53, p21, p27 and Akt leading to antiproliferative effects.104,114,117,118 Metformin was found to block the cell cycle in the “G(0)/G(1)” phase and this was observed with a sharp drop in the cyclin D1 levels, pRb phosphorylation, and elevated p27(kip) expression.112,113,118 AMPK activation causes phosphorylation of distinctive sites on TSC (Tuberous sclerosis complex)-2 as well as inhibits the mTOR pathway that is activated by insulin. It is also observed that there is a direct correlation between insulin and energy signalling in which TSC2 have a significant role in the integration of insulin and energy signalling to control cell growth and survival. In cardiac muscle cells, insulin activates Akt and thereby antagonizing the AMPK activation which further leads to the phosphorylation of AMPKα.119 In normal cell growth, IGF signaling through IGF-1R plays an important role, but it also acts as a mediator for the malignant phenotype. IGF-1R activation with their two natural ligands, such as IGF-1 and IGF-2 acts as primary risk factors for the growth of cancer.120 The binding of an IGF1 receptor ligand results in the autophosphorylation and subsequent phosphorylation of either IRS-1 (“Insulin Receptor Substrate-1”) or IRS-2 (“Insulin Receptor Substrate-2”). Both insulin receptors and IGF-1R stimulate the activation of such adapter proteins. The IRS-1/2 proteins operate as scaffolding to activate other “intermediate signalling proteins”, including MAPK/Raf/Ras and mTOR17/AKT/PI3K which are widely documented in several breast cancer subtypes.121 Overexpression of IGF1R is observed in breast cancer and promotes tumor progression by enhancing the glycolysis and production of biomass while decreasing tumor sensitivity to a minimal-glucose environment.122 The IGF1R signal promotes non-cancerous cells to transform malignantly and possesses anti-apoptotic and mitogenic activity. IGF1R also contributes to the invasion, metastasis, and angiogenesis of cancer.123,124 mTOR pathway is regulated by complexes mTORC1 and mTORC2 which comprise a catalytic subunit mTOR. Both such complexes are critical for cellular development and growth. mTORC1 signals are mediated by several pathways like IGF1, IGF2, insulin through AMPK.125,126 Downregulation of mTOR is responsible for enhanced cell growth and proliferation and the signalling pathway controlling its activity is downregulated in breast cancer. In breast cancer activating PIK3CA mutations or expression, loss of the tumor suppressor PTEN is the most common mutation which leads to uncontrolled mTOR activity.127 It is also observed that suppressing the expression of PKM2 (“Pyruvate Kinase M2”) deactivates the AKT and/or mTOR signalling pathway, thereby decreasing the SREBP-1c expression which inhibits the generation of fatty acid by inhibiting the FASN gene transcription, resulting in the inhibited tumor cell growth.128 Metformin is also responsible for inducing caspase-dependent apoptosis along with c- JNK (“Jun N-Terminal Kinase”) activation, oxidative stress and mitochondrial depolarization.113 Apart from these, metformin also blocks mitochondrial complex I, hence leading to minimizing the synthesis of ROS ultimately leading to suppression of ROS-mediated DNA damage and the development of cancers.129 Metformin has also proven beneficial in patients with diabetes receiving neoadjuvant chemotherapy. The patients who received metformin along with the chemotherapy had better pathologic responses as compared to the group without metformin.130 With this varied evidence, metformin has shown its antiproliferative activity in the cancer cell lines which can prove instrumental in cancer management but the concentration at which these effects are demonstrated are quite high as compared to the therapeutic levels in plasma and can prove toxic for humans so studies with improved protocols with lower concentration need to be tested in future to establish its effective anticancer property in clinical subjects.
Neuroprotective Role
Taking a further step to metformin’s pleiotropic effect it is observed that the conventional antidiabetic drug can also retard aging by modulating mitochondrial metabolism and signalling insulin.131 Evidence shows that metformin crosses the blood-brain barrier to enter the brain and confers diverse neuroprotective effects which can prove beneficial in cognitive impairment, HD (Huntington’s disease), stroke, and probably dementia.132–137
In PD (Parkinson’s disease), α-synuclein accumulation is considered as one of the features of neurotoxicity.138–140 In the preclinical studies, metformin was observed to decrease the expression of α-synuclein and the total of α-synuclein positive cells.141 In the C. elegans model of PD, metformin treatment has been found to reduce the α-synuclein accumulation and diminish the loss of dopaminergic neurons.142 In a similar model of rotenone-treated mice, metformin has been found to safeguard rotenone-induced dopaminergic neurons from degeneration by minimizing lipid peroxidation.143 The phosphorylation of alpha-synuclein is reduced by enhanced activity of phosphatases by metformin as observed in the substantia nigra of the MPTP-mice model.144 Based on the above evidence, metformin with its neuroprotective nature can act as a disease-modifying in PD.
Insulin resistance and a high-fat diet have been seen to promote amyloidosis and memory impairment in different animal models of AD (“Alzheimer’s Disease”).145,146 Preclinical evidence shows that rats with diabetes have been observed to have elevated levels of APP (amyloid precursor protein), Aβ, and phosphorylated tau.147 Energy metabolism is crucial in maintaining sound neural health and it has been observed that any molecule or process that improves calorie management and decreases chances of hyperglycaemia like glucagon-like peptide-1, insulin-like GF1, and even caloric restriction has been observed to have neuroprotective effects.148,149
Literature shows that altered metabolism in neural cells through aberrant insulin signalling could be a reason for the synthesis of Aβ protein and phosphorylated tau, which are two hallmark biochemical abnormalities linked with AD and proper regulation of insulin signalling has been observed to be a crucial approach in neuroprotection and guarding the synapses against noxious Aβ deposition hence improving cognition in animal models of AD.150,151 There is varied evidence on the effect of metformin on dementia in rodent models of dementia. Few studies found there is improved cognition and memory with metformin.152–155 However, there were some studies that reported otherwise.156,157 In clinical studies, metformin has been found to significantly minimize the risk of cognitive impairment in T2D.135,158
Preclinical studies conducted on nematodes have reported that metformin treatment showed amelioration in neuronal function along with enhanced touch sensitivity in models of HD through activation of AMPK and the neuroprotective effect was abolished on blocking the effect of the gene AMPKα1 (Figure 3).137 The activation of AMPK has been found to exert substantial neuroprotection if activated in early progressive phases of HD and the same was found in a clinical trial where patients with HD taking metformin had better cognition and were able to perform the tasks more easily as compared to them not taking metformin.159,160
 |
Figure 3 Neuroprotective role of metformin. AMPK- adenosine monophosphate-activated protein kinase; NOX- nicotinamide adenine dinucleotide oxidase; ROX- reactive oxygen species; PP2A- protein phosphatase 2A; BDNF- brain derived neurotrophic factor; TrkB- tyrosine kinase receptor B; PI3K- phosphatidylinositol 3 kinase; Akt- protein kinase B; GTP = guanosine triphosphate. This figure was created using the premium version of BioRender (https://biorender.com/) with License No.: GP250DJG7W. Created with BioRender.com. |
In preclinical models of MS (multiple sclerosis), metformin treatment has been observed to rise the expression of the mitofusin-2 gene, which is responsible for mitochondrial fusion. Apart from that, an increased concentration of oligodendrocytes improved the myelination of neurons along with decreased astrocytes and microglia hence leading to reduced gliosis.161 Metformin has been found to activate AMPK in a mouse model of MS, leading to decreased inflammatory and decreased demyelination lesions.162
Therapeutic Uses of Metformin
Metformin and Diabetes
Metformin gained the status of “foundation therapy” in T2D patients whose glycemic target is not achieved despite diet and other lifestyle interventions. The reason behind this glory is its effective glycemic control, weight neutrality, wide security margin, and low cost.163 In addition to the mentioned benefits, metformin also provides modest cardioprotection and improvement in various lipids and inflammatory marker profiles. Metformin’s mechanism of action is complex and is yet to be completely understood. Metformin primarily acts by reducing glucose production by inhibiting AMPK-dependent gluconeogenic enzymes in the liver, yet not all its impacts are described by its mechanism.164 It has been found that metformin also acts on the gut and increases glucose utilization and GLP-1 production in addition to alteration in the microbiome population.163–165
Metformin alone and in combination with other “glucose-lowering” agents reduce the blood glucose level effectively in T2D.166,167 It was found that combinations of metformin with all noninsulin diabetic drugs result in a similar reduction of HbA1c but with changing weight gain degrees and hypoglycemia risk. In addition to glucose control, metformin is also helpful in diabetes-related comorbidities. Diabetes has been regarded as a risk factor for coronary artery illness. UKPDS (“UK Prospective Diabetes Study”), a large randomized clinical study in the newly diagnosed T2D population found significant as well as persistent risk reduction in myocardial infarction (33%, p = 0.005), death from any cause (27%, p = 0.002) and diabetes-related endpoint (21%, p = 0.01) in metformin-treated individuals.168 Obesity and diabetes are interrelated. Metformin has a neutral influence on body weight in patients with T2D. It is associated with a reduction in weight gain when compared with sulfonylureas, thiazolidinediones, and insulin. Metformin contributes to weight loss by reducing intestinal carbohydrate absorption and insulin resistance in addition to reducing ghrelin and leptin levels.163,165 Different forms of cancers like rectum, pancreas, liver, and colon are observed to have a high incidence in T2D. The high occurrence of tumorigenesis is related to hyperinsulinemia, insulin resistance, elevated level of IGF-1, and hyperglycemia. Metformin being an insulin sensitizer reduces insulin resistance and hyperinsulinemia. AMPK activation, mTOR pathway inhibition; angiogenesis, and inflammation are the other suggestive mechanisms of metformin’s action.163,164 The usual starting metformin dose is 500mg every day with an evening meal and if required can add an additional 500 mg dose with breakfast. The dose of metformin is titrated gradually and can reach a maximum dose of 2000 mg per day; however, the dose of metformin can vary depending on the other factors including body mass index.169,170 The role of metformin in pregnant females with diabetes is controversial. A meta-analysis conducted on observational studies did not reveal a rise in neonatal deaths or congenital malformations in women taking metformin alone or in a mixture with sulfonylureas while MiG (metformin in gestational) trials suggest more incidence of preterm birth and less weight gain in women taking metformin as compared to those who were on insulin.171–173 After the MiG trial, metformin’s utility has increased in pregnancy. Metformin easily crosses the placenta and maintains a concentration in the blood of the foetus that is nearly half to as concentrated as it is in the mother’s plasma.174 There are worries about the use of metformin during pregnancy since it has anti-cell growth and pro-apoptotic effects that could harm the developing baby. Therefore, more human studies are needed to assess the safety of metformin in pregnancy.174
Recently, metformin has been hypothesized to be used as an adjunct treatment in type 1 diabetes to limit the insulin dose and to prevent the long-term difficulties of diabetes including weight gain, atherosclerotic progression, as well as elevated cholesterol levels of LDL.175 REMOVAL (“Reducing with Metformin Vascular Adverse Lesions”) trial which is one of the longest and largest trials of metformin treatment within type 1 diabetes patients reported a small but sustained reduction in LDL cholesterol and weight in middle-aged adults over 3 years. The trial also suggested the role of metformin in cardiovascular risk reduction instead of sustained glucose-reducing effect in type 1 diabetes.176 However, metformin may minimize weight gain, and somewhat improve cholesterol levels but does not lower HbA1c levels in type 1 diabetes.177–179 Yet again, this comes with a higher risk of unfavorable gastrointestinal side effects and vitamin B12 shortage. Given the ambiguity around the potential long-term advantages, it is thought that metformin has very little part to play in the treatment of type 1 diabetes.180
The most common side effect which is encountered after metformin therapy is GI disturbance including nausea, diarrhoea, and abdominal discomfort.8 These side effects are mild, and transient and occur due to the accumulation of the drug in the enterocytes. A much uncommon but more concerning side effect of biguanide treatment is lactic acidosis, which occurs due to the interference of biguanides in mitochondrial respiration and promotes anaerobic respiration and lactate generation. The incidence of lactic acidosis is extremely low in metformin therapy with a projected occurrence of 3 to 10/100,000 person-years and occurs only in individuals with advanced chronic kidney disease.181,182 Metformin may also cause vitamin B12 malabsorption; hence, it is advised that patients on long-term metformin treatment undergo routine testing of their vitamin B12 levels. Patients with renal failure, diabetic ketoacidosis, and acute illnesses that may compromise renal function should not use metformin.163,165
Metformin in Impaired Glucose Tolerance/Prediabetes
The drug for the initial therapy of T2D is metformin. In prediabetes, it was also used to delay or prevent diabetes onset.183 Recent increase in the prevalence of prediabetes emphasizes the significance of prediabetes awareness and efforts to reduce or delay the onset of T2D. The best strategy to control the risk of diabetes in prediabetes is lifestyle management followed by pharmacological interventions. The available pharmacological options include metformin, alpha-glucosidase inhibitors, thiazolidinediones, and GLP-1 agonists. However, compared to other anti-diabetic medications, metformin offers fair evidence of safety and efficacy and becomes the preferred pharmacological option for diabetes prevention.184–186 The DPP (Diabetes prevention program) trial randomized 3234 prediabetes into 3 groups, including lifestyle intervention, metformin 850mg twice daily, and placebo. After an average follow-up of 2.8 years, the incidence of diabetes dropped by 31% in the “metformin group” and by 58% in the lifestyle modification group compared to the placebo group.187 Metformin was proven to be as beneficial as a lifestyle adjustment in individuals with a BMI (“Body Mass Index”) ≥35 kg/m2 or age less than sixty years of age. In the same trial after 15-year follow-up, the mean annual occurrence of diabetes was 27% lower in patients assigned to lifestyle intervention and 18% lower in those randomized to metformin compared to placebo.187 In 2022, the ADA (American Diabetes Association) recommended metformin therapy for adults with prediabetes, specifically those between the ages of 25 and 59 with a BMI of ≥35 kg/m2, greater A1C (≥6.0%), higher fasting plasma glucose (≥110 mg/dL), and in females who have had gestational diabetes mellitus in the past. Long-term metformin use is related to a deficiency of vitamin B12; hence, the group has also proposed periodic vitamin B12 levels measurement in individuals on metformin. Despite recommendations by the ADA, metformin still has not been approved by US FDA for prediabetes patients to prevent or delay the risk of diabetes.185 Recent evidence from clinical trials suggests that SGLT2 inhibitors can be considered as the first-line therapy for the prevention of T2D.188,189
Metformin and Weight Loss
Obesity and diabetes have been found to be strongly correlated.190 Being an antidiabetic drug, metformin is also explored for weight reduction in obese patients with diabetes and without diabetes individuals. The postulated mechanism by which metformin acts as a weight-reducing agent is reducing food intake and increasing insulin sensitivity through tissue-specific AMPK action. In addition, metformin also increases GLP-1, a hormone secreted from the gut, and reduces the circulating blood glucose level by decreasing appetite and carbohydrate absorption.190–192 A study was carried out on 154 non-diabetes obese participants who had normal glucose levels irrespective of insulin sensitivity. An average dose of 2230 mg/day of metformin was given. The overall weight loss within the metformin-treated group was 5.8±7kg. The weight reduction was highly substantial as compared to the control group (p < 0.0001) who experienced an average weight gain of 0.8±3.5kg. The study also found that weight loss due to metformin was more significant in severely insulin-resistant patients as compared to insulin-sensitive participants.193
Metformin was also compared with other oral hypoglycemic agents for weight loss in patients with T2D. Kahn et al conducted a study in 4360 patients with diabetes over four years and found that oral hypoglycaemic agent rosiglitazone and glyburide cause weight gain of 4.8 and 1.6 kg respectively over a period, while metformin causes weight loss of 2.7 kg.194 However, a meta analysis by Pinto et al showed SGLT2i were better than metformin, sulphonylureas, and DPP4 inhibitors for reduction of weight (−1.04 kg, −4.76 kg and - 2.45 kg, respectively) in patients with diabetes.195 Another study by Gao et al showed GLP-1 receptor agonists and metformin decreased body weight by around 3.4±3.0 and 1.9±2.9 kg, respectively, in patients without diabetes.196 Another long-term follow-up was carried out by the DPP research group to evaluate the efficiency and sustainability of metformin in obese patients with diabetes for weight loss. The analysis has been conducted over 10 years and consists of 2.8 years of blinded phase and 7 years of unblinded or open phase. In both phases, weight loss was greater in the metformin group as compared to the placebo group and directly related to adherence to metformin therapy (blinded Phase 2.06 ±5.65 vs 0.02 ±5.52% p < 0.001; unblinded phase 2.0 vs 0.2% p < 0.001).197 A recent meta-analysis tried to summarize the metformin impact on obese patients of different populations. A total of 21 trials including 1004 patients were studied. BMI was utilized as an outcome measure to determine the impact of metformin on weight loss. The study found that metformin causes a modest drop in BMI (WMD=−0.98; −1.25 to −0.72) among study participants. The decrease in BMI in obese participants with or without diabetes was 1.1 units and 1.3 units, respectively. Results in both categories were statistically significant. Since a single unit drop in BMI (body mass index) is not sufficient to cause appreciable weight loss, the authors proposed larger clinical trials to evaluate metformin’s efficacy as a “weight loss medicine”.198 However, when compared with other drugs used in diabetes, molecules like GLP-1 analogues have superior efficacy in reducing weight as compared to metformin.196,199
Metformin and Polycystic Ovarian Syndrome (PCOS)
PCOS is a combined disorder of the metabolic and reproductive systems which is diagnosed when at least two of the following features are met: polycystic ovary on ultrasound, anovulation/ovulation, and hyperandrogenism. The pathogenesis of PCOS is mostly driven by insulin resistance; hence, insulin-sensitizing drugs like metformin have been attempted for the treatment of PCOS.200,201 Metformin has been studied as an ovulation-inducing drug in multiple clinical trials; however, the results have been contradictory.202 A meta-analysis has been conducted to examine the efficiency of metformin as an ovulation-inducing drug in women with PCOS. The evidence indicates that the metformin group has a greater ovulation rate than the placebo group (OR = 2.64; 95% CI = 1.85–3.75; I2 = 61%). The same meta-analysis compared metformin to other ovulation-inducing drugs, including CC (clomiphene citrate). When metformin was compared with CC alone, it was found that CC slightly increase the ovulation rate as compared to metformin (OR 0.45, 95% CI: 0.34 to 0.60; I2= 53%; 7 studies, 852 women). Subgroup analysis suggests that the obese women had lower pregnancy rates (OR 0.34, 95% CI: 0.21–0.55; I2=0%; 2 studies, 500 women), birth rates (OR 0.30, 95% CI: 0.17–0.52; 2 studies, 500 women) as well as ovulation rates (OR 0.29, 95% CI 0.20 to 0.43; I2 = 0%; 2 studies, 500 women) with metformin. Non-obese women show potential benefits in pregnancy (OR 1.56, 95% CI: 1.06–2.29; I2=26%; 6 studies, 530 women) and birth rates (OR 1.71, 95% CI: 1.00–2.94; I2=78%, 3 studies, 241 women) with no clear variation in the ovulation rate (OR 0.80, 95% CI 0.52–1.25; I2=0%; 5 studies, 352 women).203 In addition, metformin in combination with CC was compared to CC alone. When compared to CC alone, the combined group has a greater rate of pregnancy (OR = 1.62, 95% CI: 1.32–1.99; I2=31%; 19 studies, 1790 women) and ovulation (OR = 1.65, 95% CI: 1.35–2.03; I2=63% 21 studies, 1568 women).203 Metformin also decreased testosterone levels in PCOS patients. The effect is more evident among non-obese women. Metformin is believed to reduce testosterone levels by reducing hyperinsulinemia; however, studies suggest additional mechanisms, such as its direct inhibition of ovarian steroidogenesis. Even though metformin reduces testosterone levels, its testosterone-lowering effect is not consistently related to improvement in clinical symptoms of hyperandrogenism and menstrual abnormalities; hence, metformin is not considered a first-line treatment for hyperandrogenism in PCOS.204
Metformin and Metabolic Abnormalities of HIV
Traditionally, it was assumed that metabolic complications of HIV are caused by ART (antiretroviral therapy); however, further clinical studies suggested that host factors and direct viral effects are also linked with metabolic complications. Lipodystrophy, dyslipidemia, altered glucose metabolism, and decreased bone mineral density are frequent metabolic complications of HIV.205,206 HIV lipodystrophy is related to insulin resistance which rises the risk of cardiovascular disease in HIV-infected individuals. Metformin as an insulin-sensitizing agent is helpful in HIV-related metabolic abnormalities. Hadigan et al conducted a 3-month randomized clinical study to examine the safety and effectiveness of metformin in HIV lipodystrophy syndrome in 26 HIV-infected individuals without diabetes with fat redistribution as well as impaired glucose homeostasis.207 The study demonstrated that metformin significantly decreased insulin levels as well as AUC at 120 mins post-OGTT compared to placebo (p = 0.01). Metformin also reduces the fasting insulin level by 14%, while it remained the same in the placebo group. Patients treated using metformin displayed significant weight loss (p = 0.05), decreased waist circumference (p = 0.02), and reduced “diastolic pressure” (p = 0.009) than in the control group. In the same analysis, it was also observed that metformin treatment was linked with a drop in VAT: Visceral Abdominal Fat (−1115 [819] versus 1191 [699] mm2; p = 0.08) and a corresponding drop in SAT (“Subcutaneous Abdominal Fat”); however, the ratio of VAT-SAT remained unaltered in metformin versus placebo-treated patients. No significant adverse drug reaction was reported in the study.207 Kohli et al also conducted a study to determine the influence of metformin on HIV lipodystrophy. In univariate and linear regression analyses (p = 0.58), there was no significant variation in VAT between the metformin and placebo groups over 24 months. These results contradict those of the preceding study. The study observed no significant variation between the metformin and placebo groups regarding lipid profile or insulin sensitivity.208 A meta-analysis was conducted to determine the metformin impact on BMI as well as metabolic parameters in HIV-positive patients without diabetes. The results concluded that metformin did not substantially alter the BMI index in comparison to placebo (p = 0.46), no treatment (p = 0.32), lifestyle modifications (p = 0.06), and rosiglitazone (p = 0.14). In terms of LDL values, there were no statistically significant differences between the metformin and placebo groups. However, there was a substantial alteration in the levels of LDL between the metformin and rosiglitazone groups. Results were exactly the opposite for HDL levels. Studies comparing the metformin and placebo groups regarding triglyceride characteristics revealed a statistically substantial difference (p < 0.00001) between the 2 groups. The effect of metformin on fasting insulin was insignificant, but the mean values of insulin at 120 minutes were considerably lower within the “metformin group” than in the “placebo group”.209
Role of Metformin in Cancer
A biguanide known as metformin is a commonly recommended oral medicine for T2D. Population studies indicate that metformin reduces the risk of developing cancer and death from cancer in diabetes people.210,211
Metformin in Breast Cancer
In breast cancer cells, metformin inhibits the activation of mTOR via phosphorylating the inhibitory Ser789 site of IRS-1 through the activation of AMPK. Metformin is also involved in the repression of IGF-1R and IR promoters. IGF1R and MRP1 (“Multidrug Resistance-Associated Protein 1”) co-expression is linked to a poorer response to anticancer drugs.130 It is also observed that the lack of the tumor-suppressor kinase LKB1 makes the tumor cells even more resistant to treatment with anticancer agents. The energy-sensing enzyme AMPK has a crucial upstream kinase called LKB1.212 Metformin suppresses the mTOR via activating AMPK through an LKB1-dependent mechanism, which significantly reduces the rate of cell proliferation in many cancer cell lines.213 In hyperglycemic circumstances, metformin (1–5 mM) is shown to result in a 2-fold rise in “AMPK phosphorylation” at the T172 (Threonine 172) phosphorylation site, which is a proximate indicator for AMPK activation. Metformin also inhibits Raptor by raising its phosphorylation on the Ser792 site, which works as a scaffold to attract downstream substrates like “p70S6K” to the “mTORC1” complex through the TOR signalling motif.214,215 The effectiveness of metformin as a preventive measure in breast cancer-prone women is now being examined in many trials. The impact of metformin on obese or overweight individuals at increased risk of breast cancer depending on family history or past history of breast atypical hyperplasia is being studied in a randomized clinical trial (NCT01793948). Patients with atypical hyperplasia history, family history of breast cancer, carcinoma in situ, or high Gail Model Risk are included in another ongoing study (NCT01905046).216 Given that hormonal chemoprevention in BRCA1 mutation carriers has been debatable, metformin’s capacity to reduce the metabolic rewiring and tumor-initiating potential of BRCA1 haplo insufficient breast “epithelial cells” in vitro may point to new directions for evaluating metformin-based chemoprevention methods in mutation carriers of BRCA1.
Metformin in Endometrial Cancer
Genes involved in glycolysis and lipogenesis are more abundant in tumor-derived endometrium than in normal endometrium. Several human endometrial cancer cells have markedly increased GLUT6 glucose transporter and AKT activation as compared to nonmalignant cells.217 A pleiotropic metabolic inhibitor like metformin causes cytotoxicity in endometrial cancer cell cultures, as is clear from earlier studies. Metformin prevents the growth of both type I & II human “endometrial cancer” cell lines in vitro by preventing the entrance of serum-induced cells into the S phase.116 This arrests the G1 cell cycle. In each of these cell lines, it also reduces the expression of hTERT mRNA. Additionally, metformin causes the mTOR pathway to be inhibited since it activates AMPK, which is its direct downstream target, and reduces the phosphorylation of S6 and eIF-4E-binding proteins.218 High doses (5 or 10 mM) of metformin cause p53-deficient endometrial cancer cells to undergo apoptosis.219 Most endometrial tumors were discovered to contain telomerase, which is made up of the reverse transcriptase-active catalytic protein hTERT as well as the RNA template (hTR). In cancer cell lines, metformin reduces hTERT expression.220 The impact of metformin exposure on the emergence of endometrial cancer has drawn the attention of several researchers. According to several epidemiologic studies that have been analyzed by various organizations, metformin users had a decreased overall cancer incidence.221–226 Notably, a significant study of 478,921 Taiwanese women with diabetes found that those who took metformin had a significantly reduced risk of endometrial cancer than those who did not (HR: 0.675, 95% CI: 0.614 to 0.742).227 The decline in incidence showed a dose-response impact whether stratified by use frequency or cumulative doses. Furthermore, Tang et al's meta-analysis revealed that taking metformin was linked to a lower risk of developing endometrial cancer (RR: 0.87, 95% CI: 0.80 to 0.95).228 In addition, individuals using metformin had increased recurrence-free survival, according to Ko et al's research.229 On the other hand, a few studies did not discover any impact of “metformin” exposure on survival metrics.230,231 Others have only documented impacts on certain patient categories. For instance, Nevadunsky et al observed that metformin users had higher survival rates only among patients with “non-endometrioid” endometrial cancer and not “endometrioid endometrial” cancer.232 Only endometrioid endometrial tumors had a substantially decreased recurrence rate among metformin users, according to Hall et al233 These studies’ heterogeneity and sample size limitations are the same as those that apply to incidence research. Nevertheless, a meta-analysis of 2017 including six of the aforementioned analyses suggests that metformin users with endometrial cancer had a greater survival rate than non-users as well as patients without diabetes (HR: 0.82, 95% CI 0.70 to 0.95, I2=40%).234 The use of metformin was linked to a reduction in all-cause death in patients with concomitant diabetes for many cancer forms, including endometrial cancer (RR: 0.49, 95% CI 0.32, 0.73, p < 0.001), according to a meta-analysis of twenty-eight studies.235
Metformin in ALL (Acute Lymphoblastic Leukemia)
ALL is the most frequent type of cancer in adolescents and children, and it is distinguished by uncontrolled use of the normal systems that govern proliferation, differentiation, and apoptosis inhibition.236 Metformin promotes metabolic stress through the AMPK pathway, inhibiting many metabolic processes including the mitochondrial respiratory chain and the NF-KB (“Nuclear Factor Kappa B”) signaling pathway that is in charge of triggering the overexpression of ABCB1 genes. The ABCB1 gene was overexpressed in the majority of ALL patients.237 The mTOR kinase is a key sensor of energy and nutrition availability, and catabolic (ATP-producing) and coordinates anabolic (ATP-consuming) pathways to adapt “cell metabolism” to changes in the food supply, oxygen concentration, or energetic stress. The PI3K/Akt/mTOR pathway’s abnormal activation is one crucial characteristic of acute leukemias.238 Tumor suppressor PTEN and lipid phosphatase often limit the activation of this pathway. PTEN seems to have a role in HSC self-renewal, and animals with its loss develop leukemia.239 At the molecular level, metformin inhibits p70S6K and 4EBP1 phosphorylation, which activates AMPK and suppresses mTOR activation. Metformin prevents the recruitment of carcinogenic mRNAs to polysomes as a result, interfering with the start of translation and lowering cellular levels of cyclin D1, c-myc, as well as Bcl-xL, which are essential for cancer growth.240 Similar to this, metformin stimulates AMPK in T-ALL cells, inhibiting mTOR and causing the autophagic response, which comes before apoptosis. Metformin also altered protein synthesis in these cells, resulting in a drop in c-myc and Bcl-xL levels. Even apoptotic activity of metformin inside T-ALL requires an AMPK-dependent stimulation of ER /UPR (“Unfolded Protein Response”) cell death pathway as well as apoptotic mediators CHOP & IRE1a.241 Numerous studies support the use of metformin and its derivatives, such as phenformin, as universal adjuvants for traditional anti-leukemic medications. Increasing the potential of this intriguing anti-metabolic drug may need chemical alterations or the use of nanocarriers to facilitate metformin entrance and access its cellular targets.
Metformin in Osteosarcoma
The most prevalent primary bone tumor in children and adolescents is OS (“Osteosarcoma”), which is defined by the local invasion of bone and soft tissues and by the relatively poor response of these tissues to chemotherapy and radiation.242 Drugs that target the PI3K/Akt pathway may be utilized to treat OS invasion and migration since PI3K (“Phosphatidylinositol 3-Kinase”) and Akt are abnormally active in OS.243 There is evidence that supports that the EMT (“Epithelial-Mesenchymal Transition”) serves a significant role in metastasis initiation. EMT contributes to the invasion and metastasis of cancer cells. The expression of EMT-related markers is regulated by many transcription factors, such as Zeb, Twist, and Snail. Several substances, such as transforming growth factor-beta, epidermal growth factor, Akt, as well as extracellular-regulated kinases, may cause EMT. As a result of adversely influencing EMT, metformin displays lower levels of the mesenchymal marker vimentin as well as elevated levels of epithelial marker E-cadherin which inhibits the OS cell’s metastasis.244 It seems that a key factor in the pathogenesis of OS is the abnormal activation of several signalling pathways, like the PTEN/Akt pathway. Additionally, it has been shown that the biological processes of tumour cells, like proliferation, apoptosis, and metastasis, are significantly regulated by Akt. PTEN is a regulator that prevents the activation of Akt. Treatment with metformin upregulates the expression level of PTEN while it downregulates phosphorylation of Akt at Ser473 and might inhibit the proliferation, invasion, and migration of OS cells.245 Metformin is a possible candidate to be used in conjunction with other chemotherapeutic drugs to treat OS since it exhibits anti-metastatic as well as anti-proliferative actions on OS cells.
Metformin in Colorectal Cancer (CRC)
After lung cancer and breast cancer, the second leading cause of cancer-related death is CRC, which is the third most common malignant neoplasm.246 INHBA (Inhibin βA), which is often a TGF-superfamily member and is formed by two INHBA subunits, activates the Smad2 and/or Smad3 signal pathway via binding to threonine/serine kinase receptors. The incidence and development of CRC were linked to INHBA expression levels.247 The INHBA’s mRNA expression was consistently greater in tumor tissues and the level of INHBA is much higher in advanced-stage tumor tissue than those for the early stage, indicating INHBA as the most likely target “gene for metformin” for its antitumor function. Metformin downregulates mRNA and protein INHBA expression within CRC cells, reducing the secretion of activin A. Downregulation of INHBA by metformin mainly reduces the tumor “cell proliferation” causing G1/S arrest.248,249 We discovered that INHBA could be involved in the stimulation of the TGF-β signalling pathway by GSEA (“Gene Set Enrichment Analysis”). EGF expression and activated “PI3K/AKT” signalling via EGF are transcriptionally regulated and upregulated by pSmad2 in cancer. Treatment with metformin reduces the phosphorylation level of Smad2 and reduces the activity of TGF-β signaling which helps the metastasis and proliferation of the tumor.250
According to recent meta-analyses, metformin significantly reduced the incidence of colorectal adenoma, advanced adenoma, as well as CRC in patients with diabetes.251,252 Metformin lowers the risk of CRC indirectly by lowering the incidence of colonic adenoma in people with acromegaly and by lowering the incidence of IBD (“Inflammatory Bowel Disease”) in patients with diabetes. According to the findings of Phase II clinical studies, the anti-cancer benefits of chemotherapy drugs like 5-FU (5-Fluorouracil) as well as irinotecan in refractory CRC could be enhanced by metformin. Metformin also lowers the chance of developing new polyps as well as adenomas in people without diabetes. It is reasonable to evaluate the impact of metformin in normoglycemic individuals with CRC and broaden its therapeutic applicability for treating or preventing CRC in a high-risk group in light of the findings of prior preclinical and clinical investigations.253
Metformin in Melanoma
Among the deadliest tumours in adolescents and young adults is cutaneous melanoma, which results from the alteration of melanocytes that produce pigment. Once it spreads to distant places from the skin, metastatic melanoma is among the most aggressive human cancers, with a median survival time of only 6 to 9 months. The process, known as aerobic glycolysis, is a result of the ability of melanoma cells to change how they process glucose and produce more energy.254 Metformin is one AMPK activator that has been reported to prevent the proliferation of altered cells.255 Independent of the B-Raf or N-Ras mutational status, metformin causes cell-cycle arrest inside the cell cycle of the G0/G1 phase and a severe decrease in cell viability by inducing apoptosis and autophagy.256 The impacts of metformin on melanoma cell death are only partly inhibited by AMPK suppression by siRNA or reduction of AMPK activation by pharmacological inhibitors, indicating that these effects are mediated by an AMPK-independent mechanism. Metformin was validated in vivo, as it inhibits the formation of melanoma tumours in mice (models of melanoma xenograft and allograft) by inducing autophagy and apoptosis.257 In addition, metformin reduces the expression of the protein implicated in the epithelial-mesenchymal transition, like N-cadherin, fibronectin, SPARC, Snail, and Slug as well as the activation of MMP-2 and MMP-9. AMPK activation and the p53 tumour suppressor protein are required for this process.258
Both AMPK-dependent and AMPK-independent mechanisms are used by metformin to cause melanoma cell death. Metformin causes melanoma cells to experience cell cycle arrest by an unidentified mechanism, which in turn triggers autophagy, and apoptosis, which results in the death of the melanoma cells. Metformin’s effects, when combined with BRAF inhibitors like vemurafenib, have been studied. To prevent resistance in melanoma cells, several organizations have employed combination therapies that include BRAF inhibitors. Positive outcomes with synergistic effects for causing melanoma cell death were seen when metformin and vemurafenib (a BRAF inhibitor) were combined.259 Indeed, in vitro research demonstrates synergistic antiproliferative effects, especially in BRAFV600E mutant cell lines. In several trials, the chemotherapeutic agent cisplatin was more harmful to melanoma cells when used with metformin.260 These findings are interesting, but further research is required in this area.
Metformin’s Benefit on the Cardiovascular System
The clinical disorder known as DCM (diabetic cardiomyopathy) is linked to heart defects caused by diabetes. According to estimates, DCM may cause heart failure and mortality in as many as 12% of patients with diabetes. The 1st line of blood glucose-reducing medication with potential application to cardiovascular disorders is metformin, which is now in use. Apoptosis, inflammation, oxidative stress, cardiac hypertrophy, and uncontrolled “interstitial fibrosis” are the main characteristics of DCM.261 The common mechanisms of cardiac hypertrophic reprogramming include aberrant sarcomeric structure, brain natriuretic peptides, and accelerated protein synthesis. An important factor in DCM is the production of ROS and the activation of the apoptotic and inflammatory pathways.89 Through AMPK and glucose metabolism, metformin maintains heart function and reduces the risk of myocardial infarction.262 Metformin inhibited “cell hypertrophy” in the existence of 2DG in comparison to control H9C2 cells that were not treated.263 Metformin has anti-apoptotic effects on cardiomyoblasts via a FoxO1-dependent mechanism when they are subjected to stress. The FoxO-family proteins, which include “FoxO1, FoxO3, FoxO4, and FoxO6”, coordinate a variety of pathological and physiological processes by regulating gene expression linked to cell metabolism, oxidative stress resistance, DNA damage repair, apoptosis, and cell cycle arrest.264–266 Metformin prevents stress-induced apoptotic cell death through the FoxO1 pathway.263 Additionally, metformin leads to weight loss and a reduction in food intake to 300 kcal per day, which improves insulin sensitivity, reduces inflammation, and results in many advantageous modifications to myocardial oxygen consumption, cardiac output, and stroke volume.267 The cardiac endothelial cells that mostly express the AMPK subunit α1 and the cardiomyocytes that primarily express the AMPK subunit α2 are the myocardium’s two distinct isoforms of AMPK. The control of energy metabolism, mitochondrial health, protein synthesis, oxidative stress, autophagy, and inflammation is regulated by AMPK, which phosphorylates a wide variety of metabolic enzymes, transporters of metabolites, and signalling molecules. The activation of AMPK by the drug metformin promotes many cardioprotective pathways.268 The AMPK-dependent phosphorylation and heat shock protein-9 linkage with eNOS by metformin results in its activation. By inhibiting oxidative stress, apoptosis, and vasodilation in conjunction with increased local NO production, coronary blood flow, afterload, as well as left ventricular performance are all improved.262 Metformin has anti-hypertrophic actions that are mediated through AMPK-induced blunting of AT1R (“Angiotensin II Type 1 Receptor”) up-regulation and mitochondrial dysfunction via the “SIRT1/eNOS/p53” pathway. The p300’s histone acetyltransferase activity, which was linked to HF development, is inhibited by metformin, which prevents the acetylation of histone H3K9. Additionally, it decreases TGF-β1, TNF-α expression, and basic fibroblast growth factor in primary cardiomyocytes, which is reduced by AMPK.269,270 GLUT-4 is translocated to the cardiomyocyte membrane after AMPK activation, which increases insulin-dependent glucose absorption. Metformin significantly improves glucose absorption and restores glucose metabolism by enhancing the PI3-K (“Phosphoinositide 3-Kinase”)-protein kinase B/Akt pathway as well as AMPK activation.271
Metformin in Anti-Aging
Apart from glycemic and metabolic effects, metformin has gained tremendous attention for its extra glycemic effects including cardioprotective, hepatoprotective, anti-malignant, and geroprotective effects.272 CR (Calorie restriction) is the most promising lead in prolonging life span in various animal models, from nematodes to rodents to mammals by improving resistance to stress and toxicity, while maintaining function and vitality.273 The mechanism behind the process of calorie restriction is a reduction in insulin and the insulin-like GF-1 including an increase in insulin sensitivity. Longevity is related to the genes involved in insulin signal transduction and its transcription factors DAF-16. Down streaming genetic targets by CR memetics interacts with daf-2 and insulin signalling pathways.274 Various levels of the IGF-1 pathway, including IGF-1 release from the liver or reduction in IGF-1 signalling, are affected by metformin.275 Metformin activates AMP kinase and its activation is regulated by LKB1, a tumor suppressor gene stimulating phosphorylation of threonine 172 which in turn inhibits mTOR reducing protein synthesis.276 AMPK is also a main mediator of SIRT1 (“Sirtuin 1”) and PPAR (“Peroxisome Proliferator-Activated Receptor”) that links metabolism to longevity.277 Human vascular smooth muscle cells, macrophages, as well as endothelial cells all exhibit a dose-dependent inhibition of the “pro-inflammatory cytokines” IL-6 and IL-8 released in response to IL-1β. Metformin also reduces activation and translocation of NF-ӄB in smooth muscle cells along with suppression of activation of the proinflammatory phosphokinases Akt, Erk & p38.278 Although preclinical and cell-based studies have reported a positive response, the evidence from the majority of the clinical studies like MILES (Metformin In Longevity Study) and TAME (Targeting Aging with Metformin) seems to be controversial.279,280 Data from the “Metformin to Augment Strength Training Effective Response in Seniors (MASTERS) trial” reports that in healthy older adults, metformin impairs the hypertrophic muscle response to resistance training exercises, hence counteracting the benefits of physical activity.281 These reports from clinical studies question the effectiveness of metformin as a promising anti-aging agent. Future exploration of a detailed understanding of the aging process and modes of modifying different pathways for anti-aging action with long-term follow-up studies are required to explore and confirm the metformin’s antiaging action.
Metformin as an Endocrine Disruptor
Metformin being such a wonder drug is not free from adverse effects as evidence shows reports of remote adverse events have been reported in long-term human cohort studies, with a few major birth defects with the use of metformin.282 Similarly, in environmental studies, metformin has been found to affect the endocrine system and with concentration present in the waste water it has been shown to reduce fecundity, reduction in overall size and increase in intersex as a result of exposure from early to adult life stages of male fish.283–285 These studies point towards an associated risk; however, we need large studies with long-term follow-ups to establish the facts and conclude on the associated metabolic disruptor effect of metformin.
Limitations to the Interpretation of in vitro Studies with Metformin
There has been a tons of research on the mechanism of action of metformin, but the precise mechanism is still up for debate because the metformin concentrations used in earlier studies on preclinical models, in vitro experiments, and cell line models were much higher (milli moles) than the therapeutic concentrations used in humans (micro-moles). The concentrations employed in the preclinical study were almost 10–100 times greater than the threshold concentrations attained in T2D patients. The supra-pharmacological (>1 mM) concentrations of metformin have widely characterised mechanisms of action, such as complex I inhibition leading to AMPK activation do not exist in the usual clinical scenario. Recent research supports the fact that metformin at clinically optimal (50–100 μM) doses of the drug suppress hepatic gluconeogenesis in a substrate-selective way both in vitro and in vivo settings.10,286 These evidence limits the usefulness of metformin in various other conditions like cancer where preclinical study results reveal a pretty high concentration as compared to the therapeutic ones so in view of the varied mechanism of metformin at different concentration, modified preclinical studies with robust protocols and large trials need to be conducted in order to conclude on its usefulness in a plethora of diseases.
Conclusion
Metformin has been the cornerstone of therapy in the management of T2D. With the advent of newer molecules as oral antihyperglycaemic therapies, we do now have a varied therapeutic option; yet, metformin with pleiotropic effects still seems to have an upper hand over other drugs. The insulin-sparing effect of metformin makes it unique along with its diverse intracellular mechanisms. The drug has been found beneficial in a myriad of disorders including diabetes, obesity, cancers, cardiovascular conditions, neurological conditions, and many more but the majority of the evidence is based on the in vitro and in vivo studies with a supra-therapeutic concentration of the drug. Preliminary evidence to support the effect of metformin was collected from the preclinical and observational studies, but the drug is now also being tried in various clinical trials to prove its potential in chronic human diseases. The current evidence shows a ray of hope for metformin to be used more regularly for various conditions; yet, more future research and large clinical trials are needed on varied populations to establish the facts and make metformin an established drug for various diseases.
Acknowledgments
The figures were prepared from a licensed version of Biorender.com. We would like to thank Dr. Santosh Kumar for helping us out for the figures in this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no conflicts of interest in this work.
References
1. American Diabetes Association. Standards of care in diabetes—2023 abridged for primary care providers. Clin Diabetes. 2023;41(1):4–31. doi:10.2337/cd23-as01
2. Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108(8):1105–1107. doi:10.1172/jci14178
3. Yerevanian A, Soukas AA. Metformin: mechanisms in human obesity and weight loss. Curr Obes Rep. 2019;8(2):156–164. doi:10.1007/s13679-019-00335-3
4. Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi:10.1007/s00125-017-4318-z
5. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The multicenter metformin study group. N Engl J Med. 1995;333(9):541–549. doi:10.1056/nejm199508313330902
6. King P, Peacock I, Donnelly R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 1999;48(5):643–648. doi:10.1046/j.1365-2125.1999.00092.x
7. Engler C, Leo M, Pfeifer B, et al. Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the Diabetes Registry Tyrol 2012–2018. BMJ Open Diabetes Res Care. 2020;8(1):e001279.
8. Food and Drug Administration. Glucophage (metformin hydrochloride) Tablets; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020357s037s039,021202s021s023lbl.pdf.
9. Tadesse S. Clinical pharmacokinetics of metformin. In: Juber A, Usama A, Mohammad Irfan K, editors. Metformin. IntechOpen; 2021.
10. LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr Rev. 2021;42(1):77–96. doi:10.1210/endrev/bnaa023
11. Graham GG, Punt J, Arora M, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. doi:10.2165/11534750-000000000-00000
12. Christensen MMH, Højlund K, Hother-Nielsen O, et al. Steady-state pharmacokinetics of metformin is independent of the OCT1 genotype in healthy volunteers. Eur J Clin Pharmacol. 2015;71(6):691–697. doi:10.1007/s00228-015-1853-8
13. Triggle CR, Mohammed I, Bshesh K, et al. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133:155223. doi:10.1016/j.metabol.2022.155223
14. Sharma KS HL. Insulin and other antidiabetic drugs. In: Sharma and Sharma’s Principles of Pharmacology.
15. Electronic Medicines Compendium. Metformin: summaries of product characteristics. European Medicines Agency; 2013. Available from: https://www.medicines.org.uk/emc/product/594/smpc#CONTRAINDICATIONS.
16. Infante M, Leoni M, Caprio M, Fabbri A. Long-term metformin therapy and vitamin B12 deficiency: an association to bear in mind. World J Diabetes. 2021;12(7):916–931. doi:10.4239/wjd.v12.i7.916
17. Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022. doi:10.2337/dci22-0034
18. Logie L, Harthill J, Patel K, et al. Cellular responses to the metal-binding properties of metformin. Diabetes. 2012;61(6):1423–1433. doi:10.2337/db11-0961
19. Shitara Y, Nakamichi N, Norioka M, Shima H, Kato Y, Horie T. Role of organic cation/carnitine transporter 1 in uptake of phenformin and inhibitory effect on complex I respiration in mitochondria. Toxicol Sci. 2013;132(1):32–42. doi:10.1093/toxsci/kfs330
20. Gründemann D, Schömig E. Gene structures of the human non-neuronal monoamine transporters EMT and OCT2. Hum Genet. 2000;106(6):627–635. doi:10.1007/s004390000309
21. Koehler MR, Wissinger B, Gorboulev V, Koepsell H, Schmid M. The two human organic cation transporter genes SLC22A1 and SLC22A2 are located on chromosome 6q26. Cytogenet Cell Genet. 1997;79(3–4):198–200. doi:10.1159/000134720
22. Minematsu T, Iwai M, Umehara K, Usui T, Kamimura H. Characterization of human organic cation transporter 1 (OCT1/SLC22A1)- and OCT2 (SLC22A2)-mediated transport of 1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-(pyrazin-2-ylmethyl)- 4,9-dihydro-1H-naphtho[2,3-d]imidazolium bromide (YM155 monobromide), a novel small molecule survivin suppressant. Drug Metab Dispos. 2010;38(1):1–4. doi:10.1124/dmd.109.028142
23. Anne T, Nies HK, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. In: Martin F, Fromm RBK, editors. Handb Exp Pharmacol. Springer; 2011:108–128.
24. Shirasaka Y, Lee N, Zha W, Wagner D, Wang J. Involvement of organic cation transporter 3 (Oct3/Slc22a3) in the bioavailability and pharmacokinetics of antidiabetic metformin in mice. Drug Metab Pharmacokinet. 2016;31(5):385–388. doi:10.1016/j.dmpk.2016.04.005
25. Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–2069. doi:10.2337/diabetes.49.12.2063
26. Johnson AB, Webster JM, Sum CF, et al. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism. 1993;42(9):1217–1222. doi:10.1016/0026-0495(93)90284-u
27. Consoli A, Nurjhan N. Contribution of gluconeogenesis to overall glucose output in diabetic and nondiabetic men. Ann Med. 1990;22(3):191–195. doi:10.3109/07853899009147268
28. Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol. 2011;(201):105–167. doi:10.1007/978-3-642-14541-4_3
29. Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab. 2003;88(3):1323–1332. doi:10.1210/jc.2002-021394
30. Detaille D, Guigas B, Leverve X, Wiernsperger N, Devos P. Obligatory role of membrane events in the regulatory effect of metformin on the respiratory chain function. Biochem Pharmacol. 2002;63(7):1259–1272. doi:10.1016/s0006-2952(02)00858-4
31. El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275(1):223–228. doi:10.1074/jbc.275.1.223
32. Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494(7436):256–260. doi:10.1038/nature11808
33. Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;3:607–614.
34. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi:10.1172/jci13505
35. Larsen S, Rabøl R, Hansen CN, Madsbad S, Helge JW, Dela F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia. 2012;55(2):443–449. doi:10.1007/s00125-011-2340-0
36. LaMoia TE, Butrico GM, Kalpage HA, et al. Metformin, phenformin, and galegine inhibit complex IV activity and reduce glycerol-derived gluconeogenesis. Proc Natl Acad Sci USA. 2022;119(10):e2122287119. doi:10.1073/pnas.2122287119
37. Argaud D, Roth H, Wiernsperger N, Leverve XM. Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur J Biochem. 1993;213(3):1341–1348. doi:10.1111/j.1432-1033.1993.tb17886.x
38. Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs. 2003;63(18):1879–1894. doi:10.2165/00003495-200363180-00001
39. Miller RA, Birnbaum MJ. An energetic tale of AMPK-independent effects of metformin. J Clin Invest. 2010;120(7):2267–2270. doi:10.1172/jci43661
40. Pernicova I, Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143–156. doi:10.1038/nrendo.2013.256
41. Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem. 2011;286(1):1–11. doi:10.1074/jbc.M110.121806
42. Hardie DG. Metformin—acting through cyclic AMP as well as AMP? Cell Metab. 2013;17(3):313–314. doi:10.1016/j.cmet.2013.02.011
43. Johnson RA, Yeung SM, Stübner D, Bushfield M, Shoshani I. Cation and structural requirements for P site-mediated inhibition of adenylate cyclase. Mol Pharmacol. 1989;35(5):681–688.
44. Koch L. New metformin mechanism elucidated in vitro and in vivo. Nat Rev Endocrinol. 2013;9(3):132. doi:10.1038/nrendo.2013.11
45. Bergeron R, Russell RR 3rd, Young LH, et al. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276(5):E938–44. doi:10.1152/ajpendo.1999.276.5.E938
46. Foretz M, Ancellin N, Andreelli F, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54(5):1331–1339. doi:10.2337/diabetes.54.5.1331
47. Lochhead PA, Salt IP, Walker KS, Hardie DG, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49(6):896–903. doi:10.2337/diabetes.49.6.896
48. Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi:10.1126/science.1120781
49. Schäfer G. Guanidines and biguanides. Pharmacol Ther. 1980;8(2):275–295. doi:10.1016/0163-7258(80)90049-2
50. Wilcock C, Wyre ND, Bailey CJ. Subcellular distribution of metformin in rat liver. J Pharm Pharmacol. 1991;43(6):442–444. doi:10.1111/j.2042-7158.1991.tb03507.x
51. Meng S, Cao J, He Q, et al. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem. 2015;290(6):3793–3802. doi:10.1074/jbc.M114.604421
52. Ravera S, Cossu V, Tappino B, et al. Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells. J Cell Physiol. 2018;233(2):1736–1751. doi:10.1002/jcp.26085
53. Cao J, Meng S, Chang E, et al. Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J Biol Chem. 2014;289(30):20435–20446. doi:10.1074/jbc.M114.567271
54. He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21(2):159–162. doi:10.1016/j.cmet.2015.01.003
55. Foretz M, Hébrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120(7):2355–2369. doi:10.1172/jci40671
56. Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19(12):1649–1654. doi:10.1038/nm.3372
57. Madiraju AK, Erion DM, Rahimi Y, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510(7506):542–546. doi:10.1038/nature13270
58. He L, Sabet A, Djedjos S, et al. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137(4):635–646. doi:10.1016/j.cell.2009.03.016
59. Herzig S, Long F, Jhala US, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413(6852):179–183. doi:10.1038/35093131
60. Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25(2):463–471. doi:10.1016/j.cmet.2016.12.009
61. Hunter RW, Hughey CC, Lantier L, et al. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat Med. 2018;24(9):1395–1406. doi:10.1038/s41591-018-0159-7
62. Lv Z, Guo Y. Metformin and its benefits for various diseases. Front Endocrinol. 2020;11:191. doi:10.3389/fendo.2020.00191
63. Buse JB, DeFronzo RA, Rosenstock J, et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes Care. 2016;39(2):198–205. doi:10.2337/dc15-0488
64. Gu S, Shi J, Tang Z, et al. Comparison of glucose lowering effect of metformin and acarbose in type 2 diabetes mellitus: a meta-analysis. PLoS One. 2015;10(5):e0126704. doi:10.1371/journal.pone.0126704
65. Polianskyte-Prause Z, Tolvanen TA, Lindfors S, et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB j. 2019;33(2):2858–2869. doi:10.1096/fj.201800529RR
66. Sum CF, Webster JM, Johnson AB, Catalano C, Cooper BG, Taylor R. The effect of intravenous metformin on glucose metabolism during hyperglycaemia in type 2 diabetes. Diabet Med. 1992;9(1):61–65. doi:10.1111/j.1464-5491.1992.tb01716.x
67. Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23(7):850–858. doi:10.1038/nm.4345
68. Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24(12):1919–1929. doi:10.1038/s41591-018-0222-4
69. Lee YJ, Jeschke GR, Roelants FM, Thorner J, Turk BE. Reciprocal phosphorylation of yeast glycerol-3-phosphate dehydrogenases in adaptation to distinct types of stress. Mol Cell Biol. 2012;32(22):4705–4717. doi:10.1128/mcb.00897-12
70. Raber I, McCarthy CP, Vaduganathan M, et al. The rise and fall of aspirin in the primary prevention of cardiovascular disease. Lancet. 2019;393(10186):2155–2167. doi:10.1016/s0140-6736(19)30541-0
71. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865.
72. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi:10.1056/NEJMoa0806470
73. Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304–1311. doi:10.2337/dc12-0719
74. Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169(6):616–625. doi:10.1001/archinternmed.2009.20
75. Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. 2020;160:108001. doi:10.1016/j.diabres.2020.108001
76. Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol. 2019;18(1):96. doi:10.1186/s12933-019-0900-7
77. U.S. National Library of Medicine. MetCool ACS”- metformin “cooling” effect on metformin-naive patients treated with PCI because of acute coronary syndrome; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05305898.
78. Sena CM, Matafome P, Louro T, Nunes E, Fernandes R, Seiça RM. Metformin restores endothelial function in aorta of diabetic rats. Br J Pharmacol. 2011;163(2):424–437. doi:10.1111/j.1476-5381.2011.01230.x
79. Bacchetti T, Masciangelo S, Armeni T, Bicchiega V, Ferretti G. Glycation of human high density lipoprotein by methylglyoxal: effect on HDL-paraoxonase activity. Metabolism. 2014;63(3):307–311. doi:10.1016/j.metabol.2013.10.013
80. Sena CM, Matafome P, Crisóstomo J, et al. Methylglyoxal promotes oxidative stress and endothelial dysfunction. Pharmacol Res. 2012;65(5):497–506. doi:10.1016/j.phrs.2012.03.004
81. Durrington PN, Mackness B, Mackness MI. Paraoxonase and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21(4):473–480. doi:10.1161/01.ATV.21.4.473
82. Soran H, Schofield JD, Durrington PN. Antioxidant properties of HDL. Front Pharmacol. 2015;6:222. doi:10.3389/fphar.2015.00222
83. Sutkowska E, Fortuna P, Kałuża B, Sutkowska K, Wiśniewski J, Prof AG. Metformin has no impact on nitric oxide production in patients with pre-diabetes. Biomed Pharmacother. 2021;140:111773. doi:10.1016/j.biopha.2021.111773
84. Kato Y, Koide N, Komatsu T, et al. Metformin attenuates production of nitric oxide in response to lipopolysaccharide by inhibiting MyD88-independent pathway. Horm Metab Res. 2010;42(9):632–636. doi:10.1055/s-0030-1255033
85. Salvatore T, Pafundi PC, Galiero R, et al. Can metformin exert as an active drug on endothelial dysfunction in diabetic subjects? Biomedicines. 2020;9(1):3. doi:10.3390/biomedicines9010003
86. Kheniser KG, Kashyap SR, Kasumov T. A systematic review: the appraisal of the effects of metformin on lipoprotein modification and function. Obes Sci Pract. 2019;5(1):36–45. doi:10.1002/osp4.309
87. Machado AP, Pinto RS, Moysés ZP, Nakandakare ER, Quintão EC, Passarelli M. Aminoguanidine and metformin prevent the reduced rate of HDL-mediated cell cholesterol efflux induced by formation of advanced glycation end products. Int J Biochem Cell Biol. 2006;38(3):392–403. doi:10.1016/j.biocel.2005.09.016
88. Matsuki K, Tamasawa N, Yamashita M, et al. Metformin restores impaired HDL-mediated cholesterol efflux due to glycation. Atherosclerosis. 2009;206(2):434–438. doi:10.1016/j.atherosclerosis.2009.03.003
89. Dziubak A, Wójcicka G, Wojtak A, Bełtowski J. Metabolic effects of metformin in the failing heart. Int J Mol Sci. 2018;19:1.
90. Cittadini A, Napoli R, Monti MG, et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61(4):944–953. doi:10.2337/db11-1132
91. Kinsara AJ, Ismail YM. Metformin in heart failure patients. Indian Heart J. 2018;70(1):175–176. doi:10.1016/j.ihj.2017.05.009
92. Roberts F, Ryan GJ. The safety of metformin in heart failure. Ann Pharmacother. 2007;41(4):642–646. doi:10.1345/aph.1H523
93. Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol. 2011;38(2):94–101. doi:10.1111/j.1440-1681.2010.05470.x
94. Mohan M, Al-Talabany S, McKinnie A, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J. 2019;40(41):3409–3417. doi:10.1093/eurheartj/ehz203
95. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100(3):328–341. doi:10.1161/01.Res.0000256090.42690.05
96. Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi:10.1038/ncb839
97. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115(5):577–590. doi:10.1016/s0092-8674(03)00929-2
98. Xu CX, Zhu HH, Zhu YM. Diabetes and cancer: associations, mechanisms, and implications for medical practice. World J Diabetes. 2014;5(3):372–380. doi:10.4239/wjd.v5.i3.372
99. Ballotari P, Vicentini M, Manicardi V, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer. 2017;17(1):703. doi:10.1186/s12885-017-3696-4
100. Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi:10.2337/dc10-0666
101. Bjornsdottir HH, Rawshani A, Rawshani A, et al. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci Rep. 2020;10(1):17376. doi:10.1038/s41598-020-73668-y
102. van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. doi:10.1158/1055-9965.Epi-09-0372
103. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9(11):798–809. doi:10.1038/nrc2734
104. Soranna D, Scotti L, Zambon A, et al. Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist. 2012;17(6):813–822. doi:10.1634/theoncologist.2011-0462
105. Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS. Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism. 2013;62(7):922–934. doi:10.1016/j.metabol.2013.01.014
106. Mekuria AN, Ayele Y, Tola A, Mishore KM. Monotherapy with metformin versus sulfonylureas and risk of cancer in type 2 diabetic patients: a systematic review and meta-analysis. J Diabetes Res. 2019;7676909. doi:10.1155/2019/7676909
107. Wu Y, Liu HB, Shi XF, Song Y. Conventional hypoglycaemic agents and the risk of lung cancer in patients with diabetes: a meta-analysis. PLoS One. 2014;9(6):e99577. doi:10.1371/journal.pone.0099577
108. Alimova IN, Liu B, Fan Z, et al. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8(6):909–915. doi:10.4161/cc.8.6.7933
109. Liu B, Fan Z, Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8(13):2031–2040. doi:10.4161/cc.8.13.8814
110. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi:10.1158/0008-5472.Can-06-1500
111. Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15(3):833–839. doi:10.1677/erc-08-0038
112. Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation--implications for a novel treatment strategy. Gynecol Oncol. 2010;116(1):92–98. doi:10.1016/j.ygyno.2009.09.024
113. Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64(10):1290–1302. doi:10.1007/s00018-007-7080-4
114. Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15(1):166–178. doi:10.1111/j.1582-4934.2009.00954.x
115. Wang LW, Li ZS, Zou DW, Jin ZD, Gao J, Xu GM. Metformin induces apoptosis of pancreatic cancer cells. World J Gastroenterol. 2008;14(47):7192–7198. doi:10.3748/wjg.14.7192
116. Zakikhani M, Dowling RJ, Sonenberg N, Pollak MN. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res. 2008;1(5):369–375. doi:10.1158/1940-6207.Capr-08-0081
117. Tomimoto A, Endo H, Sugiyama M, et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 2008;99(11):2136–2141. doi:10.1111/j.1349-7006.2008.00933.x
118. Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27(25):3576–3586. doi:10.1038/sj.onc.1211024
119. Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278(41):39422–39427. doi:10.1074/jbc.M305371200
120. Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107(6):873–877. doi:10.1002/ijc.11487
121. Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26(13):1932–1940. doi:10.1038/sj.onc.1209990
122. Jones RA, Campbell CI, Gunther EJ, et al. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26(11):1636–1644. doi:10.1038/sj.onc.1209955
123. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4(5):335–348. doi:10.1038/nrc1362
124. Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98(18):10314–10319. doi:10.1073/pnas.171076798
125. Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278–2287. doi:10.1200/jco.2008.20.0766
126. Petroulakis E, Mamane Y, Le Bacquer O, Shahbazian D, Sonenberg N. mTOR signaling: implications for cancer and anticancer therapy. Br J Cancer. 2006;94(2):195–199. doi:10.1038/sj.bjc.6602902
127. Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804(3):433–439. doi:10.1016/j.bbapap.2009.12.001
128. Tao T, Su Q, Xu S, et al. Down-regulation of PKM2 decreases FASN expression in bladder cancer cells through AKT/mTOR/SREBP-1c axis. J Cell Physiol. 2019;234(3):3088–3104. doi:10.1002/jcp.27129
129. Algire C, Moiseeva O, Deschênes-Simard X, et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev Res. 2012;5(4):536–543. doi:10.1158/1940-6207.Capr-11-0536
130. Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27(20):3297–3302. doi:10.1200/jco.2009.19.6410
131. Valencia WM, Palacio A, Tamariz L, Florez H. Metformin and ageing: improving ageing outcomes beyond glycaemic control. Diabetologia. 2017;60(9):1630–1638. doi:10.1007/s00125-017-4349-5
132. Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis. 2018;65(4):1225–1236. doi:10.3233/jad-180263
133. Jiang T, Yu JT, Zhu XC, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171(13):3146–3157. doi:10.1111/bph.12655
134. Lin Y, Wang K, Ma C, et al. Evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front Aging Neurosci. 2018;10:227. doi:10.3389/fnagi.2018.00227
135. Ng TP, Feng L, Yap KB, Lee TS, Tan CH, Winblad B. Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis. 2014;41(1):61–68. doi:10.3233/jad-131901
136. Palleria C, Leporini C, Maida F, et al. Potential effects of current drug therapies on cognitive impairment in patients with type 2 diabetes. Front Neuroendocrinol. 2016;42:76–92. doi:10.1016/j.yfrne.2016.07.002
137. Vázquez-Manrique RP, Farina F, Cambon K, et al. AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum Mol Genet. 2016;25(6):1043–1058. doi:10.1093/hmg/ddv513
138. Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R. Modeling Parkinson’s disease with the alpha-synuclein protein. Front Pharmacol. 2020;11:356. doi:10.3389/fphar.2020.00356
139. Stefanis L. α-synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2(2):a009399. doi:10.1101/cshperspect.a009399
140. Wakabayashi K, Tanji K, Mori F, Takahashi H. The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology. 2007;27(5):494–506. doi:10.1111/j.1440-1789.2007.00803.x
141. Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G. Metformin prevents dopaminergic neuron death in MPTP/P-induced mouse model of parkinson’s disease via autophagy and mitochondrial ROS clearance. Int J Neuropsychopharmacol. 2016;19(9). doi:10.1093/ijnp/pyw047
142. Saewanee N, Praputpittaya T, Malaiwong N, Chalorak P, Meemon K. Neuroprotective effect of metformin on dopaminergic neurodegeneration and α-synuclein aggregation in C. elegans model of Parkinson’s disease. Neurosci Res. 2021;162:13–21. doi:10.1016/j.neures.2019.12.017
143. Ozbey G, Nemutlu-Samur D, Parlak H, et al. Metformin protects rotenone-induced dopaminergic neurodegeneration by reducing lipid peroxidation. Pharmacol Rep. 2020;72(5):1397–1406. doi:10.1007/s43440-020-00095-1
144. Katila N, Bhurtel S, Shadfar S, et al. Metformin lowers α-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2017;125:396–407. doi:10.1016/j.neuropharm.2017.08.015
145. Barini E, Antico O, Zhao Y, et al. Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener. 2016;9(11):16. doi:10.1186/s13024-016-0082-7
146. DiTacchio KA, Heinemann SF, Dziewczapolski G. Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2015;44(1):43–48. doi:10.3233/jad-141332
147. Li ZG, Zhang W, Sima AA. Alzheimer-like changes in rat models of spontaneous diabetes. Diabetes. 2007;56(7):1817–1824. doi:10.2337/db07-0171
148. Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. doi:10.1016/j.arr.2016.10.005
149. Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol. 2011;46(2–3):96–99. doi:10.1016/j.exger.2010.08.022
150. De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106(6):1971–1976. doi:10.1073/pnas.0809158106
151. Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199(2):265–273. doi:10.1016/j.expneurol.2006.01.018
152. Allard JS, Perez EJ, Fukui K, Carpenter P, Ingram DK, de Cabo R. Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res. 2016;301:1–9. doi:10.1016/j.bbr.2015.12.012
153. Chen F, Dong RR, Zhong KL, et al. Antidiabetic drugs restore abnormal transport of amyloid-β across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology. 2016;101:123–136. doi:10.1016/j.neuropharm.2015.07.023
154. Lennox R, Porter DW, Flatt PR, Holscher C, Irwin N, Gault VA. Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology. 2014;86:22–30. doi:10.1016/j.neuropharm.2014.06.026
155. Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91(11–12):409–414. doi:10.1016/j.lfs.2012.08.017
156. Li J, Deng J, Sheng W, Zuo Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav. 2012;101(4):564–574. doi:10.1016/j.pbb.2012.03.002
157. Thangthaeng N, Rutledge M, Wong JM, Vann PH, Forster MJ, Sumien N. Metformin impairs spatial memory and visual acuity in old male mice. Aging Dis. 2017;8(1):17–30. doi:10.14336/ad.2016.1010
158. Yokoyama H, Ogawa M, Honjo J, et al. Risk factors associated with abnormal cognition in Japanese outpatients with diabetes, hypertension or dyslipidemia. Diabetol Int. 2015;6(4):268–274. doi:10.1007/s13340-014-0194-7
159. Demaré S, Kothari A, Calcutt NA, Fernyhough P. Metformin as a potential therapeutic for neurological disease: mobilizing AMPK to repair the nervous system. Expert Rev Neurother. 2021;21(1):45–63. doi:10.1080/14737175.2021.1847645
160. Hervás D, Fornés-Ferrer V, Gómez-Escribano AP, et al. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS One. 2017;12(6):e0179283. doi:10.1371/journal.pone.0179283
161. Largani SHH, Borhani-Haghighi M, Pasbakhsh P, et al. Oligoprotective effect of metformin through the AMPK-dependent on restoration of mitochondrial hemostasis in the cuprizone-induced multiple sclerosis model. J Mol Histol. 2019;50(3):263–271. doi:10.1007/s10735-019-09824-0
162. Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I. AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol. 2013;183(2):526–541. doi:10.1016/j.ajpath.2013.04.030
163. Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5(1):6. doi:10.1186/1758-5996-5-6
164. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi:10.1007/s00125-017-4342-z
165. Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–1593. doi:10.1007/s00125-017-4336-x
166. Dutta S, Kumar T, Singh S, Ambwani S, Charan J, Varthya SB. Euglycemic diabetic ketoacidosis associated with SGLT2 inhibitors: a systematic review and quantitative analysis. J Fam Med Prim Care. 2022;11(3):927–940. doi:10.4103/jfmpc.jfmpc_644_21
167. Dutta S, Sharma P, Misra AK. SGLT-2 inhibitors: an evidence-based perspective. In: Frontiers in Clinical Drug Research-Diabetes and Obesity. Bentham Science Publisher; 2020:138–155.
168. Scheen AJ, Paquot N, Lefebvre PJ, et al. L'étude clinique du mois. “United Kingdom Prospective Diabetes Study”: 10 ans plus tard [United Kingdom Prospective Diabetes Study (UKPDS): 10 years later]. Rev Med Liege. 2008;63(10):624–629.
169. Wexler D Initial management of hyperglycemia in adults with type 2 diabetes mellitus: India; 2022. Available from: https://www.uptodate.com/contents/initial-management-of-hyperglycemia-in-adults-with-type-2-diabetes-mellitus.
170. Sutkowska E, Fortuna P, Wisniewski J, et al. Low metformin dose and its therapeutic serum concentration in prediabetes. Sci Rep. 2021;11(1):11684. doi:10.1038/s41598-021-91174-7
171. Gilbert C, Valois M, Koren G. Pregnancy outcome after first-trimester exposure to metformin: a meta-analysis. Fertil Steril. 2006;86(3):658–663. doi:10.1016/j.fertnstert.2006.02.098
172. Gutzin SJ, Kozer E, Magee LA, Feig DS, Koren G. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol. 2003;10(4):179–183.
173. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358(19):2003–2015. doi:10.1056/NEJMoa0707193
174. Nguyen L, Chan SY, Teo AKK. Metformin from mother to unborn child - are there unwarranted effects? EBioMedicine. 2018;35:394–404. doi:10.1016/j.ebiom.2018.08.047
175. Beysel S, Unsal IO, Kizilgul M, Caliskan M, Ucan B, Cakal E. The effects of metformin in type 1 diabetes mellitus. BMC Endocr Disord. 2018;18(1):1. doi:10.1186/s12902-017-0228-9
176. Livingstone R, Boyle JG, Petrie JR. A new perspective on metformin therapy in type 1 diabetes. Diabetologia. 2017;60(9):1594–1600. doi:10.1007/s00125-017-4364-6
177. Al Khalifah RA, Alnhdi A, Alghar H, Alanazi M, Florez ID. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: a systematic review and meta-analysis. Pediatr Diabetes. 2017;18(7):664–673. doi:10.1111/pedi.12493
178. Liu C, Wu D, Zheng X, Li P, Li L. Efficacy and safety of metformin for patients with type 1 diabetes mellitus: a meta-analysis. Diabetes Technol Ther. 2015;17(2):142–148. doi:10.1089/dia.2014.0190
179. Liu W, Yang XJ. The effect of metformin on adolescents with type 1 diabetes: a systematic review and meta-analysis of randomized controlled trials. Int J Endocrinol. 2016;2016:3854071. doi:10.1155/2016/3854071
180. BMJ Publishing Group Limited. What role for metformin in type 1 diabetes? Drug Ther Bull. 2018;56(7):78–80. doi:10.1136/dtb.2018.7.0645
181. DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65(2):20–29. doi:10.1016/j.metabol.2015.10.014
182. Heaf J. Metformin in chronic kidney disease: time for a rethink. Perit Dial Int. 2014;34(4):353–357. doi:10.3747/pdi.2013.00344
183. Aroda VR, Ratner RE. Metformin and type 2 diabetes prevention. Diabetes Spectr. 2018;31(4):336–342. doi:10.2337/ds18-0020
184. Abramowicz M, Zuccotti G, Pflomm JM. Metformin for prediabetes. JAMA. 2017;317(11):1171.
185. American Diabetes Association. Standards of medical care in diabetes-2022 abridged for primary care providers. Clin Diabetes. 2022;40(1):10–38. doi:10.2337/cd22-as01
186. Moin T. Should adults with prediabetes be prescribed metformin to prevent diabetes mellitus? Yes: high-quality evidence supports metformin use in persons at high risk. Am Fam Physician. 2019;100(3):134–135.
187. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi:10.1056/NEJMoa012512
188. Inzucchi SE, Docherty KF, Køber L, et al. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA-HF. Diabetes Care. 2021;44(2):586–594. doi:10.2337/dc20-1675
189. Rossing P, Inzucchi SE, Vart P, et al. Dapagliflozin and new-onset type 2 diabetes in patients with chronic kidney disease or heart failure: pooled analysis of the DAPA-CKD and DAPA-HF trials. Lancet Diabetes Endocrinol. 2022;10(1):24–34. doi:10.1016/s2213-8587(21)00295-3
190. Golay A. Metformin and body weight. Int J Obes. 2008;32(1):61–72. doi:10.1038/sj.ijo.0803695
191. Goel S, Singh R, Singh V, et al. Metformin: activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet. 2022;13:1022739. doi:10.3389/fgene.2022
192. Malin SK, Kashyap SR. Effects of metformin on weight loss: potential mechanisms. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):323–329. doi:10.1097/med.0000000000000095
193. Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121(1):27–31. doi:10.1055/s-0032-1327734
194. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi:10.1056/NEJMoa066224
195. Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: a systematic review and meta-analysis. Diabetol Metab Syndr. 2015;7(Suppl 1):A58. doi:10.1186/1758-5996-7-S1-A58
196. Gao L, Huang H, Zhang L, et al. Comparison of beinaglutide versus metformin for weight loss in overweight and obese non-diabetic patients. Exp Clin Endocrinol Diabetes. 2022;130(6):358–367. doi:10.1055/a-1608-0345
197. Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the diabetes prevention program outcomes study. Diabetes Care. 2012;35(4):731–737. doi:10.2337/dc11-1299
198. Pu R, Shi D, Gan T, et al. Effects of metformin in obesity treatment in different populations: a meta-analysis. Ther Adv Endocrinol Metab. 2020;11:2042018820926000. doi:10.1177/2042018820926000
199. Lyu X, Lyu T, Wang X, et al. The antiobesity effect of GLP-1 receptor agonists alone or in combination with metformin in overweight /obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Int J Endocrinol. 2021;2021:6616693. doi:10.1155/2021/6616693
200. Johnson NP. Metformin use in women with polycystic ovary syndrome. Ann Transl Med. 2014;2(6):56. doi:10.3978/j.issn.2305-5839.2014.04.15
201. Lashen H. Role of metformin in the management of polycystic ovary syndrome. Ther Adv Endocrinol Metab. 2010;1(3):117–128. doi:10.1177/2042018810380215
202. Practice Committee of the American Society for Reproductive Medicine. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertil Steril. 2017;108(3):426–441. doi:10.1016/j.fertnstert.2017.06.026
203. Sharpe A, Morley LC, Tang T, Norman RJ, Balen AH. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2019;12(12):Cd013505. doi:10.1002/14651858.Cd013505
204. Sam S, Ehrmann DA. Metformin therapy for the reproductive and metabolic consequences of polycystic ovary syndrome. Diabetologia. 2017;60(9):1656–1661. doi:10.1007/s00125-017-4306-3
205. Lisa M, Chirch PL, Mirza F. Metabolic complications of HIV infection. Case-based review. J Clin Outcomes Manag. 2017;24(12):1.
206. Wohl DA, McComsey G, Tebas P, et al. Current concepts in the diagnosis and management of metabolic complications of HIV infection and its therapy. Clin Infect Dis. 2006;43(5):645–653. doi:10.1086/507333
207. Hadigan C, Corcoran C, Basgoz N, Davis B, Sax P, Grinspoon S. Metformin in the treatment of HIV lipodystrophy syndrome: a randomized controlled trial. JAMA. 2000;284(4):472–477. doi:10.1001/jama.284.4.472
208. Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8(7):420–426. doi:10.1111/j.1468-1293.2007.00488.x
209. Harmooshi NN, Abeshtan A, Zakerkish M, Mirmomeni G, Rahim F. The effect of metformin on body mass index and metabolic parameters in non-diabetic HIV-positive patients: a meta-analysis. J Diabetes Metab Disord. 2021;20(2):1901–1911. doi:10.1007/s40200-021-00869-1
210. Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29(2):254–258. doi:10.2337/diacare.29.02.06.dc05-1558
211. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–1305. doi:10.1136/bmj.38415.708634.F7
212. Shen Z, Wen XF, Lan F, Shen ZZ, Shao ZM. The tumor suppressor gene LKB1 is associated with prognosis in human breast carcinoma. Clin Cancer Res. 2002;8(7):2085–2090.
213. Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021–2032. doi:10.1038/bjc.2013.187
214. Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–226. doi:10.1016/j.molcel.2008.03.003
215. Saini KS, Loi S, de Azambuja E, et al. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39(8):935–946. doi:10.1016/j.ctrv.2013.03.009
216. Cejuela M, Martin-Castillo B, Menendez JA, Pernas S. Metformin and breast cancer: where are we now? Int J Mol Sci. 2022;23(5):1.
217. Byrne FL, Poon IK, Modesitt SC, et al. Metabolic vulnerabilities in endometrial cancer. Cancer Res. 2014;74(20):5832–5845. doi:10.1158/0008-5472.Can-14-0254
218. Gotlieb WH, Saumet J, Beauchamp MC, et al. In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecol Oncol. 2008;110(2):246–250. doi:10.1016/j.ygyno.2008.04.008
219. Takahashi A, Kimura F, Yamanaka A, et al. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int. 2014;14:53. doi:10.1186/1475-2867-14-53
220. Yokoyama Y, Takahashi Y, Shinohara A, Lian Z, Tamaya T. Telomerase activity in the female reproductive tract and neoplasms. Gynecol Oncol. 1998;68(2):145–149. doi:10.1006/gyno.1997.4921
221. Chae YK, Arya A, Malecek MK, et al. Repurposing metformin for cancer treatment: current clinical studies. Oncotarget. 2016;7(26):40767–40780. doi:10.18632/oncotarget.8194
222. Coperchini F, Leporati P, Rotondi M, Chiovato L. Expanding the therapeutic spectrum of metformin: from diabetes to cancer. J Endocrinol Invest. 2015;38(10):1047–1055. doi:10.1007/s40618-015-0370-z
223. Febbraro T, Lengyel E, Romero IL. Old drug, new trick: repurposing metformin for gynecologic cancers? Gynecol Oncol. 2014;135(3):614–621. doi:10.1016/j.ygyno.2014.10.011
224. Gong J, Kelekar G, Shen J, Shen J, Kaur S, Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Target Oncol. 2016;11(4):447–467. doi:10.1007/s11523-016-0423-z
225. Imai A, Ichigo S, Matsunami K, Takagi H, Yasuda K. Clinical benefits of metformin in gynecologic oncology. Oncol Lett. 2015;10(2):577–582. doi:10.3892/ol.2015.3262
226. Irie H, Banno K, Yanokura M, et al. Metformin: a candidate for the treatment of gynecological tumors based on drug repositioning. Oncol Lett. 2016;11(2):1287–1293. doi:10.3892/ol.2016.4075
227. Tseng CH. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol Oncol. 2015;138(1):147–153. doi:10.1016/j.ygyno.2015.03.059
228. Tang YL, Zhu LY, Li Y, et al. Metformin use is associated with reduced incidence and improved survival of endometrial cancer: a meta-analysis. Biomed Res Int. 2017;2017:5905384. doi:10.1155/2017/5905384
229. Ko EM, Walter P, Jackson A, et al. Metformin is associated with improved survival in endometrial cancer. Gynecol Oncol. 2014;132(2):438–442. doi:10.1016/j.ygyno.2013.11.021
230. Al hilli MM, Bakkum-Gamez JN, Mariani A, et al. The effect of diabetes and metformin on clinical outcomes is negligible in risk-adjusted endometrial cancer cohorts. Gynecol Oncol. 2016;140(2):270–276. doi:10.1016/j.ygyno.2015.11.019
231. Lemańska A, Zaborowski M, Spaczyński M, Nowak-Markwitz E. Do endometrial cancer patients benefit from metformin intake? Ginekol Pol. 2015;86(6):419–423. doi:10.17772/gp/2397
232. Nevadunsky NS, Van Arsdale A, Strickler HD, et al. Metformin use and endometrial cancer survival. Gynecol Oncol. 2014;132(1):236–240. doi:10.1016/j.ygyno.2013.10.026
233. Hall C, Stone RL, Gehlot A, Zorn KK, Burnett AF. Use of Metformin in obese women with type I endometrial cancer is associated with a reduced incidence of cancer recurrence. Int J Gynecol Cancer. 2016;26(2):313–317. doi:10.1097/igc.0000000000000603
234. Meireles CG, Pereira SA, Valadares LP, et al. Effects of metformin on endometrial cancer: systematic review and meta-analysis. Gynecol Oncol. 2017;147(1):167–180. doi:10.1016/j.ygyno.2017.07.120
235. Zhang ZJ, Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2014;16(8):707–710. doi:10.1111/dom.12267
236. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi:10.1002/ijc.29210
237. Kim HG, Hien TT, Han EH, et al. Metformin inhibits P-glycoprotein expression via the NF-κB pathway and CRE transcriptional activity through AMPK activation. Br J Pharmacol. 2011;162(5):1096–1108. doi:10.1111/j.1476-5381.2010.01101.x
238. Barrett D, Brown VI, Grupp SA, Teachey DT. Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Paediatr Drugs. 2012;14(5):299–316. doi:10.2165/11594740-000000000-00000
239. Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi:10.1038/nature04703
240. Grimaldi C, Chiarini F, Tabellini G, et al. AMP-dependent kinase/mammalian target of rapamycin complex 1 signaling in T-cell acute lymphoblastic leukemia: therapeutic implications. Leukemia. 2012;26(1):91–100. doi:10.1038/leu.2011.269
241. Leclerc GM, Leclerc GJ, Kuznetsov JN, DeSalvo J, Barredo JC. Metformin induces apoptosis through AMPK-dependent inhibition of UPR signaling in ALL lymphoblasts. PLoS One. 2013;8(8):e74420. doi:10.1371/journal.pone.0074420
242. Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi:10.1007/978-3-319-07323-1_4
243. Zhang J, Yu XH, Yan YG, Wang C, Wang WJ. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 2015;444:182–192. doi:10.1016/j.cca.2014.12.041
244. Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18(10):1605–1619. doi:10.1093/annonc/mdm070
245. Gao F, Huang W, Zhang Y, et al. Hes1 promotes cell proliferation and migration by activating Bmi-1 and PTEN/Akt/GSK3β pathway in human colon cancer. Oncotarget. 2015;6(36):38667–38680. doi:10.18632/oncotarget.5484
246. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
247. He Z, Liang J, Wang B. Inhibin, beta A regulates the transforming growth factor-beta pathway to promote malignant biological behaviour in colorectal cancer. Cell Biochem Funct. 2021;39(2):258–266. doi:10.1002/cbf.3573
248. Carlton AL, Illendula A, Gao Y, et al. Small molecule inhibition of the CBFβ/RUNX interaction decreases ovarian cancer growth and migration through alterations in genes related to epithelial-to-mesenchymal transition. Gynecol Oncol. 2018;149(2):350–360. doi:10.1016/j.ygyno.2018.03.005
249. Wang Q, Wen YG, Li DP, et al. Upregulated INHBA expression is associated with poor survival in gastric cancer. Med Oncol. 2012;29(1):77–83. doi:10.1007/s12032-010-9766-y
250. Zhang K, Zhang M, Luo Z, Wen Z, Yan X. The dichotomous role of TGF-β in controlling liver cancer cell survival and proliferation. J Genet Genomics. 2020;47(9):497–512. doi:10.1016/j.jgg.2020.09.005
251. Deng M, Lei S, Huang D, et al. Suppressive effects of metformin on colorectal adenoma incidence and malignant progression. Pathol Res Pract. 2020;216(2):152775. doi:10.1016/j.prp.2019.152775
252. Hou YC, Hu Q, Huang J, Fang JY, Xiong H. Metformin therapy and the risk of colorectal adenoma in patients with type 2 diabetes: a meta-analysis. Oncotarget. 2017;8(5):8843–8853. doi:10.18632/oncotarget.13633
253. Ala M. The emerging role of metformin in the prevention and treatment of colorectal cancer: a game changer for the management of colorectal cancer. Curr Diabetes Rev. 2022;18(8):e051121197762. doi:10.2174/1573399818666211105125129
254. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
255. Petti C, Vegetti C, Molla A, et al. AMPK activators inhibit the proliferation of human melanomas bearing the activated MAPK pathway. Melanoma Res. 2012;22(5):341–350. doi:10.1097/CMR.0b013e3283544929
256. Tomic T, Botton T, Cerezo M, et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011;2(9):e199. doi:10.1038/cddis.2011.86
257. Janjetovic K, Harhaji-Trajkovic L, Misirkic-Marjanovic M, et al. In vitro and in vivo anti-melanoma action of metformin. Eur J Pharmacol. 2011;668(3):373–382. doi:10.1016/j.ejphar.2011.07.004
258. Cerezo M, Tichet M, Abbe P, et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner. Mol Cancer Ther. 2013;12(8):1605–1615. doi:10.1158/1535-7163.Mct-12-1226-t
259. Niehr F, von Euw E, Attar N, et al. Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations. J Transl Med. 2011;9:76. doi:10.1186/1479-5876-9-76
260. Hirsch HA, Iliopoulos D, Struhl K. Metformin inhibits the inflammatory response associated with cellular transformation and cancer stem cell growth. Proc Natl Acad Sci USA. 2013;110(3):972–977. doi:10.1073/pnas.1221055110
261. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301(6):H2181–90. doi:10.1152/ajpheart.00554.2011
262. Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes. 2006;55(2):496–505. doi:10.2337/diabetes.55.02.06.db05-1064
263. Loi H, Boal F, Tronchere H, et al. Metformin protects the heart against hypertrophic and apoptotic remodeling after myocardial infarction. Front Pharmacol. 2019;10:154. doi:10.3389/fphar.2019.00154
264. Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10(4):233–240. doi:10.1038/nrg2523
265. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi:10.1016/s0092-8674(04)00452-0
266. Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120(Pt 15):2479–2487. doi:10.1242/jcs.001222
267. Dei Cas A, Spigoni V, Ridolfi V, Metra M. Diabetes and chronic heart failure: from diabetic cardiomyopathy to therapeutic approach. Endocr Metab Immune Disord Drug Targets. 2013;13(1):38–50. doi:10.2174/1871530311313010006
268. Yang X, Xu Z, Zhang C, Cai Z, Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim Biophys Acta Mol Basis Dis. 2017;1863(8):1984–1990. doi:10.1016/j.bbadis.2016.09.019
269. Hernández JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med. 2014;18(4):709–720. doi:10.1111/jcmm.12220
270. Yanazume T, Morimoto T, Wada H, Kawamura T, Hasegawa K. Biological role of p300 in cardiac myocytes. Mol Cell Biochem. 2003;248(1–2):115–119. doi:10.1023/a:1024132217870
271. Bertrand L, Ginion A, Beauloye C, et al. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291(1):H239–50. doi:10.1152/ajpheart.01269.2005
272. Anisimov VN. Metformin for aging and cancer prevention. Aging. 2010;2(11):760–774. doi:10.18632/aging.100230
273. Spindler SR. Caloric restriction: from soup to nuts. Ageing Res Rev. 2010;9(3):324–353. doi:10.1016/j.arr.2009.10.003
274. Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. Transcriptional profile of aging in C. elegans. Curr Biol. 2002;12(18):1566–1573. doi:10.1016/s0960-9822(02)01146-6
275. Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–1346. doi:10.1126/science.1077991
276. Andújar-Plata P, Pi-Sunyer X, Laferrère B. Metformin effects revisited. Diabetes Res Clin Pract. 2012;95(1):1–9. doi:10.1016/j.diabres.2011.09.022
277. Verdaguer E, Junyent F, Folch J, et al. Aging biology: a new frontier for drug discovery. Expert Opin Drug Discov. 2012;7(3):217–229. doi:10.1517/17460441.2012.660144
278. Isoda K, Young JL, Zirlik A, et al. Metformin inhibits proinflammatory responses and nuclear factor-kappaB in human vascular wall cells. Arterioscler Thromb Vasc Biol. 2006;26(3):611–617. doi:10.1161/01.Atv.0000201938.78044.75
279. Glossmann Hartmut H, Lutz Oliver MD. Metformin and Aging: a Review. Gerontology. 2019;65(6):581–590.
280. Mohammed I, Hollenberg MD, Ding H, Triggle CR. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front Endocrinol. 2021;12:718942. doi:10.3389/fendo.2021.718942
281. Walton RG, Dungan CM, Long DE, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell. 2019;18(6):e13039. doi:10.1111/acel.13039
282. Wensink MJ, Lu Y, Tian L, et al. Preconception antidiabetic drugs in men and birth defects in offspring.: a nationwide cohort study. Ann Intern Med. 2022;175(5):665–673. doi:10.7326/M21-4389
283. Niemuth NJ, Jordan R, Crago J, Blanksma C, Johnson R, Klaper RD. Metformin exposure at environmentally relevant concentrations causes potential endocrine disruption in adult male fish. Environ Toxicol Chem. 2015;34(2):291–296. doi:10.1002/etc.2793
284. Niemuth NJ, Klaper RD. Emerging wastewater contaminant metformin causes intersex and reduced fecundity in fish. Chemosphere. 2015;135:38–45. doi:10.1016/j.chemosphere.2015.03.060
285. Niemuth NJ, Klaper RD. Low-dose metformin exposure causes changes in expression of endocrine disruption-associated genes. Aquat Toxicol. 2018;195:33–40. doi:10.1016/j.aquatox.2017.12.003
286. Madiraju AK, Qiu Y, Perry RJ, et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med. 2018;24(9):1384–1394. doi:10.1038/s41591-018-0125-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
