Back to Journals » Drug Design, Development and Therapy » Volume 16
Metabolism and Mass Balance in Rats Following Oral Administration of the Novel Antifibrotic Drug Fluorofenidone
Received 28 October 2021
Accepted for publication 7 February 2022
Published 30 March 2022 Volume 2022:16 Pages 973—979
DOI https://doi.org/10.2147/DDDT.S346661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Wei Wu,1 Ze-neng Cheng2
1Department of Pharmacy, The First Hospital of Changsha, Changsha, Hunan, People’s Republic of China; 2Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, Hunan, People’s Republic of China
Correspondence: Ze-neng Cheng, Xiangya School of Pharmaceutical Sciences, Central South University, No. 172 Tongzipo Road, Yuelu District, Changsha, 410013, Hunan, People’s Republic of China, Tel +86-731-82650001, Email [email protected]
Objective: Fluorofenidone (AKF-PD) is a novel antifibrotic small-molecule compound. The purpose of this study was to investigate the metabolic and excretory pathways of AKF-PD in rats.
Methods: High-performance liquid chromatography with mass spectrometric (HPLC-MS) detection was used to analyze the metabolites in rat urine. The metabolites were separated by chromatography and their structure was confirmed. HPLC was used to determine the contents of the parent compound and its metabolites in feces and urine after quantitative administration to study the excretion pathway.
Results: AKF-PD was mainly oxidized to the carboxyl group after methyl hydroxylation. After oral administration, the total amount of the prototype drug and its hydroxylated metabolites and carboxylated metabolites excreted from the urine and feces of rats was 87%. However, most of them are excreted in urine and feces in the form of carboxylated metabolites, and rarely excreted in the form of prototype drugs and hydroxylated metabolites. Which is that the urinary discharge of hydroxylated metabolites, fluorine ketones, and carboxylated metabolites were 0.2%, 1.1%, and 75.2%, respectively, while the fecal discharge were 0.2%, 0.3%, and 10.1%, respectively.
Conclusion: AKF-PD is mainly oxidized into 2-hydroxymethyl and 5-carboxyl AKF-PD through the Phase I metabolic reaction in rats. AKF-PD is a highly permeable compound classified by biopharmaceutics and is mainly excreted from the urine in the form of metabolites.
Keywords: fluorofenidone, metabolites, methyl hydroxylation, HPLC-MS
Introduction
Fluorofenidone (AKF-PD) is a novel antifibrotic small-molecule compound1–3 that is the structural analog of pirfenidone.4–6 Previous studies of the metabolic pathway of AKF-PD (see Figure 1) have found that there are two main metabolites of AKF-PD in rats:7–12 methyl hydroxylation (M1) and carboxylated (M2) products of AKF-PD.13 Based on the structural formula of AKF-PD, combined with the theory of drug metabolism and reference to the metabolic pathway of the homologous compound pirfenidone, a possible metabolic pathway can be predicted (see Figure 1). In the present study, after intragastric administration of AKF-PD to rats, samples of urine and feces were collected for the extraction of metabolites; the metabolite content was then determined in order to study the mass balance of AKF-PD.
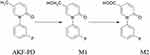 |
Figure 1 The metabolic pathway of AKF-PD. |
Materials and Methods
Chemicals
AKF-PD (batch no.: 070701, content: 99.9%), AKF-PD hydroxylated metabolites (M1, batch no.: 070930, content: 93.0%), AKF-PD carboxylic metabolites (M2, batch no.: 070715, content: 97.8%), and carboxylated pirfenidone (batch no.: 070511, content: 99.8%) were provided by the School of Pharmacy, Central South University. Methanol (batch no.: 707108) and formic acid (batch no.: 409101) were of analytical or high-performance liquid chromatography (HPLC) grade and were supplied by Tedia, USA. Sodium carboxymethyl cellulose (CMC-Na) (batch no.: 031021) was supplied by Shantou Xilong Chemical Factory, China. Purified water was supplied by Hangzhou Wahaha Group Co., Ltd, China.
Experimental Animals
Sprague–Dawley (SD) rats of both genders with a mean weight of 160–200 g were provided by the Department of Experimental Animal Science, Central South University, license no.: SCXK (Hunan) 2006-0002.
Study Design
Fourteen SD rats (seven male and seven female) were placed in metabolic cages for three days. Their feed was ground into powder, and each rat was fed 25 ± 2 g per day and given water freely. Blank urine and fecal samples of SD rats for 24h before administration were collected on the night of the third day, followed by fasting for 12h. On the morning of the fourth day, each rat was given a single gavage (120 mg), and then the urine and fecal samples of rats 24h after administration were collected.
Administration of AKF-PD and the Determination of AKF-PD and Its Hydroxylated Metabolites (M1) in Rat Urine
The AKF-PD was crushed through a 100-mesh sieve, and 0.12 g was accurately weighed and placed into a 5 mL syringe. A total of 2 mL 0.5% CMC-Na aqueous solution was added and mixed with the crushed AKF-PD. After that, the mixture was administered to the rats via gavage, and the remaining part of the syringe was washed out with methanol into a 250 mL volumetric flask (Tables 1 and 2). Water was added to the scale, which was shaken well. A total of 100 µL of this solution was added to 400 µL of internal standard carboxylated pirfenidone acetonitrile solution (5µg/mL) and mixed well. And 10ul mixing was used for the simultaneous detection of AKF-PD and its hydroxylated metabolites (M1) in rat urine samples by HPLC-UV. After that, the detection of the residual amount of AKF-PD in order to calculate an accurate dosage.
 |
Table 1 The Number, Basic Condition and Dosage of Each Tested Animal |
 |
Table 2 Dosage and Measurement of Residual Flufenidone After Administration |
Sample Collection
After administration, urine samples at 0–3, 3–6, 6–10, 10–14, 14–24, 24–48, and 48–72 hours and feces samples at 0–24, 24–48, and 48–72 hours were collected. Before each collection, the collection funnel was rinsed with approximately 3 mL of 95% ethanol. After the rinsing solution was mixed with urine, the volume was measured and the urine samples were stored at –20°C. The feces were dried at 40°C for 12 hours and stored at room temperature, and they were weighed before measurement.
Analysis of the Urine and Feces Samples
Determination of AKF-PD and Its Carboxylated Metabolites (M2) in Rat Urine
HPLC-UV Conditions
Chromatographic conditions: HPLC ultraviolet detector (Agilent 1200 with Workstation B.03.01); chromatographic column (Synergi™ 4 µm Hydro-RP 80 Å 250×4.6 mm, Phenomenex); mobile phase: acetonitrile: 0.1% formic acid aqueous solution= 14.5:85.5(v/v) (0–22 mins), acetonitrile: 0.1% formic acid aqueous solution= 90:10 (v/v) (22.1–25 mins), acetonitrile: 0.1% formic acid aqueous solution= 14.5:85.5 (v/v) (25.1–29 mins); flow rate 1.0 mL/min; wavelength 310 nm; injection volume 10 µL.
Sample Pretreatment
A total of 100 µL of rat urine was added to 400 µL of methanol solution of carboxylated pirfenidone (25µg/mL), mixed well, and centrifuged at 15,400 × g for 10 minutes; the supernatant was extracted for injection analysis. Samples with excessive concentrations were diluted before being injected. Dilution method: 100 µL of urine was added to 900 µL of water and mixed well; 100 µL was taken for injection analysis.
Simultaneous Determination of AKF-PD and Its Hydroxylated Metabolites (M1) in Rat Urine
HPLC-UV Conditions
Chromatographic conditions: HPLC ultraviolet detector (Shimadzu LC-2010AHT with LC Solution workstation); chromatographic column (Synergi™ 4 µm Hydro-RP 80 Å 250×4.6 mm, Phenomenex); mobile phase: acetonitrile: 0.1% formic acid aqueous solution = 14.5: 85.5 (v/v) (0–15 mins), acetonitrile: 0.1% formic acid aqueous solution = 40: 60 (v/v) (15.1–22 mins), acetonitrile: 0.1% formic acid aqueous solution = 14.5: 85.5 (v/v) (22.1–26.2 min); flow rate 1.0 mL/min; wavelength 310nm; injection volume 10µL.
Treatment of Samples
A total of 100 µL of rat urine was added to 400 µL of acetonitrile solution (5ug/mL) of carboxylated pirfenidone as an internal standard, mixed well, and centrifuged at 15,400 ×g for 10 minutes. The supernatant was extracted for injection analysis.
Simultaneous Determination of AKF-PD and Its Metabolites (M1 and M2) in Rat Feces
HPLC-UV Conditions
Chromatographic conditions: HPLC ultraviolet detector (Shimadzu LC-2010AHT with LC Solution workstation); chromatographic column (SynergiTM 4 µm Hydro-RP 80 Å 250x4.6 mm, Phenomenex); mobile phase: acetonitrile 0.1% formic acid aqueous solution = 14.5: 85.5(v/v) (0–21 mins), acetonitrile : 0.1% formic acid aqueous solution = 35: 65 (v/v) (21.1–28 mins), acetonitrile : 0.1% formic acid aqueous solution = 14.5: 85.5 (v/v) (28.1–34 mins); flow rate 1.0 mL/min; wavelength 310 nm; injection volume 10 µL.
Treatment of Fecal Samples
The feces samples of rat taken at 24 hours were stored and weighed at room temperature after drying. They were then soaked in 10 mL of methanol for 10 hours and ground in a mortar, before being extracted with a small amount of methanol several times. The extract was transferred to a 25mL volumetric flask, methanol was added, and the flask was shaken well, before the solution was filtered through a 0.45µm microporous membrane. A total of 100µL of internal standard carboxylated pirfenidone acetonitrile (25µg/mL) was added to 1mL of the filtrate and mixed. The residue was dried under nitrogen flow, dissolved in 100µL of 50% methanol aqueous solution, and centrifuged at 15,400 ×g for 10 minutes. The supernatant was taken for sample analysis.
Results
Dosage
Table 3 shows the basic information and administration information of each experimental animal. The actual dose was equal to the dose given with the remaining dose subtracted. After gavage of the rats, the remaining part of the syringe was washed out with methanol into a 250mL or 500mL volume flask, to which water was added. The flask was shaken well, and 100μL of the solution was added to 400ul of internal standard carboxylated pirfenidone acetonitrile solution (5µg/mL) and mixed well. AKF-PD and its hydroxylated metabolites (M1) were simultaneously detected with 10ul of the mixture through the HPLC-UV. After that, the detection of the residual amount of AKF-PD would be detected.
 |
Table 3 The Content and Total Recovery Rate of AKF-PD and Its Metabolites M1 and M2 |
Determination of AKF-PD and Its Metabolites in the Urine and Feces of Rats
The content detection results for M1, M2, and AKF-PD in the rat urine and feces samples are shown in Table 3. Flufenidone was mainly excreted in the form of carboxylated metabolites (M2) in rats urin and feces, mainly through kidneys. And the recovery rate of the drug was about 87.07%. There was no statistical difference in total excretion and carboxylated metabolites (M2) between male and female rats (Figure 2).
 |
Figure 2 The amount of excretion in rat urine (μmoL) the total excretion of rats. |
Material Balance
Total excretion was calculated as the total amount of AKF-PD and its metabolites M1 and M2 excreted in the urine and feces. Total excretion divided by actual dose and multiplied by 100% was the total recovery. The calculation results are shown in Table 3. The urinary and fecal excretion rates of AKF-PD and its main metabolites M1 and M2 are shown in Table 4.
 |
Table 4 Excretion Rates (%) of AKF-PD and Its Metabolites M1 and M2 in Urine and Feces of Rats |
Discussion and Conclusion
AKF-PD is a pyridone compound, which is a newly discovered anti-organ or tissue fibrosis drug. It has a good effect on various organ fibrotic diseases such as liver fibrosis, kidney fibrosis and so on. However, there are few reports on the AKF-PD, metabolites of AKF-PD and the quality control methods of their preparations by combining animal experiments with HPLC-MS at home and abroad. In this paper, experiment combined with HPLC-MS was established to determine the content of AKF-PD in rats after administration. The method was simple, specific, good separation and accurate, it can effectively study the effects of AKF-PD on metabolism and excretion pathway in rats.
After the rats were given AKF-PD intragastrically, 98% of the total excretion was excreted in the form of carboxylated metabolites and there were few prototype drugs and hydroxylated metabolites from the results that combines animal experiments with HPLC-MS. And the main excretion route was kidney excretion, with bile excretion only accounting for approximately 11% of the total excretion. Which was no significant difference between male and female rats. The total recovery rate in this study was 87.07%, below 90%, but the prototype drug excreted from the stool was 0.3% below the dose. In addition, AKF-PD belongs to a compound with high permeability and liposolubility, which is mainly oxidized into 2-hydroxymethyl and 5-carboxyl AKF-PD through the phase I metabolic reaction in rats. Therefore, the influence of liver drug enzyme activity on drugs should be further considered.
The metabolism and excretion mechanism of AKF-PD in rats was studied by substance balance and corresponding mechanism, in order to provide theoretical reference for further study of AKF-PD.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval
Institutional ethical issues: The study protocol for animal experiments was approved by the Institutional Animal Care and Use Committee of Xiangya School of Pharmeceutical Science, Central South University and conformed to the guidelines of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Every effort was made to minimize their pain, suffering, and death.
Funding
2021 Hunan Natural Science Foundation, Project No.: 2021JJ80017): Construction and Evaluation of PV/PD Data Quantitative Evaluation System, Quality Risk Early Warning Model Based on Period Management System.2021 Hunan Provincial Health Commission, Project No.: 202113010356: Development and Evaluation of PV/PD Data Quantitative Evaluation Information System Module for Phase I Clinical Research, and Clinical Pharmacy Research Fund of Hunan Medical Association, Project No.: HMA202001006: A real-world Evaluation of Safety of The Panax Notoginseng Saponin Injection: An Active Monitoring Study by Hospital Pharmacovigilance System.
Disclosure
The authors report no personal, financial, commercial, or academic conflicts of interest in this work.
References
1. Cao W, He L, Liu W, Huang Z, Cheng Z. Determination of AKF-PD in whole blood of rat by HPLC-UV. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;833(2):257–259. doi:10.1016/j.jchromb.2006.01.010
2. Jiang F, Li S, Jiang Y, Chen Z, Wang T, Liu W. Fluorofenidone attenuates paraquat-induced pulmonary fibrosis by regulating the PI3K/Akt/mTOR signaling pathway and autophagy. Mol Med Rep. 2021;23(6):405. doi:10.1016/j.bmcl.2012.01.073
3. Dai Q, Zhang Y, Liao X, et al. Fluorofenidone alleviates renal fibrosis by inhibiting necroptosis through RIPK3/mlKL pathway. Front Pharmacol. 2020;11:534775. doi:10.3389/fphar.2020.534775
4. Li L, Luo X, Cheng Z. In vitro inhibition and induction of human liver cytochrome P450 enzymes by a novel anti-fibrotic drug fluorofenidone. Xenobiotica. 2020;51(7):745–751. doi:10.1080/00498254.2020.1820626
5. Lv M, Liu Y, Ma S, Yu Z. Current advances in idiopathic pulmonary fibrosis: the pathogenesis, therapeutic strategies and candidate molecules. Future Med Chem. 2019;11(19):2595–2620. doi:10.4155/fmc-2019-0111
6. Liu YM, Nepali K, Liou JP. Idiopathic pulmonary fibrosis: current status, recent progress, and emerging targets. J Med Chem. 2017;60(2):527–553. doi:10.1021/acs.jmedchem.6b00935
7. Jiang Y, Quan J, Chen Y, et al. Fluorofenidone protects against acute kidney injury. FASEB J. 2019;33(12):14325–14336. doi:10.1096/fj.201901468RR
8. Liao X, Jiang Y, Dai Q, et al. Fluorofenidone attenuates renal fibrosis by inhibiting the mtROS-NLRP3 pathway in a murine model of folic acid nephropathy. Biochem Biophys Res Commun. 2020;534:694–701. doi:10.1016/j.bbrc.2020.11.017
9. Yang H, Zhang W, Xie T, Wang X, Ning W. Fluorofenidone inhibits apoptosis of renal tubular epithelial cells in rats with renal interstitial fibrosis. Braz J Med Biol Res. 2019;52(11):e8772. doi:10.1590/1414-431X20198772
10. Chen Y, Wang N, Yuan Q, et al. The protective effect of fluorofenidone against cyclosporine A-induced nephrotoxicity. Kidney Blood Press Res. 2019;44(4):656–668. doi:10.1159/000500924
11. Tang Y, Zhang F, Huang L, et al. The protective mechanism of fluorofenidone in renal interstitial inflammation and fibrosis. Am J Med Sci. 2015;350(3):195–203. doi:10.1097/MAJ.0000000000000501
12. Tang J, Li J, Li G, et al. Spermidine-mediated poly(lactic-co-glycolic acid) nanoparticles containing fluorofenidone for the treatment of idiopathic pulmonary fibrosis. Int J Nanomedicine. 2017;12:6687–6704. doi:10.2147/IJN.S140569
13. Chen J, Lu MM, Liu B, et al. Synthesis and structure-activity relationship of 5-substituent-2(1H)-pyridone derivatives as anti-fibrosis agents. Bioorg Med Chem Lett. 2012;22(6):2300–2302. doi:10.1016/j.bmcl.2012.01.073
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
