Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Metabolic Alterations and Related Biological Functions of Post-Stroke Depression in Ischemic Stroke Patients
Authors Wen L, Yan C, Zheng W, Li Y , Wang Y, Qu M
Received 31 March 2023
Accepted for publication 20 June 2023
Published 6 July 2023 Volume 2023:19 Pages 1555—1564
DOI https://doi.org/10.2147/NDT.S415141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Lulu Wen,1 Chuming Yan,1 Wancheng Zheng,1 Yi Li,2 Yuhui Wang,2 Miao Qu1
1Neurology Department, Xuan Wu Hospital Capital Medical University, Beijing, People’s Republic of China; 2Neurology Department, Third Affiliated Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
Correspondence: Miao Qu, Neurology Department, Xuan Wu Hospital Capital Medical University, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-1083198420, Email [email protected]
Background: Post-stroke depression (PSD) is one of the most common neuropsychiatric complications after stroke. However, the underlying mechanisms of PSD remain ambiguous, and no objective diagnosis tool is available to diagnose PSD. Previous metabolomic studies on PSD included patients with ischemic and hemorrhagic stroke indiscriminately, which is not conducive to elucidating and predicting the occurrence of PSD. The aim of this study is to elucidate the pathogenesis of PSD and provide potential diagnostic markers for PSD in ischemic stroke patients.
Methods: In total, 51 ischemic stroke patients at 2 weeks were included in this study. Those with depressive symptoms were assigned to the PSD group, while the others were assigned to the non-PSD group. Plasma metabolomics based on liquid chromatography–mass spectrometry (LC-MS) was performed to explore the differential plasma metabolites between the PSD and non-PSD groups.
Results: Principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) showed significant metabolic alterations between PSD patients and non-PSD patients. In total, 41 differential metabolites were screened out, mainly including phosphatidylcholines (PCs), L-carnitine and acyl carnitines, succinic acid, pyruvic acid and L-lactic acid. Metabolite-related pathway analysis revealed that alanine, aspartate and glutamate metabolism, glycerophospholipid metabolism and the citrate cycle (TCA cycle) may contribute to the pathogenesis of PSD. A panel of three signature metabolites [PC(22:5(7Z,10Z,13Z,16Z,19Z)/15:0), LysoPA(18:1(9Z)/0:0) and 1,5-anhydrosorbitol] was determined as potential biomarkers for PSD in ischemic stroke patients.
Conclusion: These findings are conducive to providing new insights into the pathogenesis of PSD and developing objective diagnostic tools for PSD in ischemic stroke patients.
Keywords: post-stroke depression, ischemic stroke, metabolomics, mechanisms, biomarker
Introduction
At present, stroke is one of the leading causes of serious long-term disability and mortality in China. One large nationally representative study showed that the incidence and mortality rates of stroke in China in 2020 were 505.2 per 100,000 person-years and 343.4 per 100,000 person-years, respectively.1 Post-stroke depression (PSD) is one of the most common neuropsychiatric complications after stroke, affecting approximately 33% of stroke patients.2,3 Specifically, one large cohort study with 157,243 stroke patients suggested that the incidence of depression in ischemic stroke patients was higher than that in hemorrhagic stroke patients within 2 years, at 28.0% and 23.3%, respectively.4 Moreover, PSD is associated with a range of poor outcomes, including cognitive impairment,5 low quality of life,6 and enhanced all-cause mortality.7 In clinical practice, PSD is always screened by subjective tools, such as the Hamilton Depression Rating Scale (HDRS) and nine-item Patient Health Questionnaire (PHQ-9), lacking objective diagnostic markers.8 Indeed, PSD is often underdiagnosed and undertreated owing to the use of subjective tools and overlaps with other complex symptoms caused by stroke.9 Therefore, it is necessary to find objective tools for the early diagnosis of PSD and thereby improve the prognosis of PSD patients.
Many studies have found that PSD is a multifactorial disease, associated with gene polymorphism,10 pre-stroke depression,11 stroke severity,12 lesion location,13 social support14 and years of education.15 Numerous studies have aimed to propose theoretical hypotheses to explain the mechanism of PSD. For instance, overactivation of the hypothalamic–pituitary–adrenal (HPA) axis induced excessive release of cortisol and regulated neuron survival and neurogenesis, which is related to late-onset PSD (3 years).16,17 Then, elevated neuroinflammation could influence every pathological domain of PSD, such as the HPA axis18 and neurotransmitter metabolism.19 Glutamate-mediated excitotoxicity was proved to be associated with the occurrence of PSD. Besides, the levels of monoamines and neurotrophic factors are decreased in PSD, and traditional antidepressants such as sertraline and nortriptyline targeting these mechanisms have been proved to be effective for PSD.20–23 However, a population-based cohort study with 60,746 patients showed that patients using antidepressants had a significantly increased risk of adverse outcomes, including hemorrhagic complications, gastrointestinal bleeding and suicide.24 Therefore, in view of the current ambiguous pathogenesis and limited targeted therapy, it is necessary to explore the mechanism of PSD in more dimensions.
Metabolomics reveals the systematic disorders of metabolites in disease states, and has been widely used to elucidate and predict the occurrence of PSD. A systematic review showed that differential urinary metabolites detected by gas chromatography–mass spectrometry (GC-MS) had good potential for the differentiation of PSD.25 One plasma metabolomic study based on liquid chromatography–mass spectrometry (LC-MS) showed that amino acid metabolism, lipid metabolism and oxidative stress were associated with PSD.26 Another plasma metabolomic study based on proton nuclear magnetic resonance (1H-NMR) indicated that disorders of neurotransmitter levels and oxidative stress were involved in the initiation of PSD.27 However, these studies often included patients with ischemic and hemorrhagic stroke indiscriminately. Although these studies can predict the occurrence of PSD to some extent, this is not conducive to the interpretation of the pathogenesis of PSD. Besides, LC-MS has higher sensitivity and a more simplified sample pretreatment process than GC-MS and NMR.28 Therefore, an LC-MS-based metabolomic study of PSD patients is required for ischemic stroke and for hemorrhagic stroke.
In this study, we intend to detect the metabolic profile in non-PSD patients and PSD patients at 2 weeks, based on the LC-MS method. The aim of this study is to elucidate the pathogenesis of PSD and provide potential diagnostic markers for PSD in ischemic stroke patients.
Methods
Participants and Procedures
This study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University (LYS [2020]096), and registered in clinical trials (ChiCTR2100041895). Our study complied with the Declaration of Helsinki. As shown in Figure S1, ischemic stroke patients were recruited within 3 days after admission, from June 2021 to April 2022. All patients signed the informed consent forms. Inclusion criteria of the subjects were as follows: 1) age 18 years or older; 2) acute ischemic stroke determined by cranial magnetic resonance imaging (MRI) within 3 days after admission; and 3) obtained informed consent. Excluded criteria for all participants were as follows: 1) history of depression; 2) cerebral hemorrhage and mixed apoplexy; 3) lack of effective cooperation, such as aphasia, agnosia or cognitive impairment; and 4) serious systemic diseases, such as heart failure, liver and kidney failure; malignant tumor, hyperthyroidism or hematological disorder. At 2 weeks of follow-up, 51 ischemic stroke patients (17 PSD patients and 34 non-PSD patients) were included. PSD patients were screened and diagnosed by experienced physicians using the Hamilton Depression Rating Scale (HDRS) and Statistical Manual of Mental Disorders-IV (DSM-IV) for depressive symptoms. The optimal cut-off value on the HDRS for PSD was 8.29 Ischemic stroke patients who did not meet the PSD diagnostic criteria were classified as the non-PSD group.
Plasma Sample Collection
At 2 weeks of follow-up, venous blood (5 mL) was extracted from the median vein and inhaled into an ethylenediaminetetraacetic acid (EDTA) anticoagulant tube under negative pressure. Then, the samples were centrifuged at 1300 rpm for 10 min at 4°C. The upper plasma was extracted and placed in 500 µL cryopreservation tubes. Finally, plasma samples were frozen in a refrigerator at −80°C.
Liquid Chromatography–Mass Spectrometry (LC-MS) Analysis
The untargeted metabolomic profile in plasma was detected by the LC-MS method. In brief, 100 μL of plasma was added to 300 μL ice-cold acetonitrile, vortexed for 10 min at 25°C and incubated at −20°C for 1 hour. After that, the mixed fluid was centrifuged at 14,000 g and 4°C for 15 min, and the supernatant fluid was added to the Accucore HILIC column (100 × 2.1 mm, 2.6 μm). The washing effluents of the positive mode were eluent A (0.1% FA in 95% ACN, 10 mM ammonium acetate) and eluent B (acetonitrile), and washing effluents of negative mode eluent A and eluent C (50% ACN, 10 mM ammonium acetate, pH 9.0). The solvent gradient was as follows: 2% B/C, 1 min; 2–50% B/C, 16.5 min; 50–2% B/C, 2.5 min. Finally, a Q-Exactive HF-X mass spectrometer (Thermo Fisher Scientific, USA) was operated under positive and negative ionization modes (ESI+, ESI−).
Data Analysis
Statistical analysis of the basic information was conducted using SPSS 26.0 (SPSS, Chicago, IL, USA). All continuous variables with a non-normal distribution were described as median (IQR), and the Mann–Whitney U-test was used to compare between-group differences. Differences in categorical variables between groups were compared by the chi-squared test. For metabolomic analysis, the content of each metabolite was expressed by the ion peak area. After eliminating deviation values and missing values, the missing values were filled up by half of the minimum value. Then, the dataset was imported to SIMCA 13.0 software package (Sartorius Stedim Data Analytics AB, Umea, Sweden) for further analysis. The data were scaled and logarithmically transformed to minimize the effects of noise and high variance of variables. After that, principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA) and orthogonal partial least-squares discriminant analysis (OPLS-DA) were performed to discriminate non-PSD and PSD patients. Variable importance in the projection (VIP) values obtained from OPLS-DA were applied to determine the plasma metabolites important for PSD discrimination. The significance of metabolites was preliminarily screened by the non-parametric Mann–Whitney U-test (P<0.05). Finally, metabolites with VIP >1.0 and P<0.05 were confirmed as significant variables. The online software MetaboAnalyst 5.0 (https://www.metaboanalyst.ca/) was used to perform pathway analysis. The conditional logistic regression analysis was conducted to screen out a panel of potential biomarkers for diagnosing PSD in ischemic stroke patients. The “pROC” R package was used to display receiver operating characteristics (ROC) curve analysis and evaluate the sensitivity and specificity of metabolites (The R Foundation; version 4.1.0).
Results
Basic Information on Participants
In total, 51 ischemic stroke patients (34 non-PSD patients, 17 PSD patients) were included in the study. The basic information on the patients is shown in Table 1. There were no differences in age, sex, National Institutes of Health Stroke Scale (NHISS), modified Rankin Scale (mRS), Trial of Org 10172 in Acute Stroke Treatment (TOAST) type or treatment therapy between the non-PSD and PSD groups. HDRS was higher in the PSD group than in the non-PSD group (P<0.001). In particular, the TOAST type was formulated in the trial of Org 10172 (Danaparoid) in acute ischemic stroke treatment in 1993.30
 |
Table 1 Basic Information on the Recruited Ischemic Stroke Patients |
Metabolic Alterations in Non-PSD and PSD Patients
PCA is an unsupervised dimensionality reduction method, which was first applied to determine the metabolic alterations between non-PSD and PSD patients (Figure 1A). PLS-DA is a multivariate statistical analysis method with a supervised pattern recognition function, which can find the variables most responsible for grouping and reduce the influence of unrelated interference factors. The PLS-DA scores plot suggested that there were marked metabolic differences distinguishing PSD patients from non-PSD patients (Figure 1B). The OPLS-DA combined orthogonal signal correction (OSC) with PLS-DA, and further removed unrelated variables. As shown in Figure 1C, the OPLS-DA model significantly divided non-PSD and PSD patients into the left and right sides of the first principal component. These results indicated that there were significant differences in metabolites between non-PSD and PSD patients.
Differential Metabolites Between Non-PSD and PSD Patients
In total, 41 plasma metabolites with VIP >1 in the OPLS-DA model and P<0.05 were confirmed as differential metabolites between non-PSD and PSD patients. The detailed information on the metabolites is summarized in Table 2. Twenty differential metabolites were classified as glycerophospholipids, of which 17 phosphatidylcholines (PCs) were down-regulated and three metabolites were up-regulated in the PSD group. Four metabolites were organic acids and derivatives, among which succinic acid increased, and N-acetyl-L-aspartic acid, L-lactic acid and pyruvic acid decreased in the PSD group. Four metabolites were carnitines and acylcarnitines, which were down-regulated in the PSD group, and comprised L-carnitine, propionyl-carnitine, butyryl-carnitine and cervonyl-carnitine. Two metabolites, 13S-hydroxyoctadecadienoic acid and 5Z-dodecenoic acid, were in the fatty acyl class; the former increased and the latter decreased in the PSD group. Three indoles and derivatives, namely 6-hydroxy-1H-indole-3-acetamide, 4-hydroxy-1H-indole-3-acetonitrile and 3-indoleacetonitrile, were reduced in the PSD group. Two sphingolipids, SM(d16:1/24:1(15Z)) and SM(d18:0/14:0), were identified; the former increased and the latter decreased in the PSD group. Other chemicals, namely 4-hydroxyphenylpyruvic acid, naringenin, 3-O-acetylepisamarcandin, oxidized adrenal ferredoxin, N-acetyl-L-aspartic acid and 1,5-anhydrosorbitol, were reduced in the PSD group, while 6,10,14-trimethyl-5,9,13-pentadecatrien-2-one was enhanced in the PSD group.
 |
Table 2 Differential Metabolites Between Non-PSD and PSD Patients |
Pathway Analysis
To explore the biological functions of these differential metabolites, the enriched pathways significantly affecting PSD were obtained using the online software MetaboAnalyst 5.0 (Figure 2). The top five enriched pathways were alanine, aspartate and glutamate metabolism (P<0.0001, impact=0.087), glycerophospholipid metabolism (P<0.0001, impact=0.252), the citrate cycle (TCA cycle) (P=0.005, impact=0.079), pyruvate metabolism (P=0.007, impact=0.207) and glycolysis/gluconeogenesis (P=0.009, impact=0.100). These results revealed the possible metabolic changes contributing to the pathogenesis of PSD.
 |
Figure 2 Enriched pathways based on differential plasma metabolites. |
Potential Metabolic Biomarkers for PSD in Ischemic Stroke Patients
To determine the potential diagnostic biomarkers for PSD in ischemic stroke patients, ROC curve analysis was performed based on the differential metabolites. The top five metabolites in terms of the area under the curve (AUC), as listed in Table 3, were PC(22:5(4Z,7Z,10Z,13Z,16Z)/14:0)) (AUC=0.815), PC(22:4(7Z,10Z,13Z,16Z)/18:1(11Z)) (AUC=0.798), succinic acid (AUC=0.782), 1,5-anhydrosorbitol (AUC=0.751) and 6,10,14-trimethyl-5,9,13-pentadecatrien-2-one (AUC=0.742). In addition, conditional logistic regression analysis was conducted based on total 41 differential metabolites to screen out a panel of potential biomarkers for PSD. Three metabolites, PC(22:5(7Z,10Z,13Z,16Z,19Z)/15:0), LysoPA(18:1(9Z)/0:0) and 1,5-anhydrosorbitol, were finally determined as the potential biomarker panel, which could effectively discriminate PSD patients from non-PSD patients with an AUC value of 0.894 (Figure 3).
 |
Table 3 Top Five Differential Metabolites, with AUC Values |
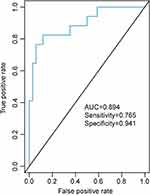 |
Figure 3 ROC curve analysis of the panel composed of three signature metabolites. |
Discussion
This study was based on using LC-MS metabolomics to explore the metabolic alterations for PSD in ischemic stroke patients. A series of differential metabolites were screened out, including PCs, L-carnitine and acyl carnitines, succinic acid, pyruvic acid and L-lactic acid. Metabolite-related pathway analysis revealed that alanine, aspartate and glutamate metabolism, glycerophospholipid metabolism and the citrate cycle (TCA cycle) may contribute to the pathogenesis of PSD. A panel of three signature metabolites [PC(22:5(7Z,10Z,13Z,16Z,19Z)/15:0), LysoPA(18:1(9Z)/0:0) and 1,5-anhydrosorbitol] was determined as potential biomarkers for PSD in ischemic stroke patients.
PCs are the most abundant phosphatides in an organism, accounting for 40–50% of the total phospholipids in all mammalian cells and subcellular organelles.31 PCs are mainly derived from dietary supplementation and biosynthesis via the choline pathway.32 Previous studies showed that PCs can inhibit the inflammatory response, and promote the expression of neurotrophic factors and synaptic function in animal models,33,34 while inflammation and synaptic dysfunction are the recognized mechanisms for PSD.2 Moreover, one study showed that phospholipid supplementation could inhibit depressive-like behaviors in mice with vaccine-related neurological manifestations.35 In this study, decreased PC-related glycerophospholipid metabolism was enriched in PSD patients, which was consistent with changes in metabolomics in the hippocampus of rats with PSD.36 Therefore, PC-related glycerophospholipid metabolism may become a potential target for intervention in PSD.
Our results suggest that L-carnitine and several acyl carnitines were decreased in PSD patients compared to non-PSD patients. About 75% of L-carnitine comes from dietary intake and 25% from biosynthesis from amino acids in the body.37 L-Carnitine and acyl carnitines play an important role in mitochondrial fatty acid oxidation, enhancing cholinergic nerve transmission, etc.38,39 A lack of L-carnitine can lead to severe symptoms in central nervous system diseases, such as encephalopathy,40 depression37 and bipolar disorder,41 while L-carnitine supplementation can significantly improve disease symptoms.42,43 Moreover, low serum L-carnitine levels have been related to first stroke in Chinese adults with hypertension.44 Thus, abnormal L-carnitine and acyl carnitine levels may provide novel clues for clarifying the mechanisms of PSD.
Energy metabolism is the cornerstone of homeostasis, including glycolysis, the TCA cycle, pyruvate metabolism and fatty acid metabolism. TCA cycle disorders have been found in stroke mouse and chronic unpredictable mild stress (CUMS) rat models.45,46 We found that pyruvic acid and L-lactic acid decreased, while succinic acid increased in the PSD group. Pyruvic acid and L-lactic acid are key final products of glycolysis, as well as substrates of the TCA cycle, while succinic acid is the crucial chemical in the TCA cycle. Therefore, disorders of glycolysis and the TCA cycle may contribute to the pathogenesis of PSD. Besides, pyruvate supplementation can reduce lesion volume and brain edema, and scavenge glutamate, thereby improving the neurological deficit and anxiety-like behavior in stroke rats;47 this finding needs further validation.
The optimal screening tools for PSD diagnosis have always been limited to subjective questionnaires, such as the Center of Epidemiological Studies Depression Scale (CES-D), HDRS and PHQ-9, and an objective diagnostic tool for PSD is lacking at present.48 Various metabolomic studies have been performed to elucidate the occurrence of PSD. Two plasma metabolomic studies showed that amino acid metabolism, lipid metabolism and oxidative stress were closely related to PSD, although no potential biomarkers for PSD were screened.26 Another plasma metabolomic study identified five plasma metabolites [phenylalanine, tyrosine, 1-methylhistidine, 3-methylhistidine and LDL CH3-(CH2)n-] distinguishing PSD from non-PSD subjects.27 Three non-invasive urine metabolomic studies determined effective diagnostic panels for PSD diagnosis.49–51 However, these studies all included ischemic stroke patients and hemorrhagic stroke patients for further screening of PSD, which may lead to great heterogeneity in the determination of potential biomarkers for PSD. Our study confirmed a panel of three signature metabolites [PC(22:5(7Z,10Z,13Z,16Z,19Z)/15:0), LysoPA(18:1(9Z)/0:0) and 1,5-anhydrosorbitol] as potential biomarkers for PSD in ischemic stroke patients.
Limitations and Strengths
Some limitations should be noted in this study. First, the sample size was small, and the results lack effective internal and external verification. Secondly, although basic information, such as the NHISS and mRS, showed no influence on our results, factors such as marriage and years of education should be further evaluated. Thirdly, the differential metabolites in plasma were not further verified by targeted metabolomic or other biochemical methods. Fourthly, since TOAST type52 and treatment methods53 may affect the pathogenesis of PSD, the effects of these factors on plasma metabolomics in PSD patients should also be explored in large samples in the future. The strengths of this study are that we explored the metabolic alterations and identified a panel of biomarkers for PSD only in ischemic stroke patients.
Conclusions
In summary, this study determined the plasma metabolomics in ischemic stroke patients to elucidate and predict the occurrence of PSD. In total, 41 differential metabolites were confirmed, and a panel of three metabolites was determined as potential biomarkers for PSD. PC-related glycerophospholipid metabolism, abnormal L-carnitine and acyl carnitine levels, and glycolysis and TCA cycle disorders contribute to the pathogenesis of PSD. These findings are conducive to providing new insights into the pathogenesis of PSD and developing objective diagnostic tools for PSD.
Data Sharing Statement
Since this is a prospective cohort study and is still in progress, the data cannot be fully disclosed. If necessary, readers can contact the corresponding author to obtain publicly available data.
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers 81973759; 82274444).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
2. Villa RF, Ferrari F, Moretti A. Post-stroke depression: mechanisms and pharmacological treatment. Pharmacol Ther. 2018;184:131–144. doi:10.1016/j.pharmthera.2017.11.005
3. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
4. Jørgensen TSH, Wium-Andersen IK, Wium-Andersen MK, et al. Incidence of depression after stroke, and associated risk factors and mortality outcomes, in a large cohort of Danish patients. JAMA Psychiatry. 2016;73(10):1032–1040. doi:10.1001/jamapsychiatry.2016.1932
5. Serrano S, Domingo J, Rodríguez-Garcia E, Castro MD, Del Ser T. Frequency of cognitive impairment without dementia in patients with stroke: a two-year follow-up study. Stroke. 2007;38(1):105–110. doi:10.1161/01.STR.0000251804.13102.c0
6. Ezema CI, Akusoba PC, Nweke MC, Uchewoke CU, Agono J, Usoro G. Influence of post-stroke depression on functional independence in activities of daily living. Ethiop J Health Sci. 2019;29(1):841–846. doi:10.4314/ejhs.v29i1.5
7. Cai W, Mueller C, Li YJ, Shen WD, Stewart R. Post stroke depression and risk of stroke recurrence and mortality: a systematic review and meta-analysis. Ageing Res Rev. 2019;50:102–109. doi:10.1016/j.arr.2019.01.013
8. Das J, Rajanikant GK. Post stroke depression: the sequelae of cerebral stroke. Neurosci Biobehav Rev. 2018;90:104–114. doi:10.1016/j.neubiorev.2018.04.005
9. Medeiros GC, Roy D, Kontos N, Beach SR. Post-stroke depression: a 2020 updated review. Gen Hosp Psychiatry. 2020;66:70–80. doi:10.1016/j.genhosppsych.2020.06.011
10. Kohen R, Cain KC, Mitchell PH, et al. Association of serotonin transporter gene polymorphisms with poststroke depression. Arch Gen Psychiatry. 2008;65(11):1296–1302. doi:10.1001/archpsyc.65.11.1296
11. Taylor-Rowan M, Momoh O, Ayerbe L, Evans JJ, Stott DJ, Quinn TJ. Prevalence of pre-stroke depression and its association with post-stroke depression: a systematic review and meta-analysis. Psychol Med. 2019;49(4):685–696. doi:10.1017/S0033291718002003
12. Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(10):2296–2301. doi:10.1161/01.STR.0000183622.75135.a4
13. Robinson RG, Szetela B. Mood change following left hemispheric brain injury. Ann Neurol. 1981;9(5):447–453. doi:10.1002/ana.410090506
14. Shi Y, Yang D, Zeng Y, Wu W. Risk factors for post-stroke depression: a meta-analysis. Front Aging Neurosci. 2017;9:218. doi:10.3389/fnagi.2017.00218
15. Backhouse EV, McHutchison CA, Cvoro V, Shenkin SD, Wardlaw JM, Priller J. Cognitive ability, education and socioeconomic status in childhood and risk of post-stroke depression in later life: a systematic review and meta-analysis. PLoS One. 2018;13(7):e0200525. doi:10.1371/journal.pone.0200525
16. Herbert J, Goodyer IM, Grossman AB, et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18(6):393–411. doi:10.1111/j.1365-2826.2006.01429.x
17. Appelros P, Viitanen M. Prevalence and predictors of depression at one year in a Swedish population-based cohort with first-ever stroke. J Stroke Cerebrovasc Dis. 2004;13(2):52–57. doi:10.1016/j.jstrokecerebrovasdis.2004.02.005
18. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi:10.1016/j.it.2005.11.006
19. Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):702–721. doi:10.1016/j.pnpbp.2010.12.017
20. Zahrai A, Vahid-Ansari F, Daigle M, Albert PR. Fluoxetine-induced recovery of serotonin and norepinephrine projections in a mouse model of post-stroke depression. Transl Psychiatry. 2020;10(1):334. doi:10.1038/s41398-020-01008-9
21. Zhang E, Liao P. Brain-derived neurotrophic factor and post-stroke depression. J Neurosci Res. 2020;98(3):537–548. doi:10.1002/jnr.24510
22. Murray V, von Arbin M, Bartfai A, et al. Double-blind comparison of sertraline and placebo in stroke patients with minor depression and less severe major depression. J Clin Psychiatry. 2005;66(6):708–716. doi:10.4088/JCP.v66n0606
23. Lipsey JR, Robinson RG, Pearlson GD, Rao K, Price TR. Nortriptyline treatment of post-stroke depression: a double-blind study. Lancet. 1984;1(8372):297–300. doi:10.1016/S0140-6736(84)90356-8
24. Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343(aug02 1):d4551. doi:10.1136/bmj.d4551
25. Cai W, Wang XF, Wei XF, et al. Does urinary metabolite signature act as a biomarker of post-stroke depression? Front Psychiatry. 2022;13:928076. doi:10.3389/fpsyt.2022.928076
26. Wang M, Gui X, Wu L, et al. Amino acid metabolism, lipid metabolism, and oxidative stress are associated with post-stroke depression: a metabonomics study. BMC Neurol. 2020;20(1):250. doi:10.1186/s12883-020-01780-7
27. Hu Z, Fan S, Liu M, et al. Objective diagnosis of post-stroke depression using NMR-based plasma metabonomics. Neuropsychiatr Dis Treat. 2019;15:867–881. doi:10.2147/NDT.S192307
28. Geisberger T, Diederich P, Steiner T, Eisenreich W, Schmitt-Kopplin P, Huber C. Evolutionary steps in the analytics of primordial metabolic evolution. Life. 2019;9(2). doi:10.3390/life9020050
29. Naarding P, Leentjens AF, van Kooten F, Verhey FR. Disease-specific properties of the rating scale for depression in patients with stroke, Alzheimer’s dementia, and Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(3):329–334. doi:10.1176/jnp.14.3.329
30. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi:10.1161/01.STR.24.1.35
31. van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, Jacobs RL. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9 Pt B):1558–1572. doi:10.1016/j.bbamem.2017.04.006
32. Kent C. Regulation of phosphatidylcholine biosynthesis. Prog Lipid Res. 1990;29(2):87–105. doi:10.1016/0163-7827(90)90010-I
33. Tan W, Zhang Q, Dong Z, et al. Phosphatidylcholine ameliorates LPS-induced systemic inflammation and cognitive impairments via mediating the gut-brain axis balance. J Agric Food Chem. 2020;68(50):14884–14895. doi:10.1021/acs.jafc.0c06383
34. Gao X, Du L, Randell E, Zhang H, Li K, Li D. Effect of different phosphatidylcholines on high fat diet-induced insulin resistance in mice. Food Funct. 2021;12(4):1516–1528. doi:10.1039/D0FO02632H
35. Kivity S, Arango MT, Molano-González N, Blank M, Shoenfeld Y. Phospholipid supplementation can attenuate vaccine-induced depressive-like behavior in mice. Immunol Res. 2017;65(1):99–105. doi:10.1007/s12026-016-8818-6
36. Jiang W, Chen J, Gong L, Liu F, Zhao H, Mu J. Alteration of glycerophospholipid metabolism in hippocampus of post-stroke depression rats. Neurochem Res. 2022;47(7):2052–2063. doi:10.1007/s11064-022-03596-y
37. Liu T, Deng K, Xue Y, et al. Carnitine and depression. Front Nutr. 2022;9:853058. doi:10.3389/fnut.2022.853058
38. Parnetti L, Gaiti A, Mecocci P, Cadini D, Senin U. Pharmacokinetics of IV and oral acetyl-L-carnitine in a multiple dose regimen in patients with senile dementia of Alzheimer type. Eur J Clin Pharmacol. 1992;42(1):89–93. doi:10.1007/BF00314926
39. Reuter SE, Evans AM. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clin Pharmacokinet. 2012;51(9):553–572. doi:10.1007/BF03261931
40. Maldonado C, Vázquez M, Fagiolino P. Potential therapeutic role of carnitine and acetylcarnitine in neurological disorders. Curr Pharm Des. 2020;26(12):1277–1285. doi:10.2174/1381612826666200212114038
41. Brennan BP, Jensen JE, Hudson JI, et al. A placebo-controlled trial of acetyl-L-carnitine and α-lipoic acid in the treatment of bipolar depression. J Clin Psychopharmacol. 2013;33(5):627–635. doi:10.1097/JCP.0b013e31829a83f5
42. Kepka A, Ochocinska A, Borzym-Kluczyk M, et al. Preventive role of L-carnitine and balanced diet in Alzheimer’s Disease. Nutrients. 2020;12(7):1987. doi:10.3390/nu12071987
43. Tashiro K, Kaida Y, Yamagishi SI, et al. L-carnitine supplementation improves self-rating depression scale scores in uremic male patients undergoing hemodialysis. Lett Drug Des Discov. 2017;14(6):737–742. doi:10.2174/1570180814666170216102632
44. Zhou Z, Zhang N, Song Y, et al. serum l-carnitine levels are associated with first stroke in Chinese adults with hypertension. Stroke. 2022;53(10):3091–3098. doi:10.1161/STROKEAHA.121.038487
45. Tanaka E, Ogawa Y, Fujii R, et al. Metabolomic analysis and mass spectrometry imaging after neonatal stroke and cell therapies in mouse brains. Sci Rep. 2020;10(1):21881. doi:10.1038/s41598-020-78930-x
46. Ling-Hu T, Liu SB, Gao Y, Han YM, Tian JS, Qin XM. Stable isotope-resolved metabolomics reveals the abnormal brain glucose catabolism in depression based on chronic unpredictable mild stress rats. J Proteome Res. 2021;20(7):3549–3558. doi:10.1021/acs.jproteome.1c00155
47. Frank D, Kuts R, Tsenter P, et al. The effect of pyruvate on the development and progression of post-stroke depression: a new therapeutic approach. Neuropharmacology. 2019;155:173–184. doi:10.1016/j.neuropharm.2019.05.035
48. Meader N, Moe-Byrne T, Llewellyn A, Mitchell AJ. Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry. 2014;85(2):198–206. doi:10.1136/jnnp-2012-304194
49. Zhang W, Zhang XA. A novel urinary metabolite signature for non-invasive post-stroke depression diagnosis. Cell Biochem Biophys. 2015;72(3):661–667. doi:10.1007/s12013-014-0472-9
50. Xie J, Han Y, Hong Y, et al. Identification of potential metabolite markers for middle-aged patients with post-stroke depression using urine metabolomics. Neuropsychiatr Dis Treat. 2020;16:2017–2024. doi:10.2147/NDT.S271990
51. Chen J, Lv YN, Li XB, et al. Urinary metabolite signatures for predicting elderly stroke survivors with depression. Neuropsychiatr Dis Treat. 2021;17:925–933. doi:10.2147/NDT.S299835
52. Wang KW, Xu YM, Lou CB, Huang J, Feng C. The etiologies of post-stroke depression: different between lacunar stroke and non-lacunar stroke. Clinics. 2022;77:100095. doi:10.1016/j.clinsp.2022.100095
53. Königsberg A, Sehner S, Arlt S, et al. Effect of intravenous alteplase on post-stroke depression in the WAKE UP trial. Eur J Neurol. 2021;28(6):2017–2025. doi:10.1111/ene.14797
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

