Back to Journals » Journal of Inflammation Research » Volume 16
Mesenchymal Stem Cells in Heterotopic Ossification in Ankylosing Spondylitis: A Bibliometric Study Based on CiteSpace and VOSViewer
Received 5 June 2023
Accepted for publication 20 September 2023
Published 4 October 2023 Volume 2023:16 Pages 4389—4398
DOI https://doi.org/10.2147/JIR.S421962
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Zhaoyi Liu,1,* Qing Yu,1,2,* Hongxiao Liu2,*
1Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Department of Rheumatology, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongxiao Liu, Tel +86-15165152738, Email [email protected]; [email protected]
Background: Heterotopic ossification is a complication in the late stage of ankylosing spondylitis (AS), and involves abnormal osteogenesis by mesenchymal stem cells (MSC). Research activity in this area has been rapidly expanding, but there is a lack of bibliometric studies that summarize the progresses.
Methods: We searched the Web of Science (WoS) for articles pertaining to the role of MSCs in heterotopic ossification in AS from the database inception to December 2022 and visualized the countries, authors, institutions, references, and keywords using CiteSpace 6.1.R6 and VOSViewer.
Results: A total of 127 publications from 188 institutions were identified, with a trend for increasing number of articles per year. China published the largest number of literature, followed by the United States and France. There were 47 core authors. The most recent research in this area mainly focused on “osteogenic differentiation”, “gene expression”, “inflammation”, “TNF-α” and “bone formation”. Current research can be broadly summarized into two topics: abnormalities in the inflammatory microenvironment and abnormalities in the MSCs. Aberrant expression of a variety of surface proteins in MSCs predisposes these cells to undergo osteogenic differentiation, and pro-inflammatory cytokines in the inflammatory milieu stimulate osteogenic differentiation of MSCs.
Conclusion: MSCs in heterotopic ossification in AS is a relatively new area of research. Research activities primarily consist abnormalities in the inflammatory microenvironment and abnormalities in the MSCs.
Keywords: ankylosing spondylitis: mesenchymal stem cells, VOSViewer, CiteSpace, heterotopic ossification, visualization analysis
Introduction
Ankylosing spondylitis (AS) is a chronic, progressive autoimmune disease that primarily involves the axial skeleton and sacroiliac joints.1 The early stage of AS manifests as tendonitis and ligamentous appendagitis, followed by articular bone destruction, fibrosis and ossification of the cartilage and ligaments. The late stage is characterized by spinal ankylosis, deformity, and severe functional impairment.2 Co-morbidities include acute anterior uveitis, psoriasis, and inflammatory bowel disease (IBD); these conditions are sometimes considered as extra-articular manifestations of AS.3
Estimated prevalence of AS ranges from 0.7 to 49 cases per 10,000 population, and caries substantially across different geographical locations. The average prevalence in North America, Europe, Asia, Latin America, and Africa is 31.9, 23.8, 16.7, 10.2, and 7.4 per 10,000 population, respectively.4 AS is more common in males; a meta-analysis that included eight studies and 2236 patients with AS showed a male predominance (70.4%) in AS.5 Disease onset is typically between 20 and 30 years of age.6
Heterotopic ossification (ossification of soft tissues outside the normal skeletal system) is a complication in the late stage of AS. In the commonly accepted framework, heterotopic ossification requires three conditions: 1) osteogenic inducers, 2) osteogenic precursor cells, and 3) a microenvironment that allows osteogenesis.7 Mesenchymal stem cells (MSC) represent an important source of osteogenic precursor cells,6 as well as a critical component of the inflammatory processes that triggers heterotopic ossification in AS.8 Research activity in this area has been rapidly expanding during the past two decades, but no bibliometric studies have been conducted to summarize the recent progresses and forecast the future trend.9
Materials and Methods
Data Retrieval and Collection
We searched the Web of Science (WoS), the most authoritative and comprehensive data source,10 from the database inception to December 2022. The article type was restricted to Original Article and Review Article, and the language was restricted to English. The search formula was (ALL=((Ankylosing spondylitis) OR (axial spondyloarthritis) OR (non-radiographic axial SpA) OR (nr-axSpA) OR (Rickshaw) OR (Bamboo Wind) OR (Turtle leeward) OR (Keung) OR (Kidney arthralgia))) AND ALL=((Bone Marrow Stromal Stem Cells OR mesenchymal stem cells)).
Data Analysis
The following information was exported from each of the articles identified in the electronic search: journal source, title, authors, research institution, abstract, and keywords. Data were visualized with countries, authors, institutions, co-cited literature, and keywords as nodes. Keywords were standardized by combining keywords with identical meaning but different expressions.
Results
Trends in the Number of Published Articles
A total of 127 articles were retrieved. Original articles accounted for 67% (85/127) of the total production. The earliest article was published in 2008. The average number of published articles per year during the index period (2008 to 2022) was 8.5. There was an overall upward trend in the number of published articles (Figure 1). The rate of growth was slow until 2017, and increased substantially after 2017.
 |
Figure 1 Trends in the number of annual publications. The y-axis on the left was the cumulative number of publications; the y-axis on the right was the number of publications in the indicated year. |
Country Analysis
Most of the published articles were conducted by researchers in East Asia, West Asia, Europe, North America, and Oceania. China (n = 75) ranked first in the total numbers of publications, followed by South Korea (n = 16), the United States (n = 16), France (n = 8), Germany and Poland (n = 6 for each) (Figure 2). Among the top ten countries in terms of number of publications, France ranked first in the average number of citations (n = 61.63), followed by the South Korea (n = 32.19), England (n = 31.40) and the United States (n = 29.94); China ranking eighth (n = 19.04) (Table 1). There was close cooperation among the research groups from North America, Europe, and East Asia (Figure 3).
 |
Table 1 Top 10 Countries with the Most Publications |
 |
Figure 2 The number of publications by country (node size indicates the number of publications). |
 |
Figure 3 Collaboration network among research groups from different countries (node size indicates strength of the collaboration). |
Institutional Analysis
A total of 188 institutions contributed to the 127 published articles. The institution with the highest number of articles was Sun Yat Sen University (n = 34), followed by the Chinese University of Hong Kong (n = 9), Yonsei University (n = 6), University of Lyon I (n = 5), and Hanyang University (n = 5) (Table 2). Figure 4 shows the inter-institutional cooperation network among research institutions.
 |
Table 2 Top 10 Institutions with Highest Number of Publications |
 |
Figure 4 Collaboration among different institutions (dot size indicates strength of association). |
Author Analysis
The author with the highest number of publications was Shen Huiyong (n = 26), followed by Wang Peng (n = 25), Wu Yanfeng (n = 25), Xie Zhongyu (n = 21), and Li Jinteng (n = 16), all from Sun Yat Sen University (Table 3). The author with the strongest collaboration strength was Shen Huiyong, followed by Wu Yanfeng, Wang Peng, Xie Zhongyu, and Li Jinteng. Among the contributing authors, Wu Yanfeng, Wang Peng, and Shen Huiyong collaborated to publish a large number of articles, most notably during a period around 2018 (Figure 5). A calculation based on the Price Law,9 34 core authors published four or more articles.
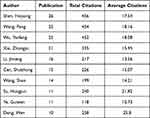 |
Table 3 Top Ten Authors in Terms of Publications |
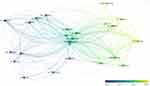 |
Figure 5 Collaboration network among the core authors (size of dots indicates strength of ties, color indicates time of posting). |
Co-Citation Analysis
Among the 127 publications, a total of 10 articles were cited 10 or more times, with a maximum of 30 citations. Figure 6 is a co-occurrence diagram of co-cited references (reference that is cited by two or more articles), and shows the authors of the most-cited references and the time of publication of the references. Most of these articles were based on research after 2010 (Figure 6A). The top ten keyword clusters with the largest distribution were as follows: tumor necrosis factor-alpha (TNF-α), infliximab, interleukin-17 (IL-17), human leukocyte antigen-B27 (HLA-B27), rheumatoid arthritis, osteogenic differentiation, mesenchymal stem cell, helper cell subsets, and bone. Other keywords with a large distribution range included IL-17 and disease activity (Figure 6B). In topic cluster analysis, clusters with large distribution range included lncRNA, MSC treatment, spondyloarthritis pathogenesis, TNF, and morphogenetic protein (Figure 6C). The earliest year of publication with top 10 outbreak intensity was in 2014 (n = 2), and the latest was 2022 (n = 3). The highest outbreak intensity was 3.2. Some of these articles will be specifically demonstrated in the discussion section (Figure 6D).
Keyword Analysis
The top ten cited keywords included: ankylosing spondylitis (64 times), mesenchymal stem cell (64 times), stromal cell (24 times), differentiation (23 times), osteogenic differentiation (20 times), rheumatoid arthritis (19 times), expression (19 times), bone formation (11 times), spondyloarthritis (9 times), and therapy (9 times) (Figure 7A). The citation frequency was five or more for 26 keywords, and 10 or more for 9 keywords. The top ten keywords were noncontact induction, neurite outgrowth, altered bone remodeling, HLA-B27-mediated activation, mesenchymal stem cell treatment, osteogenic differentiation, stromal cell, gastrointestinal malignancy, integrative genomic analysis, aberrant TGF-beta activation, and microRNA.
Changes of keywords over time are shown in Figure 7B. The top keywords during the entire index period included “osteogenic differentiation”, “symptoms” and “biomarkers”, and more recently, “disease”, “osteoporosis”, “systemic sclerosis” and “transplantation” (Figure 7C).
Keywords with increasing burst strength recently included “proliferation” “gene” and “therapy”. Keywords with high strength (≥2) include “spinal cord injury” “necrosis factor alpha” “TNF alpha” “disease” “spondyloarthritis” and “disease activity” (Figure 7D).
Discussion
Overall, the number of publications pertaining to MSCs in heterotopic ossification in AS is small and the annual growth fluctuated significantly, suggesting that this is a relatively new field. The higher rate of growth after 2017 suggested accelerated research activity in the field. The core authors published majority of their research data around 2018. Since 2008, increasing number of authors joined the field. China ranked first in terms of the number of articles published, and four of the top ten institutions in terms of number of articles published were in China. However, the average citation for these articles is relatively low, indicating that overall quality of articles in China is relatively low.
In keyword clustering, the largest cluster label was noncontact induction (differentiation of MSCs under stimulation from local microenvironment and regulatory factors).11 The study of regulators and receptors of MSCs was a hot topic after 2008. Research content can be summarized as abnormalities in inflammatory microenvironment and abnormalities in MSC itself.
Abnormalities in the Inflammatory Microenvironment
The co-citation analysis of Helix keyword analysis revealed a number of relevant clustering tags, including TNF, Treg-promoted new bone formation, HLA-B27-mediated activation, helper cell subsets, and IL-17. The analysis of outbreak words revealed spinal cord injury, TNF alpha, activation, and disease activity, all related to the inflammatory microenvironment, indicating that research on the inflammatory microenvironment accounts for a large part of the research in this field.
Th17 cells are capable of secreting the pro-inflammatory factor IL-17, whereas Treg exert their immune effects by releasing the inhibitory cytokines IL-10 and TGF-β.12 The relationship between MSC and the Th/Treg balance has been increasingly studied recently.13 In 2011, Wu published the first article on this subject, and reported diminished immunomodulation capacity of MSC derived from AS patients and its association with Th/Treg imbalance in AS patients. Wu has since published a total of 25 articles and became the second most published core author in the field with high citation. T helper cell subsets emerged as the ninth largest label in the field in terms of area when analyzed by keywords in terms of co-citations. Searching for Th17 cells and Treg cells yielded nine and six publications, respectively. In 2021, Kuca-Warnawin published a paper in the journal Cell showing that MSC induction in AS patients disrupts Th/Treg homeostasis, enhances Treg production when co-cultured with peripheral blood single nucleated cells, and may have a direct pro-inflammatory effect.14 In 2019, Fan showed that Treg in AS patients can promote ossification by suppressing Th17 cells.15
TNF-α is a key pro-inflammatory cytokine.16 In 2009, Hess found that TNF-α mediates NF-kappa B activation to support osteogenic differentiation and matrix mineralization by increasing BMP-2 expression.17 In 2011, Lencel found an association between TNF-α with heterotopic ossification in arthritis.18 In 2013, Briolay showed that anti-TNF-α treatment could limit the inflammatory phase of AS but is indirectly involved in heterotopic ossification. TNF-α significantly elevates the levels of Wnt10b and Wnt5a in MSC to stimulate ossification. The role of Wnt5a was not be limited by DKK-1.19 Since the publication of these two articles, the role of TNF-α in heterotopic ossification attracted increasing attention. In 2014, Osta published a review to summarize the role of TNF-α bone stabilization as “classical and paradoxical effects”.20 The DKK-1 regulator and the Wnt pathway associated with TNF-α have also attracted increasing interest. In 2011, Gisela showed DKK-1 is down-regulated in MSCs and osteoblasts, and activation of the Wnt pathway and enhanced osteoblast activity in AS patients.21 A total of 33 articles on TNF-α were published in this field, and TNF-α was the cluster with the largest area in the co-citation analysis of this field and became the outbreak word in this field from 2015 to 2018 with an outbreak intensity of 2.67.
Bone morphogenetic protein (BMP) is the only cytokine capable of individually inducing the differentiation of mesenchymal stem cells to osteoblasts and is the most critical factor in bone tissue formation.22 BMPs produce act mainly by regulating the SMAD pathway. Noggin is a specific antagonist of BMPs, and its expression in osteoblasts is induced by BMPs.23 In 2009, Lories emphasized the role of BMPs in a variety of bone and joint-related diseases, including AS, suggesting that inhibition of BMPs may be useful in limiting the progression of AS.18 BMP was one of the first breakout words in the field, with a total of ten directly related articles. BMP2 began to emerge as a breakout word in the field in 2019. As early as 1996, Riley studied the role of BMP, and found low concentrations of BMP can induce the migration of MSCs to the bone tissue formation area; medium concentrations of BMP can promote the differentiation of MSCs to cartilage and osteoblasts; and high concentrations of BMP can promote the proliferation of MSCs.24 Effects of BMP-2 vary significantly depending upon its concentration, suggesting a regulatory role for BMP in the full spectrum of the processes relevant to heterotopic ossification, from homing to differentiation of MSC into osteoblasts. In 2016, Xie found that MSC from AS patients had enhanced osteogenic differentiation, secreted more BMP and less Noggin, and inhibition of BMP secretion could alleviate abnormal ossification.22 This article has been cited 56 times. Xie later became a core author in the field, publishing a total of 12 articles from 2019 to 2022.
Abnormalities in the MSC
With the further development of research on ectopic ossification in AS, researchers have gradually found that the abnormal osteogenic differentiation of MSC in AS patients is not only related to the abnormal inflammatory environment, heterotopic ossification in AS is also associated with abnormalities in the MSCs themselves.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize evolutionarily conserved molecules of pathogenic microorganisms. Activation of TLRs induces a strong immune response, but overt response is detrimental. In 2008, Mo found that TLRs play an important role in the response of MSC to bacterial toxins commonly present in the human body when they studied the proliferation and osteogenic differentiation of MSC.25 Initially, TLR was thought to participate in innate immunity and seemed to be irrelevant to AS, but subsequent studies suggested otherwise. In 2018, Li found that both TLR3 and TLR4 were downregulated in AS-MSC, p38 and the ERK MAPK signaling pathway are easier to be triggered in AS-MSC.26 In 2021, Li investigated the expression profiles of long non-coding RNAs (lncRNAs) and messenger RNAs (mRNAs) in TLR4-triggered AS-MSCs, and found that the RNAs with the largest multiplicity of changes were associated with the NOD-like receptor (NLR) signaling pathway, TNF signaling pathway and NF-Kappa B signaling pathway.27
The pathogenesis of AS is strongly correlated with HLA-B27, and abnormalities in the HLA-B27 genotype have been linked to ectopic ossification. In 2009, Raimo found that in HLA-B27 positive individuals, inflammatory factors released in genitourinary injuries could act on TLRs in MSC to trigger heterotopic ossification.28 This study found an association between MSC osteogenic differentiation and AS and recognized that infection-induced inflammation can induce heterotopic ossification. More recent studies confirmed a link between HLA-B27 and ectopic ossification in AS, a process that is associated with the intracellular effects of misfolded HLA-B2729 and spliced X-box-binding protein b1 (sXBP1)/retinoic acid receptor-beta (RARB)/tissue-nonspecific alkaline phosphatase (TNAP) axis pathway correlation.30
Limitations
This study has some limitations. Firstly, only the Web of Science was searched. Secondly, studies published in non-English language were not included. Thirdly, since bibliometrics measures whether an article is important or not by the strength of its links to other articles, some content that is important but not widely recognized by researchers may have been ignored. Fourthly, studies published in 2023 were not included.
Conclusion
MSCs in heterotopic ossification in AS is currently in the early stages of development. Research activity has been increasingly focused on regulatory factors and pathways, and the treatment of heterotopic ossification via MSC transplantation. These studies suggest heterotopic ossification in AS involves both abnormal MSCs and pro-inflammatory cytokines. The proinflammatory factors in the environment promote MSC osteogenic differentiation. AS-MSC have their own pathological changes that make them more sensitive to the promotional effects of inflammation. In addition, the proinflammatory nature of AS-MSC can further exacerbate this process. Also, multiple molecular pathways interact with each other to form a complex network.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):210770. doi:10.1136/annrheumdis-2016-210770
2. Ma L, Li Y. Progress of combined diagnosis and treatment of bone loss in ankylosing spondylitis with Chinese and Western medicine. Chin J Osteop. 2017;23(01):029. doi:10.3969/j.issn.1006-7108.2017.01.029
3. Mielants H, Van den Bosch F. Extra-articular manifestations. Clin Exp Rheumatol. 2009;27(4):S56–61.
4. Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology. 2014;53(4):650–657. doi:10.1093/rheumatology/ket387
5. de Winter JJ, van Mens LJ, van der Heijde D, et al. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther. 2016;18(1):196. doi:10.1186/s13075-016-1093-z
6. Liu Y, Zhao Y, Xia Q. Advances in the diagnosis and treatment of ankylosing spondylitis. Chin J Orthop. 2015;23(03):235–238. doi:10.3977/j.issn.1005-8478.2015.03.09
7. Mao Y, Wang M, Wu X. Heterotopic ossification. Chin J Orthop Trauma. 2004;2004:78–82.
8. Lu X, Liu T, Meng W, Zhu H-L, Xi Y-M, Liu Y-M. Immunomodulatory effects of human bone marrow mesenchymal stem cells on T lymphocytes. J Exp Hematol. 2005;13:651–655. doi:10.3969/j.issn.1009-2137.2005.04.025
9. Wang CD. Quantitative study of journal authors. Infor Sci. 1998;16(6):471–475.
10. Meho LI, Yang K. Impact of data sources on citation counts and rankings of LIS faculty: web of science versus Scopus and google scholar. J Am Soc Inf Sci Technol. 2007;58:2105–2125. doi:10.1002/asi.20677
11. Tian L, Liang X, Wang Z. Experimental observation on the induction and differentiation of MSC to chondrocytes by non-contact co-culture in vitro. Chin Med Herald. 2008;117(19):16–18. doi:10.3969/j.issn.1673-7210.2008.19.012
12. Yang J, Liu X, Li Q. Progress of the role of Th17/Treg balance in rheumatoid arthritis. Chin Pharmacol Bull. 2013;29(08):1045–1048. doi:10.3969/j.issn.1001-1978.2013.08.003
13. Wu Y, Ren M, Yang R, et al. Reduced immunomodulation potential of bone marrow-derived mesenchymal stem cells induced CCR4+CCR6+ Th/Treg cell subset imbalance in ankylosing spondylitis. Arthritis Res Ther. 2011;13(1):R29. doi:10.1186/ar3257
14. Kuca-Warnawin E, Janicka I, Bonek K, et al. Modulatory impact of adipose-derived mesenchymal stem cells of ankylosing spondylitis patients on T helper cell differentiation. Cells. 2021;10(2):280. doi:10.3390/cells10020280
15. Xu F, Guanghao C, Liang Y, et al. Treg-promoted new bone formation through suppressing TH17 by secreting Interleukin-10 in ankylosing spondylitis. Spine. 2019;44(23):E1349–E1355. doi:10.1097/BRS.0000000000003169
16. Huang F. Progress in the treatment of ankylosing spondylitis with TNFα inhibitors. Chin J New Drugs. 2006;853–857. doi:10.3321/j.issn:1003-3734.2006.11.005
17. Hess K, Ushmorov A, Fiedler J, et al. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45(2):367–376. doi:10.1016/j.bone.2009.04.252
18. Lories RJ, Luyten FP. Bone morphogenetic protein signaling and arthritis. Cytokine Growth Factor Rev. 2009;20(5–6):467–473. doi:10.1016/j.cytogfr.2009.10.009
19. Briolay A, Lencel P, Bessueille L, et al. Autocrine stimulation of osteoblast activity by Wnt5a in response to TNF-α in human mesenchymal stem cells. Biochem Biophys Res Commun. 2013;430(3):1072–1077. doi:10.1016/j.bbrc.2012.12.036
20. Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48. doi:10.3389/fimmu.2014.00048
21. Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(4):572–574. doi:10.1136/annrheumdis-2011-200216
22. Xie Z, Wang P, Li Y, et al. Imbalance between bone morphogenetic protein 2 and noggin Induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheumatol. 2016;68(2):430–440. doi:10.1002/art.39433
23. Riley EA, Lane JM, Urist MR, et al. Bone morphogenetic protein-2: biology and Application. Clinic Orthop. 1996;324:39. doi:10.1097/00003086-199603000-00006
24. Riley EH, Lane JM, Urist MR, et al. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res. 1996;324:39–46.
25. Mo IF, Yip KH, Chan WK, et al. Prolonged exposure to bacterial toxins downregulated expression of toll-like receptors in mesenchymal stromal cell-derived osteoprogenitors. BMC Cell Biol. 2008;9:52. doi:10.1186/1471-2121-9-52
26. Li Y, Huang L, Cai Z, et al. A Study of the immunoregulatory function of TLR3 and TLR4 on mesenchymal stem cells in ankylosing spondylitis. Stem Cells Dev. 2019;28(20):1398–1412. doi:10.1089/scd.2019.0039
27. Li YX, Liu T, Liang YW, et al. Integrative analysis of long non-coding RNA and messenger RNA expression in toll-like receptor 4-primed mesenchymal stem cells of ankylosing spondylitis. Ann Transl Med. 2021;9(20):1563. doi:10.21037/atm-21-5020
28. Pöllänen R, Sillat T, Pajarinen J, et al. Microbial antigens mediate HLA-B27 diseases via TLRs. J Autoimmun. 2009;32(3–4):172–177. doi:10.1016/j.jaut.2009.02.010
29. Navid F, Holt V, Colbert RA. The enigmatic role of HLA-B27 in spondyloarthritis pathogenesis. Semin Immunopathol. 2021;43(2):235–243. doi:10.1007/s00281-021-00838-z
30. Liu CH, Raj S, Chen CH, et al. HLA-B27-mediated activation of TNAP phosphatase promotes pathogenic syndesmophyte formation in ankylosing spondylitis.The. J Clin Invest. 2019;129(12):5357–5373. doi:10.1172/JCI125212
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


