Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 13
Mesenchymal Stem Cells Application in Wound Tissue Healing in Old Animals
Authors Silina E , Manturova N , Stupin V
Received 16 June 2020
Accepted for publication 19 October 2020
Published 11 November 2020 Volume 2020:13 Pages 103—116
DOI https://doi.org/10.2147/SCCAA.S267967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Bernard Binetruy
Ekaterina Silina,1 Natalia Manturova,2 Victor Stupin3
1Department of Human Pathology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia; 2Department of Plastic and Reconstructive Surgery, Cosmetology and Cell Technologies, Pirogov Russian National Research Medical University, Moscow, Russia; 3Department of Hospital Surgery №1, Pirogov Russian National Research Medical University, Moscow, Russia
Correspondence: Ekaterina Silina
Department of Human Pathology, I.M. Sechenov First Moscow State Medical University (Sechenov University), Trubetskaya Street, 8, Moscow 119991, Russia
Email [email protected]
Purpose: An assessment of the effectiveness of progenitor mesenchymal stem cell as injections and as part of a polymer hydrogel for the wounds treatment.
Materials and Methods: Fixed-size wounds (average area of 135.8 mm2) were modeled on the back of white Wistar rats, aged 9 months. Mesenchymal stem cells (MSC) isolated from a human umbilical cord were injected into the wounds once on the modeling day (SC group). In other animals, MSC were periodically applied externally as one of the components in the polymer hydrogel (Polymer_sc group). The systemic effect of the cells was assessed via the analysis of intact contralateral wounds located on the opposite side of the same animal’s back (groups Control_sc and Control_Psc, respectively). The reference intact wounds belonged to the Control_0 group. The wound area was studied in dynamics. Descriptive microscopy was supplemented by an assessment of the collagen fibers’ maturity, the epidermal layers, and the number of fibroblasts and leukocytes in different parts of the wounds.
Results: Both the local and systemic application of MSC led to an improvement in wound regeneration. During the acute inflammatory phase (up to 3 days), the method and place of application did not affect the dynamics of wound healing. The use of Polymer_sc ultimately demonstrated the best effectiveness. The anti-inflammatory effect of MSC was confirmed by a decrease in leukocyte infiltration in the wound centers (Polymer_sc and SC groups) and edges (all groups, with the greatest extent in the Polymer_sc group). The proliferative phase that expresses itself via accelerated growth in fibroblast number and collagen production was affected in the Control_Psc group and mostly in the Polymer_sc group.
Conclusion: The applications of MSC in various ways improve and accelerate wound healing even in old animals. The best performance was achieved in the Polymer_sc group.
Keywords: stem cells, umbilical cord cells, adult animals, fibroblasts, leukocytes, wound, wound treatment, regeneration, wound area, collagen, skin, epidermis, epithelium, polymers, injections, local and systemic action of stem cells
Introduction
Even though the term “stem cell” (SC) was introduced into biology by A. Maximow in 1908,1 this field of cellular biology achieved the status of great science only in the last decade of the 20th century. In 1999, the “Science” journal recognized stem cell discovery to be the third most important event in biology after DNA double helix decoding and the Human Genome project. During this period, a large scientific study database demonstrated the importance and potential of mesenchymal stem cells (MSCs) in the regeneration of damaged tissues.2–5 Moreover, stem cells demonstrated their effectiveness in skin wound treatment,6–10 due to their anti-inflammatory, immunomodulatory, and plasticity properties.5,11,12 Although significant progress has been made in understanding the nature and mechanisms of stem cell actions, this topic remains widely debated. The issue of SC vitality time, SC practical use, and the optimal method of SC application in wound treatment remains unresolved. There is still no consensus on the best source of SC for wound treatment. Some scientists have evidence of the benefits of using autologous stem cells to heal wounds.13–15 In other studies prove the benefits of using allogeneic SC for wound healing.16–18 Obviously, the use of allogeneic cells is more convenient and faster compared to the autologous material. Among allogeneic sources of SCs, the MSCs from the human umbilical cord are distinguished by an advantage. The advantage is the ease of preparation and minimization of bioethical problems. At the same time, the use of MSCs isolated from the human umbilical cord also promotes regeneration and improves wound healing.19–22
The ambiguity of the results obtained by scientists may be associated with the primary material from which the SC was isolated, with the frequency of passaging before the inclusion of cell material in the study, the difference in cell media, the number of cells injected and the method of their application, as well as the age of the SC recipient. Tissue regeneration potential decreases with age.23,24 Thus, to ensure an adequate selection of patients and to increase the effectiveness of cell therapy, preclinical studies on adult and old animals should be conducted. These studies will increase knowledge related to the physiological and pathophysiological age-related dynamics of the potential and mechanisms of SCs, help develop new effective therapeutic strategies, and increase the clinical applications of SCs, thereby improving treatment outcomes in regenerative medicine.
The purpose of this study is to assess the effectiveness of progenitor mesenchymal stem cells’ systemic and local activities during the treatment of deep and wide wound tissues when the stem cells are injected or applied externally as a polymer hydrogel compound.
Materials and Methods
Research Design
An experimental prospective randomized pilot control–comparative blind study was conducted on white Wistar rats of the same age (9 months) and sex (males) with an average body weight of 416.7 ± 41.6 grams (Me = 410 g). Micropreparate blinding was done for histologists who did not know about the study groups and worked with the labeled and encoded materials. This means that the histologists did not know about the therapeutic methods of treating wounds.
We used the age of the second half of life (the average lifespan of a rat is 12–18 months), because it is during this period that problems of wound healing arise.
The animal observations lasted for 30 days. The animals were kept in standard vivarium conditions for the first 2 weeks (adaptation period). At the end of the period, the animals were randomized into groups. Immediately after randomization, the animals were each kept alone in a cage, which made it possible to exclude the external effects on wounds from other animals during the experiment.
Randomization of the experimental animals was carried out according to their weight. One day before the wounds modeling, the animals were weighed and labeled according to the withdrawal time (euthanasia) and groups. In total, 15 subgroups (5 groups, 3 euthanasia points) were identified, 7 wounds in each. Thus, all subgroups before the start of the experiment were statistically indistinguishable from each other (the animals were the same age, sex and weight).
Control points of the experiment: day 0 (wound modeling, measurements and instrumental studies, and wound treatment in the corresponding groups); on the 1st, 3rd, 5th, 7th, and 14th days, the wound sizes and healing dynamics were assessed, and on the withdrawal days (the 3rd, 7th, and 14th days), instrumental studies were performed, and the wounds were prepared for the subsequent morphometry.
Wound modeling was performed under general anesthesia (chloral hydrate, 300 mg/kg, intraperitoneally). A piece of the back skin was shaved, and a standardized wound with a predetermined size was modeled (Patent RF No. 79,701/10.01.2009). Two square wounds at the same distance to the left and right of the spine, deep into the fascia, were created on each animal for the two weeks of studies. On average (median), the wound area, measured 30–40 minutes after modeling, was 135.8 mm2 and did not statistically differ within the groups. The wounds were deep (all layers of the epidermis and dermis were removed down to the muscle fascia; the bottom of the wound was fascia, but the muscles were not damaged) and wide (it was modeled square wounds that distinguish them from cut wounds) (Figure 1).
 |
Figure 1 A macroscopic photograph of the wound a few minutes after modeling (day 0). |
Research Groups and Treatment Methods
In the SC group, the stem cells were injected into wounds once on the day of modeling. In the Polymer_sc group, they were applied externally as a polymer hydrogel compound. The treatment result analysis of these groups made it possible to assess the local effects of stem cells. The systemic effects of cells were assessed via the intact contralateral wounds located on the opposite side of the rats’ bodies.
On the modeling day, 0.1 mL of SCs was injected into the lateral edge, and 0.1 mL of SC into the caudal edge (a total of 100,000 SC per wound), of each wound of the SC group through a single puncture via a thin needle inserted into the wound corners. The systemic effect of a single stem cell injection was assessed via the intact wounds located on the opposite side of the rats’ bodies (Control_sc group).
The local effect of the cells’ external application was analyzed in the Polymer_sc group. The Polymer stem cell biological drug (1 mL) was applied onto the wound, and the concentration of the stem cells was 50000 cells/cm2 (the initial wound area). The application of Polymer_sc was performed under standard laboratory conditions (room temperature 20–23°C; air humidity 50% ± 5%). Polymer stem cells were extracted via a needleless syringe with volume gradations. The gel was accurately transferred to the wound surface so that only the gel touched the edges or bottom of the wound. Within 10 minutes, the surface of the polymer stem cells formed a biomembrane that reliably sealed the wound. This transformation protected the wound from external factors and also created a favorable environment for stem cell preservation. Thus, there was no need for plasters. Polymer stem cells were applied on days 0, 3, and 7. The systemic effect of the local external application of SCs was determined via the intact contralateral wounds located on the opposite side of rats’ bodies (Control_Psc group).
The Control_0 group had reference wounds that remained intact (without any treatment) throughout the whole study.
Before the start of the experiment, the rats were labeled according to groups and the timing of euthanasia at the rate of 7 wounds for each point of removal in each group. At the same time, at least 25 wounds were initially modeled in each group. The total number of animals in the experiment: 80.
Stem Cell Extraction Technology
The SCs used in this study were isolated from a human umbilical cord that was obtained after a normal birth with a 38–40 week gestation period. The mandatory conditions for SC isolation were a healthy examined mother, a normal course of pregnancy, no less than 8 points on the Apgar scale for the newborn, and the informed consent of the donor.
The preparation and cultivation technology of cellular material from the human umbilical cord consisted of the following steps: (1) Before giving birth, the donor was tested for hepatitis viruses; human immunodeficiency viruses; herpes simplex types 1, 2, and 6; Epstein–Barr; cytomegalovirus; Toxoplasma Gondi; and syphilis and signed their informed consent. (2) The umbilical cord fragment was taken within the first 10 minutes after cutting the cord. Biomaterial containing at least 10 mL of whole blood (for subsequent testing) was placed in a sterile container filled with Hanks solution and antibacterial agents. (3) Biomaterial transportation was carried out in the same container as soon as possible, no more than 24 hours from the moment of umbilical cord ligation. During transportation, the container temperature was +2 to + 8°C. (4) The cell culture isolation and cultivation were performed according to a previously described and tested method.10,25,26 By the end of the isolation process, the stem cells had a standard morphological structure of cells grown in a MEM culture medium (Lonza), StemPro ™ MSC (Gibco, USA), at a concentration of 500,000 cells/1 mL. (5) Sorting the cell culture by types was done under microscopy of unpainted preparations. Several specific protein markers of cytophenotypes were detected by immunocytochemistry and flow cytofluorometry. The resulting cell culture was examined for the presence of histocompatibility antigens (HLA-ABC, HLA-DR), as well as surface marker proteins of the mesenchymal stem cells CD34, CD44 (HCAM), CD45, CD49b (α2β1 – integrin), CD54 (ICAM), CD90 (ICAM) (Thy-1), CD105 (endoglin), CD106, and CD117 (c-kit). (6) The obtained culture of umbilical cord cells was tested for (virological and bacteriological testing) the presence of microflora; markers of hepatitis B and C viruses; HIV; herpes simplex virus types 1, 2, and 6; Epstein–Barr; cytomegalovirus; and toxoplasmosis (via an enzyme-linked immunosorbent assay and microscopy).
A Polymer Bioproduct Containing Stem Cells for External Use
A culture of progenitor mesenchymal stem cells was prepared according to the technology described above. Then, the culture was added to our biological product based on natural and synthetic polymers27,28 under the standard conditions. In total, 1 mL of the stem cell suspension was introduced drop by drop into 10 mL of gel in a bottle at room temperature and was stirred on a magnetic stirrer at a low speed for 15 minutes.
The hydrogel base of the product, containing chitosan, alginate, agar, and pectin, was designed to ensure that the stem cells would stay in an active state, as well as gradually flow from the gel into the wound.
Recording Research Results Methods
The wound tissue area was evaluated for its dynamics on days 0, 1, 3, 5, 7, and 14; the final result was recorded as mm2. For this evaluation, photo documentation of the wounds was performed (Canon EOS550D camera (Japan), Canon EF-S18-55 lens, the distance to the object was 30 cm). To ensure the right calibration, a paper with millimeter gradations was placed next to the wound in the frame at the same location. The wound area, precisely limited by its edges, was calculated using the program JMicroVision 1.2.7 (Switzerland).
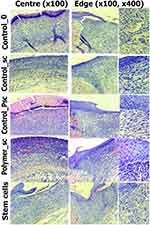
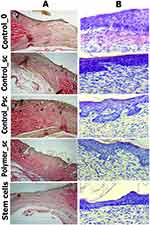
Morphometry and an assessment of 6–7 wounds in each wound group were performed at each control point of the study (days 3, 7, and 14). Three sides of each wound were studied: the center and two opposite edges. The numbers at the wound edges were summarized and averaged. Histological sections and nuclei were stained with hematoxylin–eosin, Mallory (Van Gieson). Descriptive microscopy was performed using a Leica CME microscope optical system (Germany) and Levenhuk D740 (USA) at x40, x100, and x400 magnifications. To determine the inflammation phase, the presence and severity of edema, the presence of blood stasis in the blood vessels, the severity and gradient of leukocyte infiltrate; the presence, maturity, and spatial organization of collagen fibers; and the presence and severity of epidermal layers covering the interstitial tissue formed by fibroblasts were analyzed and taken into account (Figure 2).
During the Van Gieson staining, the ratio of mature and oxyphilically stained young collagen was compared. The cell number and characteristics in all granulation layers were also assessed. We used the Image-J morphometry package (National Institutes of Health, USA), which is widely used by morphologists for image analysis and processing. The program is written in Java and allows to determine the density and number of cells per unit area, including the discretization of the nuclei by their generally recognized visual characteristics (shape and size). As a result, two main cell types were determined: resident cells (fibroblasts with different maturity) and non-resident cells (leukocytes, granulocytes, macrophages, lymphocytes, and monocytes that flowed to the regeneration area). We analyzed their absolute value (number of cells per mm2) and their percentage ratio.
Statistical analysis of the experiment results was performed using the SPSS 23.0 program (SPSS: An IBM Company, USA). Standard parametric and nonparametric criteria for statistical significance assessments were used. Descriptive statistics of continuous quantitative data are presented as the mean, standard error of the mean, median (Me), and values of the lower Q1 (25%) and upper Q3 (75%) quartiles. A Mann–Whitney test was used to analyze two independent nonparametric samples, while a Wilcoxon test was used to analyze two dependent samples. Qualitative variables were compared using a χ2 test (contingency tables analysis). Differences were considered to be statistically significant at p <0.05.
Ethical Considerations
The experiment was performed under the principles of laboratory animal handling and complied with the provision of the “European Convention for the Protection of Vertebral Animals Used for Experimental and Other Scientific Purposes. CETS 123.” This study was approved at the meeting of the Regional Ethics Committee of the Kursk State Medical University under the Ministry of Health of the Russian Federation (Protocol No. 5 dated 02.11.17).
MSCs from human umbilical cord: The study complies with the requirements of the Russian Federation in terms of purity of the cellular material and ethical standards. The MSCs used in the experiment were isolated from a human umbilical cord under ethical principles with the informed consent of the donor. The use of MSCs from the human umbilical cord for experimental studies on animals was approved by the local ethical committee of the Institute of Plastic Surgery and Cosmetology (Protocol No. 03/1 dated January 29, 2020).
Results
During the experiment, a significant increase in the wound area of the Control_0 group was documented within 24 hours after modeling; on average, the increase was 1.21 times (p <0.05). By day 3, the wounds returned to their initial size. A statistically significant regression in the wound areas of the control wounds relative to the modeling day was recorded on days 5, 7, and 14. On the 7th day, the wound area of the Control group was 1.32 times smaller (p <0.01), and on the 14th day, it was 5.94 times smaller (p <0.001) compared to its initial size. The different applications of mesenchymal stem cells thus showed their effectiveness (Figure 3).
Single local injections of stem cells resulted in an inhibition of the wound expansion period. The wound area of the SC group gradually diminished and became 1.1 times, 1.35 times, and 8.75 times larger, on average, on the modeling day compared to day 3 (p <0.05), day 7 (p <0.01), and day 14 (p <0.001). Significant differences in the wound area index between the SC and Control_0 groups were observed on days 1 and 3, thus demonstrating the effectiveness of SC treatment.
Nevertheless, on days 5 and 7, SC effectiveness was observed to fall, presenting a gradual regression or complete absence of therapeutic effect, and the wound area became comparable with that of the control groups. The stem cells’ short lifetime may be associated with the fact that they were injected only once on the modeling day. Nevertheless, even if the cells were active for only the first 3 days, this active action produced a significant wound size reduction by day 14. Compared with the SC group, the Control_0 group wound area was 1.44 times larger (p = 0.043) on day 14.
The systemic effect of SCs that was assessed in comparison with the contralateral intact wound (Control_sc group) was comparable to the direct action of SCs. The wounds of the Control_sc group did not show a statistically significant increase during the first 24 hours after modeling, and statistically significant differences with the Control_0 group (p <0.05) were noted on days 1 and 3. The absence of additional injection trauma in the Control_sc group led to a significant reduction in the wound area by the last day of observations, while on day 0, this group’s wounds were 11.93 times larger (p <0.001) on average. Compared with the Control_sc group, the Control_0 group’s wound area was 1.96 times larger (p = 0.039) by the last day of the observations.
A comparative analysis of the area index in the SC and Control_sc groups on days 3 and 5 showed no statistically significant differences, revealing the same effectiveness of SC in both single local and systemic applications, mainly limited to 3 days. This effective-yet-short active period is associated with the lifetime of SCs in rats, which is about 3–4 days.
A similar systemic effect was recorded in the Control_Psc group wounds, where the wound area did not statistically differ in dynamics from that of the SC and Control_sc groups (p> 0.05) throughout the study but was lower compared to the Control_0 group (p <0.05) on days 1 and 3. The wound area of the Control_Psc group decreased by 11.84 times on average by the end of the experiment (as in the Control_sc group).
The best wound area dynamics were demonstrated by the Polymer_sc group. From the 3rd day of observations, the wound area index of the Polymer_sc group was the lowest within the groups. This result significantly differed from the Control_0 group starting on the 1st day, from the SC group on the 5th day, and from the Control_sc and Control_Psc groups on the 7th day. Thus, the Polymer_sc wounds did not have a period of inflammatory expansion (just as in all other groups containing SC), and their areas were significantly less than those in the Control_0 group (p = 0.002) by the end of the 1st day. On day 3, the Polymer_sc wound area was 1.16 times less than that in the Control_0 group (p = 0.002). On day 5, the Polymer_sc wound area was 1.13 times less than that in the Control_0 group (p = 0.007) and 1.12 times less than that in the SC group (p = 0.015). On day 7, the Polymer_sc wound area was 1.25 times less than that in the Control_0 group (p = 0.004), 1.24 times less than that in the SC group (p = 0.001), 1.25 times less than that in the Control_sc group (p = 0.002), and 1.26 times less than that in the Control_Psc group (p = 0.012). On day 14, the Polymer_sc wound area was 8.5 mm2, which is 16.73 times smaller compared to day 0 (p <0.001). These numbers are higher than those in all other groups. Compared with the Polymer_sc wound area on day 14, the Control_0 group wound area was 2.60 times larger (p = 0.004), the SC wound area was 1.33 times larger (p = 0.019), the Control_sc wound area was 1.80 times larger (p = 0.024), and the Control_Psc wound area was 1.40 times larger (p = 0.047).
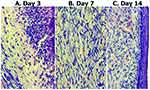
To support the numeric wound size data we give a photo of the wounds at different time points (Figure 4).
 |
Figure 4 The photos of the wounds at different time points in different groups. |
The remaining positive effects of SC applications in different forms after 3 days can be explained by the results of the morphological studies.
By day 3, leukocyte cells prevailed in all group wounds, comprising 61–65%, on average (median), in the center of the wounds and 70–83% (median) in the wound edges. At the same time, the number of leukocytes was significantly higher at the center of the wounds than at the periphery, which highlights the active inflammatory phase of regeneration, running intensively at the bottom of the wound. However, the inflammation intensity varied.
The smallest number of leukocytes was observed in the wounds treated externally by a polymer gel (Polymer _sc group) or internally by injection (SC group). The largest number was observed in the control groups. On day 3, in the Control_0 group wound center, the absolute average (median) number of leukocytes was 197 cells/mm2, which is 1.17 times more than that in the Polymer_sc group (Me = 168 leukocytes/mm2, p <0.05) and 1.10 times more than that in the SC group (Me = 179 leukocytes/mm2, p <0.05). On day 3, in the Control_sc wound centers, the absolute number of leukocytes was 228 cells/mm2, which is 1.36 times more than that in the Polymer_sc group (p <0.05) and 1.27 times more than that in the SC group (p <0.05). Although the number of leukocytes in all control groups’ wound centers was not statistically different, it was the smallest in the Control_Psc group (Me = 184 white blood cells/mm2, p> 0.05) and did not statistically differ from the SC and Polymer_sc groups (Figure 5).
 |
Figure 5 The number of leukocytes in different groups’ wound centers on the 3rd day of the study. |
Similar results were obtained after analysis of the leukocyte numbers at the wound edges. However, a significant difference in leukocyte numbers in the Polymer_sc group was noted. This number was significantly less than that in any other group (Me = 90 leukocytes/mm2)—on average, 1.77 times less (p <0.05) than that in the Control_0 group, 1.94 times less (p <0.05) than that in the Control_sc group, 1.31 times less (p <0.05) than that in the Control_Psc group, and 1.44 times less (p <0.05) than that in the SC group. The absolute numbers of leukocytes at the wound edges of the SC group (Me = 130 leukocytes/mm2) and the Control_Psc group (Me = 118 leukocytes/mm2) did not statistically differ from each other (p> 0.05) but were significantly less than those in the Control_0 and Control_sc groups (p <0.05) (Figure 6).
 |
Figure 6 The number of leukocytes in different groups’ wound edges on the 3rd day of the study. |
The acute inflammatory processes correlating with the leukocyte numbers continued on day 3 in the Control_0 and Control_sc groups and decreased in the Control_Psc group, followed by the SC group; in the Polymer_sc group, they were active to a minimum extent. Based on the obtained data, it can be concluded that the most effective anti-inflammatory effect of SCs is observed after their local application. This may be explained by the higher concentration of SCs, the anti-inflammatory cytokines induced by SCs in the wounds along with other biologically active substances, and the direct contact of the wound tissues with the SCs. The joint use of cells in the polymer gel demonstrated the best effect during systemic application.
The acute inflammation phase changed to the proliferation phase on the 7th day, and the leukocytes became smaller in number than the fibroblasts, prevailing in the centers and edges of the wounds. However, the leukocyte numbers remained at a high level when the macrophages began to prevail among them. It was recorded that the leukocyte numbers on day 7 dropped only in the Polymer_sc and Control_Psc groups, which may have been caused by repeated external SC applications to the wounds and an increase of the cell lifespan in Polymer_sc. Thus, in the wound edges of the Polymer_sc and Control_Psc groups, the average numbers were 158 leukocytes/mm2 and 181 leukocytes/mm2, respectively. These numbers differ significantly from those of the Control_0 group, where the absolute leukocyte number was maximum (Me = 204 leukocytes/mm2, p <0.05). A single local injection of SCs resulted in a decrease of leukocyte wound infiltration by day 7; however, it did not significantly differ between the Control_0 group and the Polymer_sc group (Figure 7).
 |
Figure 7 The number of leukocytes in different groups’ wound edges on the 7th day of the study. |
During the proliferation phase, fibroblasts became the dominant cell population in the wound. In the wound center, the number of fibroblasts exceeded the number of leukocytes by 1.2–1.8 times (p <0.05) on average. The highest numbers were observed in the Polymer_sc group (Me = 601 fibroblasts/mm2) and Control_Psc group (Me = 562 fibroblasts/mm2) and were 1.43 times and 1.34 times (p <0.05) more than those in the Control_0 group, 1.42 times and 1.33 times (p <0.05) more than those in the Control_sc group, and 1.34 times and 1.25 times (p <0.05) more than those in the SC group (Figure 8). These numbers prove that regeneration accelerated in the wounds of the Polymer_sc and Control_Psc groups, whose proliferation phase began earlier and proceeded more efficiently by day 7.
 |
Figure 8 The number of fibroblasts in the different group’ wound centers on the 7th day of the study. |
Similar results were obtained at the wound edges. The ratio of fibroblasts over the resident cells was, on average (in descending order), 4.53 times more in the Polymer_sc group, 4.13 times more in the Control_Psc group, 3.38 times more in the Control_sc group, 3.34 times more in the Control_0 group, and 3.14 times more in the SC group. These numbers are higher than those of the wound centers due to the predominance of marginal epithelization processes (Figure 9).
Fibroblasts are the cells responsible for extracellular matrix maturation and deposition, which ultimately leads to a reduction in wound size and accelerates its epithelization. Therefore, collagenization of the wounds on day 14 was an important indicator for the experiment. It was found that thePolymer_sc contained the most mature collagen, while Control_0 and Control_sc presented the poorest collagen maturation. Moreover, young collagen prevailed over mature collagen (Table 1).
 |
Table 1 Wound Collagenization in Different Groups on the 14th Day of the Study |
Microscopic evaluation of the histological sections was used to visualize the collagen fibers, which were mostly organized and more mature in the Polymer_sc group. The epidermis covered the wound over the collagen fibers. Moreover, the epidermis was evenly organized, had all layers restored, and covered the entire wound only in the Polymer_sc group. In the other groups, the collagen fibers and epidermis were thinner and less organized; the weakest organization was observed in the Control_0 group (Figure 10).
Discussion
This study demonstrated that the mesenchymal stem cell treatment has a systemic effect. The application method and region do not affect the wound healing dynamics during the initial stages of regeneration up to 3 days (inclusive). Introduced to the edges of wounds via injection or applied externally to the animal’s body, significantly accelerated the onset of wound regeneration in the control group. Unfortunately, the activity of the cells in this setting did not last more than 3–5 days. This was likely due to the short lifespan of the stem cells, but this hypothesis was not instrumentally tested in the present study.
Wound treatment on days 0, 3, and 7 with an external agent of polymer hydrogel with mesenchymal stem cells accelerated regeneration starting from the 1st day of use. The polymer gel with compound stem cells was more effective than single local injections when regularly applied to the surface of the wound without additionally injuring it. The gel was preferred over the injection due to the limited lifetime of SCs once injected.
The main issues that arise during SC treatment are not only regular bandaging but the preparation of the agent immediately before use, without violating the storage rules of the stem cells at low positive temperatures. This fact significantly complicates the process of managing patients with wounds, burns, or trophic ulcers, as it will require an ongoing cultural laboratory in each of the hospitals or clinics using this technology. The creation of a single laboratory with ongoing culture cultivation will lead to transportation problems and a poorly optimized course of treatment.
Therefore, to transfer the cell technologies from scientific laboratories to clinics, it will be necessary to develop new methods for maintaining the activity of the stem cell cultures using injection and non-injection methods or to find a way of isolating biological substrates synthesized by the cell that directly activate the regeneration process. Fundamentally similar technologies are used in the production of antibiotics. However, the substances synthesized by the stem cells may be less stable and require adjustments in their storage regimes.
Despite the difficulties discussed above, we have already shown the effectiveness of stem cell technologies and their great potential not only in the field of general surgery but also within the framework of aesthetic medicine, where great importance is given to the wound closure process and the formation of the scar. Skin regeneration after deep peeling or laser resurfacing also seems to be a prospective segment for the use of stem cell technology, which seems to represent a reasonable and effective treatment method under the joint use of injections and bandages containing the elements of the stem cell therapy. We believe that for patients with somatic diseases accompanied by tissue ischemia, this kind of treatment will be the most effective, as embryo development under conditions of relative hypoxia makes stem cells differentiate and provides rapid cell mass growth.29–32 Our assumptions are consistent with those in33–35 and demonstrate the potential of stem cell treatment in different areas of regenerative medicine.
The systemic responses in the animal control wounds treated with human umbilical cord mesenchymal stem cells should also be noted. In both control groups, the systemic response was active on the very first day of the treatment and was only slightly weaker compared to the direct action of the cell substances. It is necessary to continue this line of research and investigate the possibilities of creating resistant strains of cell cultures, as well as develop the clinical conditions needed for the most effective synthesis of biologically active substances both in vitro and in vivo.
Financial Aid
This manuscript does not have specific sponsorship. The materials presented were obtained as a part of the Foundation for the Promotion of the Development of Small Forms of Enterprises in the Scientific and Technical Field grant (232GRNTIS5/35,963), devoted to the development of prototypes of a biomedical polymer product that accelerates wound healing.
Acknowledgments
The authors thank Artyushkova EB, Anikanov AV, Dudka VT, Furman YuV, Gladchenko MP, Ivanov AV, Kaplin AN, Khokhlov NV, Kryukov AA, Naimzada M.D.Z., Suzdaltseva Y.G., Vasin VI for help and participation in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maximow A. Der Lymphozyt als gemeinsame Stammzelle der verschiedenen Blutelemente in der embryonalen Entwicklung und im postfetalen Leben der Säugetiere archive copy dated June 29, 2009 at Wayback Machine. Originally in: folia Haematologica 8.1909, 125—134. Cell Ther Transplant. 2009;1. doi:10.3205/ctt-2008-en-000040.01
2. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi:10.1002/jcb.20886
3. Shaer A, Azarpira N, Aghdaie MH, Esfandiari E. Isolation and characterization of human mesenchymal stromal cells derived from placental decidua basalis; umbilical cord Wharton’s jelly and amniotic membrane. Pak J Med Sci. 2014;30:1022.
4. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi:10.1186/s13287-016-0363-7
5. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. doi:10.1038/s41536-019-0083-6
6. Takeo M, Lee W, Ito M. Wound healing and skin regeneration. Cold Spring Harb Perspect Med. 2015;5(1):a023267. doi:10.1101/cshperspect.a023267
7. Ojeh N, Pastar I, Tomic-Canic M, Stojadinovic O. Stem cells in skin regeneration, wound healing, and their clinical applications. Int J Mol Sci. 2015;16(10):25476–25501. doi:10.3390/ijms161025476
8. Iwata Y, Akamatsu H, Hasebe Y, Hasegawa S, Sugiura K. Skin-resident stem cells and wound healing. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):1–11. doi:10.2177/jsci.40.1
9. Kosaric N, Kiwanuka H, Gurtner GC. Stem cell therapies for wound healing. Expert Opin Biol Ther. 2019;19(6):575–585. doi:10.1080/14712598.2019.1596257
10. Suzdaltseva Y, Zhidkih S, Kiselev SL, Stupin V. Locally delivered umbilical cord mesenchymal stromal cells reduce chronic inflammation in long-term nonhealing wounds: a randomized study. Stem Cells Int. 2020;2020:5308609. doi:10.1155/2020/5308609
11. Zomer H, Vidane A, Gonçalves N, Ambrosio C. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015;8:125–134. doi:10.2147/SCCAA.S88036
12. Borges FT, Convento MB, Schor N. Bone marrow-derived mesenchymal stromal cell: what next? Stem Cells Cloning. 2018;11:77–83. doi:10.2147/SCCAA.S147804
13. Chang Y-W, Wu Y-C, Huang S-H, Wang H-MD, Kuo Y-R, Lee S-S. Autologous and not allogeneic adipose-derived stem cells improve acute burn wound healing. PLoS One. 2018;13(5):e0197744. doi:10.1371/journal.pone.0197744
14. Hamada T, Matsubara H, Yoshida Y, Ugaji S, Nomura I, Tsuchiya H. Autologous adipose-derived stem cell transplantation enhances healing of wound with exposed bone in a rat model. PLoS One. 2019;14(5):e0214106. doi:10.1371/journal.pone.0214106
15. Badiavas AR, Badiavas EV. Potential benefits of allogeneic bone marrow mesenchymal stem cells for wound healing. Expert Opin Biol Ther. 2011;11(11):1447–1454. doi:10.1517/14712598.2011.606212
16. Cao SJ, Wang LF, Ba T, Fu X, Li F, Hao CG. Effects of allogeneic mouse adipose-derived mesenchymal stem cell-microporous sheep acellular dermal matrix on healing of wound with full-thickness skin defect in mouse and the related mechanism. Zhonghua Shao Shang Za Zhi. 2018;34(12):901–906. doi:10.3760/cma.j.issn.1009-2587.2018.12.015
17. Textor JA, Clark KC, Walker NJ, et al. Allogeneic stem cells alter gene expression and improve healing of distal limb wounds in horses. Stem Cells Transl Med. 2018;7(1):98–108. doi:10.1002/sctm.17-0071
18. Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–1312. doi:10.1089/ten.2006.0278
19. Wang S, Yang H, Tang Z, Long G, Huang W. Wound dressing model of human umbilical cord mesenchymal stem cells-alginates complex promotes skin wound healing by paracrine signaling. Stem Cells Int. 2016;2016:3269267. doi:10.1155/2016/3269267
20. Milan PB, Lotfibakhshaiesh N, Joghataie MT, et al. Accelerated wound healing in a diabetic rat model using decellularized dermal matrix and human umbilical cord perivascular cells. Acta Biomater. 2016;45:234–246. doi:10.1016/j.actbio.2016.08.053
21. Han Y, Sun T, Han Y, et al. Human umbilical cord mesenchymal stem cells implantation accelerates cutaneous wound healing in diabetic rats via the Wnt signaling pathway. Eur J Med Res. 2019;24(1):10. doi:10.1186/s40001-019-0366-9
22. Xu H, Huang S, Wang J, et al. Enhanced cutaneous wound healing by functional injectable thermo-sensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. Int J Biol Macromol. 2019;137:433–441. doi:10.1016/j.ijbiomac.2019.06.246
23. West MD, Sternberg H, Labat I, et al. Toward a unified theory of aging and regeneration. Regen Med. 2019;14(9):867–886. doi:10.2217/rme-2019-0062
24. Josephson AM, Bradaschia-Correa V, Lee S, et al. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019;116(14):6995–7004. doi:10.1073/pnas.1810692116
25. Suzdal’tseva YG, Burunova VV, Vakhrushev IV, Yarygin VN, Yarygin KN. Capability of human mesenchymal cells isolated from different sources to differentiation into tissues of mesodermal origin. Bull Exp Biol Med. 2007;143(1):114–121. doi:10.1007/s10517-007-0030-1
26. Yarygin KN, Suzdal’tseva YG, Burunova VV, et al. Comparative study of adult human skin fibroblasts and umbilical fibroblast-like cells. Bull Exp Biol Med. 2006;141(1):161–166. doi:10.1007/s10517-006-0117-0
27. Silina EV, Khokhlov NV, Stupin VA, et al. Multicomponent polysaccharide essential formula of wound healing medicines enriched with fibroblast growth factor. Int J Biomed. 2019;9(3):247–250. doi:10.21103/Article9(3)_OA12
28. Silina EV, Manturova NE, Vasin VI, et al. Efficacy of a novel smart polymeric nanodrug in the treatment of experimental wounds in rats. Polymers. 2020;12:
29. Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9(4):285–296. doi:10.1038/nrm2354
30. Taguchi T, Cho JY, Hao J, Nout-Lomas YS, Kang KS, Griffon DJ. Influence of hypoxia on the stemness of umbilical cord matrix-derived mesenchymal stem cells cultured on chitosan films. J Biomed Mater Res B Appl Biomater. 2018;106(2):501–511. doi:10.1002/jbm.b.33864
31. Zhao D, Liu L, Chen Q, et al. Hypoxia with Wharton’s jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells. Stem Cell Res Ther. 2018;9(1):158. doi:10.1186/s13287-018-0902-5
32. Chang CW, Wakeland AK, Parast MM. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J Endocrinol. 2018;236(1):R43–R56. doi:10.1530/JOE-17-0402
33. Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant. 2011;26:1474–1483. doi:10.1093/ndt/gfr015
34. Muscari C, Giordano E, Bonafè F, Govoni M, Pasini A, Guarnieri C. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013;20(1):63. doi:10.1186/1423-0127-20-63
35. Hawkins KE, Corcelli M, Dowding K, et al. Embryonic stem cell-derived Mesenchymal Stem Cells (MSCs) have a superior neuroprotective capacity over fetal MSCs in the hypoxic-ischemic mouse brain. Stem Cells Transl Med. 2018;7(5):439–449. doi:10.1002/sctm.17-0260
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.