Back to Journals » Journal of Inflammation Research » Volume 16
Mesenchymal Stem Cell Transplantation: Neuroprotection and Nerve Regeneration After Spinal Cord Injury
Authors Chen SY , Yang RL, Wu XC, Zhao DZ, Fu SP, Lin FQ, Li LY, Yu LM, Zhang Q, Zhang T
Received 1 July 2023
Accepted for publication 3 October 2023
Published 20 October 2023 Volume 2023:16 Pages 4763—4776
DOI https://doi.org/10.2147/JIR.S428425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Si-Yu Chen,1 Rui-Lin Yang,1 Xiang-Chong Wu,2 De-Zhi Zhao,1 Sheng-Ping Fu,2 Feng-Qin Lin,1 Lin-Yan Li,1 Li-Mei Yu,1 Qian Zhang,3 Tao Zhang1,2
1Key Laboratory of Cell Engineering of Guizhou Province, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, People’s Republic of China; 2Department of Orthopaedic Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, People’s Republic of China; 3Department of Human Anatomy, Zunyi Medical University, Zunyi, Guizhou, People’s Republic of China
Correspondence: Tao Zhang; Qian Zhang, Email [email protected]; [email protected]
Abstract: Spinal Cord Injury (SCI), with its morbidity characteristics of high disability rate and high mortality rate, is a disease that is highly destructive to both the physiology and psychology of the patient, and for which there is still a lack of effective treatment. Following spinal cord injury, a cascade of secondary injury reactions known as ischemia, peripheral inflammatory cell infiltration, oxidative stress, etc. create a microenvironment that is unfavorable to neural recovery and ultimately results in apoptosis and necrosis of neurons and glial cells. Mesenchymal stem cell (MSC) transplantation has emerged as a more promising therapeutic options in recent years. MSC can promote spinal cord injury repair through a variety of mechanisms, including immunomodulation, neuroprotection, and nerve regeneration, giving patients with spinal cord injury hope. In this paper, it is discussed the neuroprotection and nerve regeneration components of MSCs’ therapeutic method for treating spinal cord injuries.
Keywords: spinal cord injury, mesenchymal stem cell, neuroinflammation, neuroprotection, nerve regeneration
Introduction
Spinal cord injury (SCI) is a devastating neurological disease, which can lead to temporary or permanent impairment of motor, sensory, and autonomic nerve functions. It is estimated that the global prevalence of SCI has been increasing over the past 30 years, with 236 to 1298 patients per million people in different countries,1,2 and each SCI patient suffers from both physical and psychological torment and also faces difficulties such as lack of financial resources and broken social relationships.3 The pathogenesis of SCI can be divided into two stages, the first stage of injury is primary injury (such as from a fall or a traffic accident), and secondary injury ensues as a complex series of abnormal molecular signaling, inflammatory cell infiltration, inflammatory factor release, oxidative stress, vascular changes, and secondary cellular dysfunction hierarchical association reaction, which ultimately leads to apoptotic necrosis of neurons and glial cells, forming a microenvironment unfavorable to nerve regeneration and injury recovery microenvironment, aggravating the injury,4 and when the injury enters the chronic phase of secondary damage, the already formed glial scar blocks nerve regeneration.5,6 Patients with SCI experience sensory loss and functional defects below the injured spinal cord level as a result of primary injury and subsequent injury, which influence how severe the condition is.1,7 No treatment has been able to properly treat SCI and improve the prognosis of SCI patients as of yet. Methylprednisolone decreases oxidative stress and inhibits lipid peroxidation in addition to treat post-SCI neuroinflammation effectively.8,9 However, using methylprednisolone can lead to serious side effects as sepsis, pneumonia, wound infection, and gastrointestinal bleeding10. The glycolipid molecule gangliosides, which are found in neuronal membranes, are used as a neuroprotective agent in the treatment of SCI has several effects, including the prevention of apoptosis and anti-excitotoxic activity. However, studies have shown that after six months of ganglioside treatment, there is no difference in neurological recovery.6,11 Mesenchymal stem cell (MSC) transplantation, is a novel therapy full of optimism and potential development, has evolved precisely because the currently available medicines to suppress neuroinflammation and neuroprotective drugs do not achieve the optimal standard of treatment for SCI.
Mesenchymal stem cells (MSCs) are pluripotent stem cells that can be derived from a variety of tissues, including bone marrow, adipose, human umbilical cord blood, and others.12,13 MSCs have the capacity for multidirectional differentiation and self-renewal, and they can differentiate into end-stage cells such as lipogenic cells, chondrogenic cells, and neuronal cells in vitro when subjected to various stimulating factors and induction media.14–16 These qualities have caused MSCs to gradually gain attention in the fields of medicine and tissue engineering in recent decades, and numerous experiments have now demonstrated that MSCs are a very promising research area and have extensive research significance for the regeneration of various tissues and cells, such as bone, skin, and nerves.7,17,18 The emergence and growth of MSCs have greatly aided the search for novel treatments for several disorders. MSC transplantation is a frequent first step in the investigation of disease therapeutic techniques that function through direct physical contact between cells, paracrine secretion, transfer of mitochondria, transfer of RNA, and other molecules, among other mechanisms.19,20 Through these modes of action, MSCs can decrease inflammatory responses, alter immune cell activity, reduce tissue damage and induce regeneration.21,22
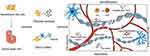
Numerous studies have demonstrated the capacity for MSCs from various sources (bone marrow, fat, umbilical cord blood, dental pulp, etc.) to treat SCI, which is consistent with a similar mechanism17 (Figure 1). Different tissue-derived MSCs have varying capacities for differentiation and proliferation.17,23 Under typical differentiation settings, bone marrow mesenchymal stem cells have good osteogenic and chondrogenic properties, while synovial-derived cells have a higher capacity for chondrogenesis than BM- MSCs.24 MSCs from synovial and adipose tissue had greater adipogenic potential than MSCs from bone marrow.24,25 Comparison of the proliferative potential of bone marrow, adipose, and umbilical cord-derived MSCs revealed that umbilical cord blood mesenchymal stem cells were found to have the highest cell proliferation rate and clonogenicity.26 Different types of MSCs secrete various bioactive substances. For illustration, umbilical cord mesenchymal stem cells (UC-MSCs) secrete more neurotrophic substances (bFGF, NGF, NT3, NT4, and GDNF), whereas bone marrow mesenchymal stem cells (BM-MSCs) and adipose- derived mesenchymal stem cells (Ad-MSCs) secrete more pro-angiogenic substances.27 Inhibiting the inflammatory response at the injury site, reducing the formation of peripheral glial scar to slow the process of spinal cord injury, enhancing neuroprotection and promoting axon regeneration, and finally reducing neuralgia and promoting functional recovery in patients with SCI are all important functions of various bioactive factors.28 Many MSCs transplant clinical trials are already in the first stages (Phase I/II), and their feasibility and safety have already been tentatively established.29,30 We will discuss the therapeutic mechanism of MSCs on SCI, concentrating on the role of neuroprotection and nerve regeneration in the mechanism of mesenchymal stem cell transplantation, in order to better understand the therapeutic effect of MSCs on spinal cord injury and simplify follow-up research.
Pathophysiology of Spinal Cord Injury
The first stage of spinal cord injury (SCI) is the primary injury event, also described as primary injury, which is an injury to the spinal cord from physical forces such as compression, shear, laceration, acute stretch/distraction, and large area impact.11,27,31 The blood vessels at the wounded site are ruptured and leak during this phase, which also results in damage to the nerve parenchyma and glial structure.32,33 The secondary injury is the second stage of spinal cord injury (SCI), which is brought on by the primary injury event to start a secondary response that lasts almost the entire duration of SCI. The secondary injury event also causes the spinal cord injury area to grow through a series of complex and related cascades, aggravating SCI34,35 (Figure 2).
According to the unique characteristics of the various damage periods, the secondary injury occurrences have been divided into three categories: acute, subacute, and chronic.31,32 After an injury, the acute phase lasts for 48 hours and is characterized by symptoms such as vascular dysfunction, free radical generation, increased calcium inward flow, inflammation, excitotoxicity, and edema.36 The course of spinal cord injury enters a subacute phase (2–14 days) if the acute phase is not interrupted, which is marked by axonal demyelination, Wallerian degeneration, axonal remodeling, and other symptoms.32,36,37 It subsequently reaches a chronic phase that persists for the rest of the individual’s lifetime, with the chronic phase featuring cystic cavity formation, axonal blight, gliosis, and scar formation after extracellular matrix deposition.27,38
With ongoing apoptosis or necrosis of neurons and glial cells throughout the response, the many molecular reactions in the secondary injury event are interrelated and interact with one another, all aggravating spinal cord injury to differing degrees.36,39 Reduced blood flow and extravasation of erythrocytes and leukocytes follow spinal cord injury due to variable degrees of structural malfunction of the blood arteries in the affected area.32 The extravasation of blood leads to a sustained increase in pressure at the damaged site, combined with the infiltration of peripheral inflammatory cells such as neutrophils, bone marrow-derived macrophages, and lymphocytes into the damaged tissue, the release of inflammatory cytokines (interleukin-1β (IL-1β), interleukin-1α (IL-1α), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)), and vasospasm, micro-thrombosis, persistent bleeding, and other conditions, and eventually blood flow supply is interrupted, damaged tissues become ischemic and hypoxic, and the blood-spinal cord barrier becomes dysfunctional.36,40,41 In conclusion, the loss of vascular function and structure restricts anticipated restoration techniques and prevents endogenous tissue repair after spinal cord injury.41,42
Neuroinflammation contributes significantly to secondary injury, and following SCI, processes associated with inflammation are activated.43,44 The primary effector cells of the inflammatory response following SCI are activated microglia and recruited macrophages, which can cause neurons and glial cells at the injured region to undergo apoptosis or necrosis.45 Infiltrating the damaged site sequentially, neutrophils (peaking 1 day after injury), macrophages/microglia (peaking 7 days after injury), and lymphocytes (peaking 9 days after injury) secrete various inflammatory mediators and aid in clearing up cellular debris.46,47 Tissue levels of inflammatory cytokines peak 6 to 12 hours after injury.31,48 The subsequently released tissue degradation enzymes, proteases, reactive oxygen species (ROS), and apoptosis-inducing chemicals create a neurotoxic milieu that extends the injury to neighboring healthy tissue and speeds up neuronal and neuronal cell death or necrosis.49,50 Both positive and negative effects can be attributed to inflammation that develops at the site of the injury. While infiltrating inflammatory cells and releasing inflammatory factors aid in patient repair in the early stages of SCI, their protracted presence worsens neurotoxicity and exacerbates neurological dysfunction.51
After blood-spinal cord barrier dysfunction, cell membrane permeability increases, and combined with endothelial injury and inflammatory factors, the ion concentration inside and outside the neuronal and glial cell membranes become imbalanced, with increased extracellular potassium (K+) concentrations and increased intracellular sodium (Na+) and calcium (Ga2+) concentrations, leading to cytotoxic, ionic, and vasogenic edema and persistent edema will eventually lead to massive cellular necrosis.32,52,53 As a result of the rapid rise in intracellular calcium ion concentration, mitochondria malfunction, and slowed oxidative phosphorylation in an effort to buffer the excess calcium ions.54,55 Mitochondria are an integral part of the energy metabolism of nerve cells, and their mitochondrial dysfunction has resulted in a lack of energy on which nerve cells depend and an increase in the number of nerve cell deaths.56 A considerable amount of evidence proves that oxygen radicals are important mediators of secondary damage events in SCI and are involved in oxidative stress to neural tissue, mainly in the form of lipid peroxidation.54,57 Most of the oxidative stress in damaged neuronal cells initially start with the production of peroxynitrite (PN, an oxidant) in the mitochondria, which produces “oxidative damage” to cellular lipids and proteins, mainly in the form of oxidative attack on polyunsaturated fatty acids of the cell membrane.58,59 The disruption of mitochondrial respiratory function occurs before, or at least simultaneously with, the production of free radicals in mitochondria, and existing studies have found that mitochondrial dysfunction leads to the emergence of PN, and the rest of the undamaged mitochondria exposed to PN also experience respiratory dysfunction after oxidative stress, with the two interacting to exacerbate neuronal impairment.60–62 Caused by several factors like physical shock, cell necrosis or apoptosis, and lipid peroxidation, which continually creates cytotoxicity and disrupts cellular ion homeostasis, glutamate, an excitatory neurotransmitter, increases in concentration during SCI.32,53
The occurrence of the aforementioned chain of events results in immediate axonal degeneration, oligodendrocyte apoptosis, and persistent apoptosis or necrosis of neurons and glial cells at the site of injury. The stability of axonal function is impacted by the apoptosis of oligodendrocytes, the myelin-forming cells that encourage myelin proliferation and myelin synthesis.63–65 It also slows down information transmission along the axon and results in axonal demyelination. When secondary injury reaches the chronic stage, astrocytes proceed to proliferate and hypertrophy, migrate along the edges of the severely damaged tissue sites, and secrete a great number of growth inhibitory chondroitin sulfate proteoglycans (CSPGs) to deposit in the microenvironment, eventually forming the central role of the glial scar around the injury’s center. At the same time, fibroblasts also infiltrate the area surrounding the lesion, replacing the extracellular matrix with fibrous connective tissue, and creating the final chronic stage scar.31,66–68 The body’s natural process of glial scar formation, which initiates and initiates healing after SCI, but some researchers believe it to be one of the barriers to neuronal axon regeneration in the CNS (central nervous system).69–71 Lesions grow and create cysts as the spinal cord injury progresses, leaving behind microcystic cavities from lingering necrotic or apoptotic cells that eventually form spinal cord cavities, inflicting permanent harm.31,71
MSCs Transplantation
Cell therapy has become a cutting-edge therapeutic approach for spinal cord injuries, and several early clinical trials have shown that cell transplantation is typically possible. However, its effectiveness and long-term safety has not yet been established.72–74 Among the numerous alternative cells, stem cells have attracted attention because of their capacity for self-renewal and multidirectional differentiation, followed by the selection of different stem cells, mesenchymal stem cells (MSCs) are distinguished by easy isolation, easy preservation, rapid proliferation, low immunogenicity, not involving ethical issues.27,75 Currently, local injection, intravenous injection, and intrathecal injection are the three most used methods for directly injecting MSCs to treat spinal cord injury.76–78 While intrathecal and intravenous injections are less invasive but require a large amounts of cells, and the proportion of mesenchymal stem cells reaching the injury site is low.76,77 local injections can directly transplant a sufficient amount of stem cells to the site of spinal cord injury,78 However, they may further damage the spinal cord and increase the risk of wound infection.78 The “homing” capacity of MSCs has garnered interest in experimental experiments using intrathecal and intravenous injections.79,80 The migration of bone marrow MSCs to the site of injury is regulated by both chemical factors (cytokines, growth factors, etc.) and mechanical factors (mechanical strain, shear stress, etc.).81–86 Vascular endothelial growth factor-A (VEGF-A) has been shown to stimulate platelet-derived growth factor receptors (PDGFRs) and thereby regulate the migration of human BM-MSCs.87 Growth factors (PDGF or IGF-1) released at the level of injury attract MSCs to homing, and an increase in inflammatory factors or chemokines at the site of injury also promotes this cellular behavior.27,88,89 Although local injections can result in spinal cord re-injury, intravenous and intrathecal injections can prevent this. However, cellular localization failure for MSCs moving through “homing” is a possibility. This might have anything to do with the injection time, dose, etc. Further research is required to determine the precise “homing” mechanism and the relationships between the variables.
Cell survival after MSC transplantation influences to some extent the functional improvement after SCI, and in fact, in the damaged spinal cord, the poor microenvironment leads to a low survival rate of transplanted cells.90,91 Studies are now concentrating not only on direct transplantation of MSCs but also on pretreatment, co-transplantation, and transplantation after genetic modification. This is done to improve the post-transplantation microenvironment and cell survival as well as to enhance the repairing effect of MSC transplantation after spinal cord injury.92 The secretion and impact of some bioactive substances can be enhanced by MSC transplantation after gene alteration. Glial-derived neurotrophic factor can be expressed more effectively thanks to the microRNA-383 gene, and altering microRNA-383 and its related genes can enhance MSCs’ ability to treat spinal cord injuries.91,93 A medication, biological scaffold, or other cells can be co-transplanted with MSCs to enhance the microenvironment and have a synergistic effect to promote functional recovery.90,92,94 Pretreating the injury before transplanting MSCs is another option. While the biological scaffold can offer neurotrophic factors, protective growth factors, etc. to promote MSCs to their benefit, the medicine of choice frequently has its own antioxidant, anti-inflammatory, and neurotrophic actions.94,95 The active ingredient in plumbagin, plumbagin, has been shown in pharmacological studies to have antimicrobial, anti-inflammatory, and anti-cancer effects. Treatment with plumbagin combined with mesenchymal stem cells can significantly improve the recovery of motor function in SCI rats by undoing the inhibition of Nrf2, p-Akt, and p-ERK and the promotion of p-p38 MAPK to exert anti-inflammatory and antioxidant effects.96 The survival rate of stem cells was increased when they were injected into SCI mice in the form of chitosan (CS) hydrogels loaded with MSCs. As a result, they were able to release a large number of growth factors and anti-inflammatory cytokines to support neural tissue repair and significantly lessen the glial scar, which encouraged axonal growth and nerve regeneration.97,98 In addition, biological scaffolds are also able to improve difficulties in colonization due to excessive cell spreading after direct transplantation, and collagen scaffolds prepared from fresh bovine tendon membranes have appropriate porosity and nanoscale linear fibers with good adhesion to MSCs, and data suggest that combined appeal scaffolds for rat bone marrow-derived MSC transplantation can not only inhibit chronic scar formation and provide linear neural regeneration priming, but also facilitate the polarization of macrophages to M2 type for better anti-inflammatory effects.99 Therefore, the MSC transplantation modality may be an essential research direction for the treatment of spinal cord injury.
Paracrine secretion and directed differentiation are the two crucial functions of MSCs in repairing injured tissue, however in models of spinal cord injury, paracrine secretion is more likely to occur than directed differentiation.28,100 Numerous bioactive substances, including as the neurotrophic compounds GDNF and NGF as well as the anti-inflammatory cytokines TNF-β1 and IL-13, are secreted by MSCs.101–103 MSCs have differentiation potential and can be stimulated to differentiate into neuron-cells in vitro.103,104 To replace dead cells and restore the integrity of neuronal conduction pathways, researchers have tried to develop MSCs into neuronal cells and glial cells following transplantation into the spinal cord lesion site. However, recent research indicates that there is still a lack of proof for distinction.105
Existing research suggests that transplanted MSCs may primarily exert neuroprotective and neuro- regenerative effects through cell-cell interactions and paracrine effects, supporting morphological and functional recovery following spinal cord injury.106,107 Animals treated with MSCs after transplantation improved motor and sensory capabilities and encouraged the restoration of hind limb function in SCI mice or SCI rats, according to research on animal models of spinal cord injury.108–110 Clinical trials using MSCs for spinal cord injury has also been conducted recently, and despite their limited frequency, they have so far produced encouraging outcomes. Some SCI patients demonstrated improvement in neurological function when adipose-derived MSCs were extracted from patients’ adipose tissue for intrathecal delivery via lumbar puncture.111 The available data have demonstrated to researchers that MSC transplantation is effective in the treatment of spinal cord injury, even after clinical trial’s challenges with a small number of SCI patients who experienced adverse effects (headache, urinary tract infection, nausea and vomiting), as well as a small and heterogeneous number of patients.112,113
MSCs in the Treatment of SCI: Neuroprotection
Neuroprotection is defined as the protection of the structure and function of the injury site and surrounding neurons from further damage by alleviating and attenuating specific events in secondary injury in an attempt to reduce the rate of injury occurrence and mitigate the extent of injury.11,32 In the acute and subacute phases of spinal cord injury, nerve protection is a crucial treatment goal and the first line of defense that should be put in place as soon as feasible.42,114 Anti-inflammatory, antioxidant, anti-apoptotic, anti- excitotoxic, and channel blocking can be exploited as breakthrough points for neuroprotection depending on the various events in the secondary cascade response.115,116 A channel blocker called riluzole will stop excitotoxic cell death by preventing sodium inward flow in injured neurons and restricting presynaptic glutamate release.31,117 Granulocyte colony-stimulating factor (G-CSF) has been found to increase cell survival and decrease the expression of inflammatory factors (TNF-α, IL-1β) in the central nervous system.118 The existing neuroprotective therapies are not limited to this. The therapeutic effects of MSCs on SCI are gaining attention as research advances because they exhibit significant autocrine and paracrine activities, exerting anti-inflammatory and antioxidant effects, preventing neurodegeneration and apoptosis, promoting axonal and myelin regeneration, preventing vascular damage, and enhancing angiogenesis119–122 (Figure 3).
Anti-inflammatory: Tumor necrosis factor (TNFβ1), interleukin (IL-13), IL-18 binding protein, and other substances are secreted by mesenchymal stem cells. MSCs can also control cytokine production in the location of the injury and enhance the inflammatory microenvironment.122 Mesenchymal stem cells from umbilical cord blood promotes the polarization of M2 macrophages and reduces IL-7 and IFN- γ, TNF- α, at the same time, they increased the expression of IL-4 and IL-13.47,75 Transplantation of murine adipose-derived mesenchymal stem cells inhibit macrophage infiltration and reduce the expression of TNF-α, IL-1β, and IL-6.22,47 Rat bone marrow mesenchymal stem cells suppress the expression of pro-inflammatory cytokines such as TNF- α and IL-1β.21 The research that is currently available indicates that transplanted MSCs can also change the macrophage phenotype from M1 to M2. Microglia and macrophage phenotypes are classified as M1 type (neurotoxic and pro-inflammatory) and M2 type (immune regulation). The bioactive substances released by the latter encourage myelin sheath development and axon growth.123,124 Reduce the number of M1 macrophages to reduce the production of the inflammatory response, which has a beneficial effect on neuroprotection.125,126 BM-MSCs can activate M2 macrophages, suppress M1 macrophages, elevate IL-4, and IL-13 levels, and decrease TNF-α, IL-6, and IL-1β levels for immune modulation.127,128
Antioxidation: One of the efficient damage processes engaged in secondary damage events is lipid peroxidation brought on by oxygen radicals.54 The constitutive production of the antioxidant enzymes SOD1, SOD2, catalase (CAT), and glutathione peroxidase (GPX), as well as high levels of the antioxidant glutathione, have been linked to MSCs’ resistance to oxidative and nitrous stimulation in vitro (GSH). In the animal model of spinal cord injury, MSCs have also been shown to perform an antioxidant role by a wealth of evidence.129,130 By scavenging free radicals, boosting host antioxidant defenses, and changing cellular bioenergetics, MSCs are currently assumed to lessen oxidative damage.129,131,132 After adipose MSCs were transplanted, 3- NT, a PN marker, and PC, a protein oxidative stress-related product, significantly decreased at the spinal cord lesion site, trying to show that lipid peroxidation and protein oxidation were reduced after cell transplantation and that MSCs can reduce oxidative stress after injury.133 The modification of the redox environment and oxidative stress by MSCs, which together promote cytoprotection, also has an anti-inflammatory effect.134
Anti-apoptotic: After SCI, neuronal and glial cells die as a result of both primary and secondary injury, and the activation of apoptotic pathways also plays a role in cell death.135 Both the receptor-dependent extrinsic pathway and the intrinsic system, which is influenced by cell-intrinsic events such as DNA damage, hypoxia, and oxidative stress, are involved in apoptosis.136 In addition to the anti-apoptotic genes, Bcl-2 and the apoptosis-inducing gene Bax are also involved in the regulation of apoptosis after SCI. Caspase-3 and Caspase-8 activation and apoptosis are temporally similar, and Caspase-8 activation is an important step in initiating exogenous pathways after SCI.137 By lowering apoptosis, mesenchymal stem cell implantation can aid neurological rehabilitation. On days 14 and 28 following the transplantation of olfactory sheath cells combined with bone marrow Mesenchymal stem cells (BM-MSCs), it was discovered that the levels of the proteins caspase-9 and caspase-3 were significantly decreased and the levels of the protein Bcl-2 were significantly increased in the spinal cord of SCI rats.137 Bone marrow MSCs can also mediate protection against apoptotic injury by secreting protective factors that stimulate neuronal endogenous survival signaling pathways, namely PI3K/Akt and MAPK/ERK1/2 cascade responses.138 Additionally, the interaction between stressed neurons and BM-MSCs improved neuroprotection even more.139
Revascularization: Leaky or nonexistent blood vessels cause ischemia, which prevents endogenous regeneration of damaged tissue. Blood vessels play a critical role in spinal cord injury. The ischemia cascade that results from the vascular injury ultimately speeds up cell death and tissue damage by increasing cytotoxic proteolytic enzymes and reactive oxygen species.40 Whereas endogenous vascular regeneration occurs in the organism during the early stages of spinal cord injury, it is still challenging to re-establish functioning blood vessels at the site of injury.41,42 When it comes to neuroprotection and nerve regeneration, blood flow reconstruction is essential. Additionally, a healthy blood supply creates a microenvironment that is conducive to the survival of residual tissue and nerve regeneration, which supports functional recovery after spinal cord injury.41,140,141 MSCs induce angiogenesis by paracrine secretion of vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and others.27 The blood spinal cord barrier (BSCB) leakage was reduced, the density of microvasculature/repair-neovascularization at the injury site was increased, there was extensive remyelination around the injury epicenter, and finally improved functional recovery in SCI rats treated with adult bone marrow-derived mesenchymal stem cells.142
MSCs in the Treatment of SCI: Nerve Regeneration
In the acute and subacute phases of spinal cord injury, prompt neuroprotection can be very beneficial; however, for patients in the chronic phase, nerve regeneration is now more important than neuroprotection.31 Promoting axonal regeneration after damage is a crucial goal in the treatment of the chronic phase of spinal cord injury since the disruption of central nerve linkages is one of the causes for ongoing dysfunction after SCI.67,143 The functional recovery of patients with spinal cord injuries may significantly improves with even a little amount of axonal regrowth.144
Axon lengthening, axonal sprouting and growth of new axons, the remyelination of nerve cells, and other processes that entail the regeneration and repair of damaged neural tissue (neurons, axons, synapses, and glial cells) after injury are all examples of nerve regeneration.32,145 Mammalian CNS regeneration is challenging, cannot upregulate the genetic program required for axonal growth as in the regeneration of neurons within the peripheral nervous system, and the capacity for regeneration declines with age due to limited plasticity.31,144,146 After spinal cord injury, ischemia and hypoxia increase the amount of oxygen free radicals present at the site of injury and lead to the formation of myelin fragments,40 where it contains inhibitory molecules like Nogo-A protein or myelin-associated glycoprotein (MAG) that stops axon growth in animal models.27,147
Collectively, these factors build a microenvironment that is not conducive to axonal regeneration, also referred to as a non-permissive environment. The glial scar, of which astrocytes are the main component, forms a physical barrier that isolates damaged tissue from healthy tissue, leading to impaired axonal regeneration.31 Although glial scarring has long been considered detrimental to the repair of the injured spinal cord, recent studies have also shown that glial scarring can be protective of the damaged spinal cord,98,148 and that this discrepancy may be because glial scarring isolates healthy tissue from further damage by inflammatory cells and various toxic molecules in the early stages of injury, but hinders endogenous or treatment-induced of axonal regeneration.98,149 Related investigations have shown how functional recovery in animals with spinal cord injuries are facilitated by enhanced glial scar permeability.148 By secreting numerous growth-inhibiting chondroitin sulfate proteoglycans (CSPGs), such as Neurocan, Versican, Brevican, PhosphaCan, and NG2, which create a chemical barrier, astrocytes also prevent post-injury repair or regeneration.31 Therefore, therapies to encourage axonal regeneration within the CNS have concentrated on increasing the intrinsic ability of neurons to renew, improving the environment that is non-permissive for their regeneration, or minimizing the impacts of the double barrier created by astrocytes.67,144
Early research into MSC-based regenerative therapies concentrated on their ability to differentiate into neurons or glial cells after transplantation. However, there is currently a lack of conclusive experimental evidence for MSC differentiation in vivo.10,105,150 Nevertheless, transplanted MSCs are still capable of performing a variety of tasks, such as supplying nutritional support, regulating the inflammatory response in the acute phase, and lowering scar tissue inhibition in the subacute and chronic phases to create an environment that is favorable for axonal regeneration.31,139 Neurotrophic factors have been proven to enhance the growth potential of CNS neurons after injury, and the enhanced ability of neurons exposed to neurotrophic factor (BDNF) or glial-derived neurotrophic factor (GDNF) to overcome the non-permissive environment is mediated by elevated intracellular cAMP levels.144,151 MSCs are capable of secreting brain-derived growth factor (BDNF), glial cell-derived growth factor (GDNF), nerve growth factor (NGF), NT-1, NT-3, CNTF, and basic fibroblast growth factor (bFGF), leading to the speculation that transplanted MSCs may enhance the intrinsic growth propensity of damaged neurons by secreting neurotrophic factors.27 In addition to improving intrinsic growth propensity, neurotrophic substances help ameliorate the existing nonpermissive environment by acting as antioxidants, anti-inflammatory agents, and BDNF can act against oxidative stress to increase neuronal survival.148,152 The glial scar that astrocytes created after transplanting MSCs might also be modified.153 According to studies, transplanting MSCs into SCI rats prevents the creation of glial scars and alters the reactive astrocytes’ shape, which combined create an ideal microenvironment for axonal regeneration.153,154
Furthermore, another experiment using human bone marrow-derived MSCs to treat SCI rats showed that the treatment group had a lower density of GFAP-positive scars than the control group, which formed a loose glial scar.148 The attractive effect can be seen in experiments using dogs as a model of SCI, where adipose mesenchymal stem cells and chondroitinase ABC (a bacterial enzyme) work together to degrade CSPGs. The results of this experiment showed a significant reduction in the reactive astrocyte marker GFAP and a reduction in scar formation at the injury site.155 Researchers were motivated to investigate the causes of these effects after learning that BM-MSCs could improve motor function by reducing the activation of TGF-B/Smads signaling in astrocytes. Since TGF- can mediate the formation of glial scars by activating Smads, it is hypothesized that BM-MSCs can prevent scarring after injury by controlling the TGF-B/Smads signaling conduction pathway.153,156,157 Further research is required because the precise and intricate mechanism is still unknown.
Conclusions
Spinal Cord Injury (SCI) is a serious, prolonged and irreversible injury. As a result, a great deal of academics and professionals in the medical field are eager to discover secure and efficient treatments for spinal cord injuries. Numerous preclinical and clinical studies have demonstrated the effectiveness of Mesenchymal stem cell (MSC) in the treatment of SCI. The effectiveness of MSC for the treatment of SCI has now been established by a large number of preclinical and clinical studies. The anti-inflammatory, anti-oxidant, anti-apoptotic, and increased blood flow that MSC transplantation provides protects the nerves. It also increases intrinsic neuronal growth potential, boosts non-permissive settings, and alters glial scarring to support regeneration. Future studies may need to further investigate more specific therapeutic mechanisms as well as better methods of transplanting MSC (pre-treatment, gene modification and combination therapy, and others) because the therapeutic mechanism of MSC transplantation is not fully understood and because issues like inaccurate cell localization and a low survival rate after direct cell transplantation exist.
Acknowledgments
Si-Yu Chen: Writing - Original Draft. Rui-Lin Yang, Xiang-Chong Wu, De-Zhi Zhao, Sheng-Ping Fu, Feng-Qin Lin, Lin-Yan Li, Li-Mei Yu and Qian Zhang: Writing - Review & Editing. Tao Zhang: Writing - Review & Editing, Funding acquisition.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.81960299).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
1. Sterner RC, Sterner RM. Immune response following traumatic spinal cord injury: pathophysiology and therapies. Front Immunol. 2022;13:1084101. doi:10.3389/fimmu.2022.1084101
2. Chen C, Qiao X, Liu W, Fekete C, Reinhardt JD. Epidemiology of spinal cord injury in China: a systematic review of the Chinese and English literature. Spinal Cord. 2022;60(12):1050–1061. doi:10.1038/s41393-022-00826-6
3. Borg SJ, Geraghty T, Arora M, et al. Employment outcomes following spinal cord injury: a population- based cross-sectional study in Australia. Spinal Cord. 2021;59(10):1120–1131. doi:10.1038/s41393-021-00639-z
4. Xia Y, Zhu J, Yang R, Wang H, Li Y, Fu C. Mesenchymal stem cells in the treatment of spinal cord injury: mechanisms, current advances and future challenges. Front Immunol. 2023;14:1141601. doi:10.3389/fimmu.2023.1141601
5. Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. doi:10.1038/s41467-021-25585-5
6. Clifford T, Finkel Z, Rodriguez B, Joseph A, Cai L. Current advancements in spinal cord injury research- Glial scar formation and neural regeneration. Cells. 2023;12(6):853. doi:10.3390/cells12060853
7. Sykova E, Cizkova D, Kubinova S. Mesenchymal Stem cells in treatment of spinal cord injury and amyotrophic lateral sclerosis. Front Cell Dev Biol. 2021;9:695900. doi:10.3389/fcell.2021.695900
8. Jin Y, Yang S, Zhang X. Retraction: reduction of neuronal damage and promotion of locomotor recovery after spinal cord injury by early administration of methylprednisolone: possible involvement of autophagy pathway. RSC Adv. 2022;12(41):26565. doi:10.1039/D2RA90087D
9. Chio JCT, Xu KJ, Popovich P, David S, Fehlings MG. Neuroimmunological therapies for treating spinal cord injury: evidence and future perspectives. Exp Neurol. 2021;341:113704.
10. Canseco JA, Karamian BA, Bowles DR, et al. Updated review: the steroid controversy for management of spinal cord injury. World Neurosurg. 2021;150:1–8. doi:10.1016/j.wneu.2021.02.116
11. Yang CH, Quan ZX, Wang GJ, et al. Elevated intraspinal pressure in traumatic spinal cord injury is a promising therapeutic target. Neural Regen Res. 2022;17(8):1703–1710. doi:10.4103/1673-5374.332203
12. Teoh PL, Mohd Akhir H, Abdul Ajak W, Hiew VV. Human mesenchymal stromal cells derived from perinatal tissues: sources, characteristics and isolation methods. Malays J Med Sci. 2023;30(2):55–68. doi:10.21315/mjms2023.30.2.5
13. Barbon S, Rajendran S, Bertalot T, et al. Growth and differentiation of circulating stem cells after extensive ex vivo expansion. Tissue Eng Regen Med. 2021;18(3):411–427. doi:10.1007/s13770-021-00330-7
14. Pievani A, Scagliotti V, Russo FM, et al. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16(7):893–905. doi:10.1016/j.jcyt.2014.02.008
15. Laloze J, Fievet L, Desmouliere A. Adipose-derived mesenchymal stromal cells in regenerative medicine: state of play, current clinical trials, and future prospects. Adv Wound Care (New Rochelle). 2021;10(1):24–48. doi:10.1089/wound.2020.1175
16. Moayeri A, Alizadeh R, Ghasemi Hamidabadi H, et al. Transdifferentiation of human umbilical cord-derived mesenchymal stem cells in dopaminergic neurons in a three-dimensional culture. Basic Clin Neurosci. 2022;13(5):625–636. doi:10.32598/bcn.2021.973.3
17. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886. doi:10.3390/cells8080886
18. Kim M, Kim KH, Song SU, et al. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduce fibrotic scar formation in a rat spinal cord injury model. J Tissue Eng Regen Med. 2018;12(2):e1034–e1045. doi:10.1002/term.2425
19. Liao Y, Ming J, Song W, et al. Mitochondrial transplantation and immune response of human bone marrow mesenchymal stem cells for the Therapeutic of ischemic stroke. Curr Stem Cell Res Ther. 2023. doi:10.2174/1574888X18666230505103407
20. Spees JL, Lee RH, Gregory CA. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res Ther. 2016;7(1):125. doi:10.1186/s13287-016-0363-7
21. Maeda Y, Otsuka T, Takeda M, et al. Transplantation of rat cranial bone-derived mesenchymal stem cells promotes functional recovery in rats with spinal cord injury. Sci Rep. 2021;11(1):21907. doi:10.1038/s41598-021-01490-1
22. Zhou Z, Tian X, Mo B, et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem Cell Res Ther. 2020;11(1):212. doi:10.1186/s13287-020-01724-5
23. Li L, Cao J, Li S, et al. M2 macrophage-derived sEV regulate pro-inflammatory CCR2(+) macrophage subpopulations to favor post-AMI cardiac repair. Adv Sci (Weinh). 2023;10(14):e2202964. doi:10.1002/advs.202202964
24. Lin H, Sohn J, Shen H, Langhans MT, Tuan RS. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi:10.1016/j.biomaterials.2018.06.026
25. Sun J, Xing F, Zou M, Gong M, Li L, Xiang Z. Correction: comparison of chondrogenesis-related biological behaviors between human urine-derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res Ther. 2022;13(1):514. doi:10.1186/s13287-022-03193-4
26. Shirin M, Agharezaeei M, Alizadeh S, et al. A comparative study of the bone marrow- and umbilical cord-derived Mesenchymal Stem Cells (MSCs) Efficiency on Generating MSC-Educated Macrophages (MEMs). Asian Pac J Cancer Prev. 2022;23(9):3083–3092. doi:10.31557/APJCP.2022.23.9.3083
27. Cofano F, Boido M, Monticelli M, et al. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. doi:10.3390/ijms20112698
28. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8(8):784. doi:10.3390/cells8080784
29. Tahmasebi F, Barati S. Effects of mesenchymal stem cell transplantation on spinal cord injury patients. Cell Tissue Res. 2022;389(3):373–384. doi:10.1007/s00441-022-03648-3
30. Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. 2012;114(7):935–939. doi:10.1016/j.clineuro.2012.02.003
31. Ahuja CS, Nori S, Tetreault L, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80(3S):S9–S22. doi:10.1093/neuros/nyw080
32. Anjum A, Yazid MD, Fauzi Daud M, et al. Spinal cord injury: pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int J Mol Sci. 2020;21(20):7533. doi:10.3390/ijms21207533
33. Lee CY, Chooi WH, Ng SY, Chew SY. Modulating neuroinflammation through molecular, cellular and biomaterial-based approaches to treat spinal cord injury. Bioeng Transl Med. 2023;8(2):e10389. doi:10.1002/btm2.10389
34. Hachem LD, Fehlings MG. Pathophysiology of spinal cord injury. Neurosurg Clin N Am. 2021;32(3):305–313. doi:10.1016/j.nec.2021.03.002
35. Zhang Y, Al Mamun A, Yuan Y, et al. Acute spinal cord injury: pathophysiology and pharmacological intervention (Review). Mol Med Rep. 2021;23(6). doi:10.3892/mmr.2021.12056
36. Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282.
37. Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15(6):394–409. doi:10.1038/nrn3680
38. O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 2017;127(9):3259–3270. doi:10.1172/JCI90608
39. Eli I, Lerner DP, Ghogawala Z. Acute traumatic spinal cord injury. Neurol Clin. 2021;39(2):471–488. doi:10.1016/j.ncl.2021.02.004
40. Haggerty AE, Maldonado-Lasuncion I, Oudega M. Biomaterials for revascularization and immunomodulation after spinal cord injury. Biomed Mater. 2018;13(4):044105. doi:10.1088/1748-605X/aaa9d8
41. Yao C, Cao X, Yu B. Revascularization after traumatic spinal cord injury. Front Physiol. 2021;12:631500. doi:10.3389/fphys.2021.631500
42. Oudega M. Molecular and cellular mechanisms underlying the role of blood vessels in spinal cord injury and repair. Cell Tissue Res. 2012;349(1):269–288. doi:10.1007/s00441-012-1440-6
43. Wu X, Yan Y, Zhang Q. Neuroinflammation and Modulation role of natural products after spinal cord injury. J Inflamm Res. 2021;14:5713–5737. doi:10.2147/JIR.S329864
44. Yao XQ, Liu ZY, Chen JY, et al. Proteomics and bioinformatics reveal insights into neuroinflammation in the acute to subacute phases in rat models of spinal cord contusion injury. FASEB J. 2021;35(7):e21735. doi:10.1096/fj.202100081RR
45. Garcia E, Aguilar-Cevallos J, Silva-Garcia R, Ibarra A. Cytokine and growth factor activation in vivo and in vitro after spinal cord injury. Mediators Inflamm. 2016;2016:9476020. doi:10.1155/2016/9476020
46. Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133(Pt 2):433–447. doi:10.1093/brain/awp322
47. Pang QM, Chen SY, Fu SP, et al. Regulatory role of mesenchymal stem cells on secondary inflammation in spinal cord injury. J Inflamm Res. 2022;15:573–593. doi:10.2147/JIR.S349572
48. Ulndreaj A, Chio JC, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 2016;16(10):1127–1129. doi:10.1080/14737175.2016.1207532
49. David S, Lopez-Vales R. Bioactive lipid mediators in the initiation and resolution of inflammation after spinal cord injury. Neuroscience. 2021;466:273–297. doi:10.1016/j.neuroscience.2021.04.026
50. Dolma S, Kumar H. Neutrophil, extracellular matrix components, and their interlinked action in promoting secondary pathogenesis after spinal cord injury. Mol Neurobiol. 2021;58(9):4652–4665. doi:10.1007/s12035-021-02443-5
51. Miron VE, Franklin RJ. Macrophages and CNS remyelination. J Neurochem. 2014;130(2):165–171. doi:10.1111/jnc.12705
52. Fehlings MG, Nakashima H, Nagoshi N, Chow DS, Grossman RG, Kopjar B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): a randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016;54(1):8–15. doi:10.1038/sc.2015.95
53. Vanzulli I, Butt AM. mGluR5 protect astrocytes from ischemic damage in postnatal CNS white matter. Cell Calcium. 2015;58(5):423–430. doi:10.1016/j.ceca.2015.06.010
54. Hall ED, Wang JA, Bosken JM, Singh IN. Lipid peroxidation in brain or spinal cord mitochondria after injury. J Bioenerg Biomembr. 2016;48(2):169–174. doi:10.1007/s10863-015-9600-5
55. Pandya JD, Musyaju S, Modi HR, et al. Comprehensive evaluation of mitochondrial redox profile, calcium dynamics, membrane integrity and apoptosis markers in a preclinical model of severe penetrating traumatic brain injury. Free Radic Biol Med. 2023;198:44–58. doi:10.1016/j.freeradbiomed.2023.02.001
56. Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther. 2017;23(1):5–22. doi:10.1111/cns.12655
57. Scholpa NE, Schnellmann RG. Mitochondrial-based therapeutics for the treatment of spinal cord injury: mitochondrial biogenesis as a potential pharmacological target. J Pharmacol Exp Ther. 2017;363(3):303–313. doi:10.1124/jpet.117.244806
58. Hall ED. Antioxidant therapies for acute spinal cord injury. Neurotherapeutics. 2011;8(2):152–167. doi:10.1007/s13311-011-0026-4
59. Lee BJ, Jeong JH. Review: steroid use in patients with acute spinal cord injury and guideline update. Korean J Neurotrauma. 2022;18(1):22–30. doi:10.13004/kjnt.2022.18.e21
60. Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26(11):1407–1418. doi:10.1038/sj.jcbfm.9600297
61. Sullivan PG, Krishnamurthy S, Patel SP, Pandya JD, Rabchevsky AG. Temporal characterization of mitochondrial bioenergetics after spinal cord injury. J Neurotrauma. 2007;24(6):991–999. doi:10.1089/neu.2006.0242
62. Wang B, Huang M, Shang D, Yan X, Zhao B, Zhang X. Mitochondrial behavior in Axon Degeneration and regeneration. Front Aging Neurosci. 2021;13:650038. doi:10.3389/fnagi.2021.650038
63. Cohen-Adad J, El Mendili MM, Lehericy S, et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 2011;55(3):1024–1033. doi:10.1016/j.neuroimage.2010.11.089
64. Domingues HS, Portugal CC, Socodato R, Relvas JB. Oligodendrocyte, astrocyte, and microglia crosstalk in myelin development, damage, and repair. Front Cell Dev Biol. 2016;4:71. doi:10.3389/fcell.2016.00071
65. Ginet V, Spiehlmann A, Rummel C, et al. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy. 2014;10(5):846–860. doi:10.4161/auto.28264
66. Anderson MA. Targeting central nervous system regeneration with cell type specificity. Neurosurg Clin N Am. 2021;32(3):397–405. doi:10.1016/j.nec.2021.03.011
67. Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–647. doi:10.1038/nn.4541
68. Moretti M, Caraffi R, Lorenzini L, et al. ”Combo” multi-target pharmacological therapy and new formulations to reduce inflammation and improve endogenous remyelination in traumatic spinal cord injury. Cells. 2023;12(9):1331. doi:10.3390/cells12091331
69. Li L, Acioglu C, Heary RF, Elkabes S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain Behav Immun. 2021;91:740–755. doi:10.1016/j.bbi.2020.10.007
70. Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541–553. doi:10.1007/s13311-018-0631-6
71. Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol Rev. 2018;98(2):881–917. doi:10.1152/physrev.00017.2017
72. Gong Z, Xia K, Xu A, et al. Stem cell transplantation: a promising therapy for spinal cord injury. Curr Stem Cell Res Ther. 2020;15(4):321–331. doi:10.2174/1574888X14666190823144424
73. Nurkovic J, Dolicanin Z, Mustafic F, et al. Mesenchymal stem cells in regenerative rehabilitation. J Phys Ther Sci. 2016;28(6):1943–1948. doi:10.1589/jpts.28.1943
74. Shao A, Tu S, Lu J, Zhang J. Crosstalk between stem cell and spinal cord injury: pathophysiology and treatment strategies. Stem Cell Res Ther. 2019;10(1):238. doi:10.1186/s13287-019-1357-z
75. Bao CS, Li XL, Liu L, Wang B, Yang FB, Chen LG. Transplantation of Human umbilical cord mesenchymal stem cells promotes functional recovery after spinal cord injury by blocking the expression of IL-7. Eur Rev Med Pharmacol Sci. 2018;22(19):6436–6447. doi:10.26355/eurrev_201810_16056
76. Yamazaki K, Kawabori M, Seki T, Houkin K. Clinical trials of stem cell treatment for spinal cord injury. Int J Mol Sci. 2020;21(11):3994. doi:10.3390/ijms21113994
77. Mothe AJ, Bozkurt G, Catapano J, et al. Intrathecal transplantation of stem cells by lumbar puncture for thoracic spinal cord injury in the rat. Spinal Cord. 2011;49(9):967–973.44. doi:10.1038/sc.2011.46
78. Oh SK, Jeon SR. Current concept of stem cell therapy for spinal cord injury: a review. Korean J Neurotrauma. 2016;12(2):40–46.42. doi:10.13004/kjnt.2016.12.2.40
79. Zachar L, Bačenková D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231–240. doi:10.2147/JIR.S121994
80. Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal stem/stromal cells and their paracrine activity-immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics. 2022;14(2):381. doi:10.3390/pharmaceutics14020381
81. Deng QJ, Xu XF, Ren J. Correction to: effects of SDF-1/CXCR4 on the repair of traumatic brain injury in rats by mediating bone marrow derived mesenchymal stem cells. Cell Mol Neurobiol. 2021;41(3):617–618. doi:10.1007/s10571-020-00932-0
82. Dubon MJ, Yu J, Choi S, Park KS. Transforming growth factor beta induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J Cell Physiol. 2018;233(1):201–213. doi:10.1002/jcp.25863
83. Liu L, Luo Q, Sun J, Song G. Cytoskeletal control of nuclear morphology and stiffness are required for OPN-induced bone-marrow-derived mesenchymal stem cell migration. Biochem Cell Biol. 2019;97(4):463–470. doi:10.1139/bcb-2018-0263
84. Schmidt A, Ladage D, Schinkothe T, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24(7):1750–1758. doi:10.1634/stemcells.2005-0191
85. Zhou SB, Wang J, Chiang CA, Sheng LL, Li QF. Mechanical stretch upregulates SDF-1alpha in skin tissue and induces migration of circulating bone marrow-derived stem cells into the expanded skin. Stem Cells. 2013;31(12):2703–2713. doi:10.1002/stem.1479
86. Gebreyesus EA, Park A, Guldberg RE, Ong KG. In vitromagnetohydrodynamics system for modulating cell migration. Biomed Phys Eng Express. 2023;9(2):025007. doi:10.1088/2057-1976/acb711
87. Ball SG, Shuttleworth CA, Kielty CM. Vascular endothelial growth factor can signal through platelet- derived growth factor receptors. J Cell Biol. 2007;177(3):489–500. doi:10.1083/jcb.200608093
88. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi:10.1634/stemcells.2007-0054
89. Hagmeijer MH, Korpershoek JV, Crispim JF, et al. The regenerative effect of different growth factors and platelet lysate on meniscus cells and mesenchymal stromal cells and proof of concept with a functionalized meniscus implant. J Tissue Eng Regen Med. 2021;15(7):648–659. doi:10.1002/term.3218
90. Li X, Zhan J, Hou Y, et al. Coenzyme Q10 regulation of apoptosis and oxidative stress in H2O2 induced BMSC death by modulating the Nrf-2/NQO-1 signaling pathway and its application in a model of spinal cord injury. Oxid Med Cell Longev. 2019;2019:6493081. doi:10.1155/2019/6493081
91. Sayad Fathi S, Zaminy A. Stem cell therapy for nerve injury. World J Stem Cells. 2017;9(9):144–151. doi:10.4252/wjsc.v9.i9.144
92. Jin MC, Medress ZA, Azad TD, Doulames VM, Veeravagu A. Stem cell therapies for acute spinal cord injury in humans: a review. Neurosurg Focus. 2019;46(3):E10. doi:10.3171/2018.12.FOCUS18602
93. Wei GJ, An G, Shi ZW, et al. Suppression of MicroRNA-383 enhances therapeutic potential of human bone-marrow-derived mesenchymal stem cells in treating spinal cord injury via GDNF. Cell Physiol Biochem. 2017;41(4):1435–1444. doi:10.1159/000468057
94. Lv B, Zhang X, Yuan J, et al. Biomaterial-supported MSC transplantation enhances cell-cell communication for spinal cord injury. Stem Cell Res Ther. 2021;12(1):36. doi:10.1186/s13287-020-02090-y
95. Silva D, Sousa RA, Salgado AJ. Hydrogels as delivery systems for spinal cord injury regeneration. Mater Today Biol. 2021;9:100093. doi:10.1016/j.mtbio.2021.100093
96. Yang W, Yang Y, Yang JY, Liang M, Song J. Treatment with bone marrow mesenchymal stem cells combined with plumbagin alleviates spinal cord injury by affecting oxidative stress, inflammation, apoptotis and the activation of the Nrf2 pathway. Int J Mol Med. 2016;37(4):1075–1082. doi:10.3892/ijmm.2016.2498
97. Boido M, Ghibaudi M, Gentile P, Favaro E, Fusaro R, Tonda-Turo C. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci Rep. 2019;9(1):6402. doi:10.1038/s41598-019-42848-w
98. Pang QM, Chen SY, Xu QJ, et al. Neuroinflammation and scarring after spinal cord injury: therapeutic roles of MSCs on inflammation and Glial Scar. Front Immunol. 2021;12:751021. doi:10.3389/fimmu.2021.751021
99. Peng Z, Gao W, Yue B, et al. Promotion of neurological recovery in rat spinal cord injury by mesenchymal stem cells loaded on nerve-guided collagen scaffold through increasing alternatively activated macrophage polarization. J Tissue Eng Regen Med. 2018;12(3):e1725–e1736. doi:10.1002/term.2358
100. Kim YJ, Seo DH, Lee SH, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 2018;16:96–102. doi:10.1016/j.bbrep.2018.10.007
101. Boido M, Piras A, Valsecchi V, et al. Human mesenchymal stromal cell transplantation modulates neuroinflammatory milieu in a mouse model of amyotrophic lateral sclerosis. Cytotherapy. 2014;16(8):1059–1072. doi:10.1016/j.jcyt.2014.02.003
102. Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 2014;9(10):e109305. doi:10.1371/journal.pone.0109305
103. Teixeira FG, Carvalho MM, Neves-Carvalho A, et al. Secretome of mesenchymal progenitors from the umbilical cord acts as modulator of neural/glial proliferation and differentiation. Stem Cell Rev Rep. 2015;11(2):288–297. doi:10.1007/s12015-014-9576-2
104. Hou L, Cao H, Wang D, et al. Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. Int J Hematol. 2003;78(3):256–261. doi:10.1007/BF02983804
105. Fu Q, Liu Y, Liu X, et al. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am J Transl Res. 2017;9(9):3950–3966.
106. Lindsay SL, Barnett SC. Therapeutic potential of Niche-specific mesenchymal stromal cells for spinal cord injury repair. Cells. 2021;10(4):901. doi:10.3390/cells10040901
107. Nagaoki T, Kumagai G, Nitobe Y, et al. Comparison of the Anti-inflammatory effects of mouse adipose- and bone-marrow-derived multilineage-differentiating stress-enduring cells in acute-phase spinal cord injury. J Neurotrauma. 2023. doi:10.1089/neu.2022.0470
108. Mammana S, Gugliandolo A, Cavalli E, et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J Tissue Eng Regen Med. 2019;13(7):1109–1121. doi:10.1002/term.2857
109. Wang W, Huang X, Lin W, et al. Hypoxic preconditioned bone mesenchymal stem cells ameliorate spinal cord injury in rats via improved survival and migration. Int J Mol Med. 2018;42(5):2538–2550. doi:10.3892/ijmm.2018.3810
110. Zhilai Z, Biling M, Sujun Q, et al. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. doi:10.1016/j.brainres.2016.04.025
111. Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–664. doi:10.1179/2045772315Y.0000000048
112. Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A Phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 2016;78(3):436–447. doi:10.1227/NEU.0000000000001056
113. Satti HS, Waheed A, Ahmed P, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a Phase I pilot study. Cytotherapy. 2016;18(4):518–522. doi:10.1016/j.jcyt.2016.01.004
114. Ahuja CS, Mothe A, Khazaei M, et al. The leading edge: emerging neuroprotective and neuroregenerative cell-based therapies for spinal cord injury. Stem Cells Transl Med. 2020;9(12):1509–1530. doi:10.1002/sctm.19-0135
115. Bryk M, Karnas E, Mlost J, Zuba-Surma E, Starowicz K. Mesenchymal stem cells and extracellular vesicles for the treatment of pain: current status and perspectives. Br J Pharmacol. 2022;179(17):4281–4299. doi:10.1111/bph.15569
116. Hellenbrand DJ, Quinn CM, Piper ZJ, Morehouse CN, Fixel JA, Hanna AS. Inflammation after spinal cord injury: a review of the critical timeline of signaling cues and cellular infiltration. J Neuroinflammation. 2021;18(1):284. doi:10.1186/s12974-021-02337-2
117. Martins BC, Torres BBJ, de Oliveira KM, et al. Association of riluzole and dantrolene improves significant recovery after acute spinal cord injury in rats. Spine J. 2018;18(3):532–539. doi:10.1016/j.spinee.2017.10.067
118. Wallner S, Peters S, Pitzer C, Resch H, Bogdahn U, Schneider A. The Granulocyte-colony stimulating factor has a dual role in neuronal and vascular plasticity. Front Cell Dev Biol. 2015;3:48. doi:10.3389/fcell.2015.00048
119. Ghasemi N, Razavi S, Mardani M, Esfandiari E, Salehi H, Zarkesh Esfahani SH. Transplantation of human adipose-derived stem cells enhances remyelination in lysolecithin-induced focal demyelination of rat spinal cord. Mol Biotechnol. 2014;56(5):470–478. doi:10.1007/s12033-014-9744-2
120. Razavi S, Ghasemi N, Mardani M, Salehi H. Remyelination improvement after neurotrophic factors secreting cells transplantation in rat spinal cord injury. Iran J Basic Med Sci. 2017;20(4):392–398. doi:10.22038/IJBMS.2017.8580
121. Rbia N, Bulstra LF, Lewallen EA, Hovius SER, van Wijnen AJ, Shin AY. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: an in vitro analysis of the gene expression and growth factors produced. J Plast Reconstr Aesthet Surg. 2019;72(8):1316–1325. doi:10.1016/j.bjps.2019.04.014
122. Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi:10.3390/ijms18091852
123. Devanney NA, Stewart AN, Gensel JC. Microglia and macrophage metabolism in CNS injury and disease: the role of immunometabolism in neurodegeneration and neurotrauma. Exp Neurol. 2020;329:113310. doi:10.1016/j.expneurol.2020.113310
124. Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015;1619:1–11. doi:10.1016/j.brainres.2014.12.045
125. Brockie S, Hong J, Fehlings MG. The role of microglia in modulating neuroinflammation after spinal cord injury. Int J Mol Sci. 2021;22(18):9706. doi:10.3390/ijms22189706
126. Miranpuri GS, Bali P, Nguyen J, et al. Role of microglia and astrocytes in spinal cord injury induced neuropathic pain. Ann Neurosci. 2021;28(3–4):219–228. doi:10.1177/09727531211046367
127. Nakajima H, Uchida K, Guerrero AR, et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29(8):1614–1625. doi:10.1089/neu.2011.2109
128. Papa S, Vismara I, Mariani A, et al. Mesenchymal stem cells encapsulated into biomimetic hydrogel scaffold gradually release CCL2 chemokine in situ preserving cytoarchitecture and promoting functional recovery in spinal cord injury. J Control Release. 2018;278:49–56. doi:10.1016/j.jconrel.2018.03.034
129. Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19(12):1885–1893. doi:10.1089/scd.2010.0093
130. Buzoglu HD, Burus A, Bayazit Y, Goldberg M. Stem cell and oxidative stress-inflammation cycle. Curr Stem Cell Res Ther. 2023;18(5):641–652. doi:10.2174/1574888X17666221012151425
131. DeSantiago J, Bare DJ, Banach K. Ischemia/Reperfusion injury protection by mesenchymal stem cell derived antioxidant capacity. Stem Cells Dev. 2013;22(18):2497–2507. doi:10.1089/scd.2013.0136
132. Mahrouf-Yorgov M, Augeul L, Da Silva CC, et al. Mesenchymal stem cells sense mitochondria released from damaged cells as danger signals to activate their rescue properties. Cell Death Differ. 2017;24(7):1224–1238. doi:10.1038/cdd.2017.51
133. Kim Y, Jo SH, Kim WH, Kweon OK. Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Res Ther. 2015;6:229. doi:10.1186/s13287-015-0236-5
134. Stavely R, Nurgali K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl Med. 2020;9(9):985–1006. doi:10.1002/sctm.19-0446
135. Sobrido-Camean D, Barreiro-Iglesias A. Role of Caspase-8 and Fas in cell death after spinal cord injury. Front Mol Neurosci. 2018;11:101. doi:10.3389/fnmol.2018.00101
136. Cai W, Shen WD. Anti-apoptotic mechanisms of acupuncture in neurological diseases: a review. Am J Chin Med. 2018;46(3):515–535. doi:10.1142/S0192415X1850026X
137. Wu S, Cui G, Shao H, Du Z, Ng JC, Peng C. The cotransplantation of olfactory ensheathing cells with bone marrow mesenchymal stem cells exerts antiapoptotic effects in adult rats after spinal cord injury. Stem Cells Int. 2015;2015:516215. doi:10.1155/2015/516215
138. Isele NB, Lee HS, Landshamer S, et al. Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem Int. 2007;50(1):243–250. doi:10.1016/j.neuint.2006.08.007
139. Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells. 2014;6(2):120–133. doi:10.4252/wjsc.v6.i2.120
140. Li Y, Lucas-Osma AM, Black S, et al. Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med. 2017;23(6):733–741. doi:10.1038/nm.4331
141. Lu Y, Zhou Y, Zhang R, et al. Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front Neurosci. 2019;13:209. doi:10.3389/fnins.2019.00209
142. Morita T, Sasaki M, Kataoka-Sasaki Y, et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–231. doi:10.1016/j.neuroscience.2016.08.037
143. Filous AR, Silver J. ”Targeting astrocytes in CNS injury and disease: a translational research approach”. Prog Neurobiol. 2016;144:173–187. doi:10.1016/j.pneurobio.2016.03.009
144. Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strategies to promote neural repair and regeneration after spinal cord injury. Spine (Phila Pa 1976). 2005;30(17 Suppl):S3–13. doi:10.1097/01.brs.0000175186.17923.87
145. Wu X, Xu XM. RhoA/Rho kinase in spinal cord injury. Neural Regen Res. 2016;11(1):23–27. doi:10.4103/1673-5374.169601
146. Kwon BK, Liu J, Messerer C, et al. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99(5):3246–3251. doi:10.1073/pnas.052308899
147. Schwab ME, Strittmatter SM. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 2014;27:53–60. doi:10.1016/j.conb.2014.02.011
148. Urdzikova LM, Ruzicka J, LaBagnara M, et al. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15(7):11275–11293. doi:10.3390/ijms150711275
149. Bradbury EJ, Burnside ER. Moving beyond the glial scar for spinal cord repair. Nat Commun. 2019;10(1):3879. doi:10.1038/s41467-019-11707-7
150. Sultan I, Lamba N, Liew A, et al. The safety and efficacy of steroid treatment for acute spinal cord injury: a systematic review and meta-analysis. Heliyon. 2020;6(2):e03414. doi:10.1016/j.heliyon.2020.e03414
151. Gordon T. The role of neurotrophic factors in nerve regeneration. Neurosurg Focus. 2009;26(2):E3. doi:10.3171/FOC.2009.26.2.E3
152. Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004;190(1):17–31. doi:10.1016/j.expneurol.2004.05.045
153. Anderson MA, O’Shea TM, Burda JE, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561(7723):396–400. doi:10.1038/s41586-018-0467-6
154. Okuda A, Horii-Hayashi N, Sasagawa T, et al. Bone marrow stromal cell sheets may promote axonal regeneration and functional recovery with suppression of glial scar formation after spinal cord transection injury in rats. J Neurosurg Spine. 2017;26(3):388–395. doi:10.3171/2016.8.SPINE16250
155. Lee SH, Kim Y, Rhew D, et al. Effect of the combination of mesenchymal stromal cells and chondroitinase ABC on chronic spinal cord injury. Cytotherapy. 2015;17(10):1374–1383. doi:10.1016/j.jcyt.2015.05.012
156. Hellal F, Hurtado A, Ruschel J, et al. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331(6019):928–931. doi:10.1126/science.1201148
157. Lv C, Zhang T, Li K, Gao K. Bone marrow mesenchymal stem cells improve spinal function of spinal cord injury in rats via TGF-beta/Smads signaling pathway. Exp Ther Med. 2020;19(6):3657–3663. doi:10.3892/etm.2020.8640
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.