Back to Journals » Journal of Inflammation Research » Volume 16
Mesenchymal Stem Cell-Derived Exosomes: A Novel Approach to Diabetes-Associated Cognitive Impairment
Authors Ran Q, Tian H, Lin J, Wang H, Wang B, Chen Z, Song D, Gong C
Received 26 July 2023
Accepted for publication 12 September 2023
Published 21 September 2023 Volume 2023:16 Pages 4213—4228
DOI https://doi.org/10.2147/JIR.S429532
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Qingsen Ran,1 He Tian,1 Jian Lin,1 Han Wang,2 Bo Wang,1 Zhixin Chen,1 Da Song,1 Chunzhu Gong1
1Department of Science and Education, Shenzhen Pingle Orthopedic Hospital (Shenzhen Pingshan Traditional Chinese Medicine Hospital), Shenzhen, Guangzhou Province, 518118, People’s Republic of China; 2Department of Gastroenterology, Affiliated Hospital of Changchun University of Chinese Medicine, Changchun, Jilin Province, 130021, People’s Republic of China
Correspondence: Chunzhu Gong, Email [email protected]
Abstract: The progression of diabetes frequently results in a myriad of neurological disorders, including ischemic stroke, depression, blood-brain barrier impairment, and cognitive dysfunction. Notably, diabetes-associated cognitive impairment, a prevalent comorbidity during the course of diabetes, progressively affects patients’ cognitive abilities and may reciprocally influence diabetes management, thereby severely impacting patients’ quality of life. Extracellular vesicles, particularly nanoscale exosomes, have garnered considerable attention in recent years. These exosomes carry and transfer various functional molecules, such as proteins, lipids, and diverse non-coding RNAs, serving as novel regulators and communicators in intercellular interactions. Of particular interest, mesenchymal stem cell-derived exosomes (MSC-Exos) have been reported to traverse the blood-brain barrier and ameliorate intracerebral pathologies. This review elucidates the role of MSC-Exos in diabetes-related cognitive impairment, with a focus on their applications as biomarkers, modulation of neuronal regeneration and synaptic plasticity, anti-inflammatory properties, antioxidative effects, and their involvement in regulating the functionality of β-amyloid proteins during the course of cognitive impairment. The immense therapeutic potential of MSC-Exos in the treatment of diabetes-induced cognitive dysfunction is emphasized.
Keywords: diabetes-associated cognitive impairment, mesenchymal stem cell-derived exosomes, blood-brain barrier, neuronal regeneration, synaptic plasticity
Introduction
Diabetes mellitus is a prevalent metabolic disorder, characterized principally by elevated blood glucose levels surpassing normal thresholds.1,2 Over recent decades, there has been a marked upsurge in global diabetes incidence, particularly that of type 2 diabetes mellitus, attributable not solely to lifestyle transformations such as dietary habits, obesity, and physical activity levels,2,3 but also to increased life expectancy and population aging. Categorization by etiology delineates diabetes into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), with the latter constituting the most ubiquitous form worldwide, accounting for approximately 90–95% of diabetic individuals.4,5 T1DM results from autoimmune-mediated attacks on pancreatic β-cells, culminating in their loss and concomitant insulin secretion defects,6,7 whereas T2DM primarily arises from diminished insulin sensitivity in peripheral tissues. T2DM’s initial stages are often accompanied by compensatory insulin secretion from pancreatic β-cells, hence its advanced phase typically manifests with concomitant hyperglycemia and hyperinsulinemia.8 The onset of T2DM is frequently accompanied by myriad complications, primarily classified into microvascular and macrovascular diseases.9,10 Microvascular complications chiefly involve the microvasculature and generally affect organs such as the eyes, kidneys, and the nervous system, encompassing conditions such as diabetic retinopathy, diabetic nephropathy, diabetic peripheral neuropathy, diabetic foot, and diabetic pulmonary arterial hypertension.11,12 Macrovascular complications predominantly encompass diabetic cardiovascular diseases and diabetic peripheral vascular alterations, frequently leading to sequelae such as coronary artery disease, myocardial infarction, and arteriosclerosis.13,14
Cognitive impairment and dementia patient populations often exhibit similar demographic trends, which are not coincidental; epidemiological studies have corroborated the association between diabetes and dementia.15 Furthermore, diabetes is significantly correlated with the onset of mild cognitive impairment, a common early symptom of Alzheimer’s disease.16,17 Diabetic-related cognitive dysfunction is not a singular disorder, as its manifestations and prognoses tend to vary according to diabetes type and age.18,19 Notably, the severity of cognitive impairment engendered by T1DM and T2DM seems to differ. In T1DM, cognitive dysfunction is often influenced by insulin levels, vascular risk factors, and blood glucose levels,20 whereas in T2DM, it is associated with vascular risk factors, diabetes treatment modalities, and disease duration.21 Research findings suggest that cognitive impairment and magnetic resonance imaging alterations in T1DM patients are not as pronounced as in age-matched T2DM patients,18,22 implying that the pathophysiological differences between T1DM and T2DM exert distinct impacts on the genesis of cognitive impairment in the brain. However, the precise pathogenic mechanisms of T2DM-related brain disorders remain multifactorial, complex, and not entirely understood.23
Mesenchymal stem cells (MSCs) are a class of multipotent stem cells originating from mesenchymal tissue, possessing self-renewal, multilineage differentiation, and immunomodulatory functions.24–26 In addition to directly participating in tissue regeneration and repair, MSCs mediate intercellular communication and signal transduction via the production of extracellular vesicles (EVs).27,28 The most crucial constituents of EVs are exosomes, small vesicles formed by the cell membrane, with diameters ranging from 30–150nm.29 MSC-derived exosomes contain various bioactive molecules, including proteins, nucleic acids, and metabolic byproducts, capable of influencing the biological processes of recipient cells through the transfer of these molecules. They possess a wide range of biological functions, such as cell proliferation, migration, angiogenesis, immunomodulation, and repair.28,30,31 The application prospects of mesenchymal stem cell exosomes are vast, as they can serve as a novel extracellular matrix-based therapeutic approach for promoting tissue repair and regeneration.29 Furthermore, MSC exosomes can act as an effective drug delivery system, targeting specific biologically functional molecules to particular recipient cells, thereby enabling precision therapy.32,33 However, to date, the preparation and purification of mesenchymal stem cell-derived exosomes remain challenging tasks.
Different Types of Brain Disorders Caused by Diabetes
In addition to the various microvascular and macrovascular complications mentioned earlier, T2DM is often accompanied by a multitude of brain disorders closely associated with vascular dysfunction.34 T2DM frequently leads to a significant increase in the risk of ischemic and hemorrhagic strokes and is highly correlated with the onset of dementia and depression. In the development of these brain complications, factors such as poor glycemic control, vascular diseases, oxidative stress, genetic predisposition, insulin resistance, and amyloid protein production may all play crucial roles.22,35,36
Ischemic Stroke
Ischemic stroke is a primary cause of morbidity and mortality in patients with T2DM. Studies have shown that endothelial dysfunction and inflammation are highly correlated with the risk, recurrence, and adverse prognosis of stroke.37,38 In diabetic patients, insulin resistance, endothelial dysfunction, and inflammation impair stroke recovery, thereby compromising the integrity of cerebral vasculature.39 Large vessel strokes can result in severe neurological deficits and even death due to extensive cerebral infarction caused by embolism, while strokes caused by end-terminal small artery disease lead to “lacunar stroke” infarctions in deep brain structures, often associated with small area brain infarctions, damaging regions such as the lentiform nucleus, internal capsule, thalamus, and pons.40 Lacunar strokes may be more common than large vessel strokes in diabetic patients with ischemic stroke,41,42 and stroke patients with diabetes have longer hospital stays, worse functional outcomes, and a higher risk of stroke recurrence.43–45 Compared to non-diabetic stroke patients, the one-year mortality rate for stroke patients with diabetes is twice as high,46,47 and hemoglobin A1c (HbA1c) is significantly associated with stroke prognosis, with a 33% increase in the likelihood of post-stroke death for every 1% increase in HbA1c.48 Additionally, the risk of cognitive impairment or dementia caused by lacunar strokes is higher in diabetic patients.49,50
Blood-Brain Barrier Damage
The blood-brain barrier (BBB) is a specialized structural barrier that regulates the exchange between blood and the brain microenvironment, maintaining internal homeostasis.51 The BBB is located at the interface between blood and brain parenchyma and is composed of tight brain microvascular endothelial cells (BMVECs), pericytes, a single-layered basement membrane, and astrocyte end-feet.52,53 Experimental models have reported increased BBB permeability under diabetic conditions,54 accompanied by impaired BBB integrity, primarily due to damage and inflammation in brain vascular endothelial cells.55 Under high glucose conditions or in the presence of saturated fats and cholesterol, oxidative stress and inflammatory responses in endothelial cells increase,56 leading to changes in the expression and function of tight junction proteins and transmembrane proteins within cells, thereby increasing BBB permeability. Additionally, high glucose levels also elevate brain inflammatory cytokines and free radicals, further damaging BBB integrity, and resulting in increased BBB permeability and neuroinflammation in patients with T2DM.57 Enhanced magnetic resonance imaging shows that the extent of BBB damage caused by diabetes is also highly age-related and leads to neuronal injury,58 suggesting an association between BBB damage and cognitive impairment and dementia. Currently, antioxidants, such as vitamin C, can be used to reduce the harmful effects of glucose,59 and anti-inflammatory and lipid-lowering drugs can be used to reduce the BBB damage caused by inflammation and oxidative stress.60
Depression
T2DM, depression, and cognitive dysfunction often occur together. Compared with individuals without T2DM, the risk of depression in patients with T2DM is doubled, and the risk of T2DM in patients with depression is increased by 1.5 times.61 The potential mechanisms of the relationship between T2DM, depression, and cognitive dysfunction are complex and multifactorial. The connections between these diseases may include common risk factors (such as obesity, physical inactivity, and psychosocial stress) and common underlying mechanisms (such as insulin resistance, activation of the hypothalamic-pituitary-adrenal and sympathetic-adrenal systems, and inflammatory factors).
Additionally, the increased blood-brain barrier permeability caused by T2DM62 and the reduced cerebral vascular reactivity63 can regulate the emotional regulation of the frontal and subcortical brain regions, which may lead to the development of depression. The neurons in the brains of patients with depression may be damaged due to inflammation and changes in neurotransmitters, affecting cognitive function.64 Moreover, research suggests that depression may be associated with microvascular complications of T2DM: in a meta-analysis, the risk of depression was increased in patients with diabetes-related microvascular complications,65 but the impact of psychological stress could not be ruled out.
Depression may also cause changes in patients’ self-awareness and emotional experience, making it difficult for them to concentrate, pay attention, and remember information. Additionally, patients with depression may experience sleep disorders and fatigue, which can also affect cognitive function.
Cognitive Impairments Caused by T2DM
In adults with T2DM, cognitive deficits can be broadly classified into three distinct stages based on severity: diabetes-related cognitive decline, mild cognitive impairment, and dementia23 (Table 1). The first stage typically involves subtle changes in cognitive function and cognitive discomfort without affecting daily activities or diabetes self-management. The second stage, mild cognitive impairment, is characterized by a significant decline in cognitive function but not severe enough to be considered dementia. At this stage, patients may struggle with self-management tasks such as medication management, blood glucose monitoring, and dietary planning. In the third stage, dementia caused by T2DM affects patients’ daily life and independence. Dementia may involve multiple cognitive domains, including memory, communication, attention, reasoning, judgment, and visual-spatial skills.18,66
 |
Table 1 Symptoms and Incidence of Diabetes-Related Cognitive Impairment |
The etiology of T2DM-associated cognitive dysfunction is likely multifactorial, with insulin resistance, inflammation, oxidative stress, gut microbiota dysregulation, metal ion imbalance, and lymphocyte dysfunction potentially contributing to cognitive impairment.34 Dementia has also been linked to transcriptional and proteomic alterations in diabetes. The prevalence of cognitive impairment in T2DM patients is significantly increased,22 with a higher incidence of dementia associated with T2DM as the duration of illness and age increase.66 Concomitant research demonstrates a significant positive correlation between cognitive impairment and the incidence of dementia in T2DM patients, with both HbA1c levels and diabetes duration serving as salient factors.67
Despite the connection with other diabetes complications, there is currently no definitive evidence that the increased risk of cognitive impairment can be solely attributed to elevated blood sugar levels.23 Reduced cerebral vascular reactivity and resting cerebral blood flow changes are associated with worse cognitive test scores.40 In dementia caused by diabetes, multiple disease manifestations are present, including the accumulation of amyloid and tau proteins in the brain, leading to neuronal loss and vascular brain injury.68 T2DM has serious adverse effects on the cardiovascular system, increasing the risk of stroke and small vessel disease in the brain.69
Managing cognitive dysfunction in diabetes patients is crucial as there is a bidirectional relationship between cognition and diabetes management, with cognitive dysfunction adversely affecting diabetes management and poor diabetes management increasing the risk of cognitive dysfunction.70 Current therapies, such as anti-diabetic medications, weight loss surgery, lifestyle interventions, and anti-oxidant and anti-inflammatory compounds, may improve cognitive function in T2DM patients. Patients with T2DM-related cognitive impairment often present with older age and frailty, characterized by weight loss, fatigue, reduced muscle mass and strength, and decreased mobility.71,72 As a result, older individuals with diabetes are more likely to exhibit depression, chronic pain, and medication intolerance.73,74 Identifying these issues is critical for developing personalized management plans, requiring early clinical diagnosis and screening, combined with appropriate treatment approaches and psychological assessments.75
Mesenchymal Stem Cell-Derived Exosomes
MSCs are a type of adult stem cell found in various tissues, first discovered in bone marrow in the 1960s. MSCs, derived from bone marrow, umbilical cord, adipose tissue, placenta, and other tissues, have self-renewal and multilineage differentiation capabilities, enabling them to differentiate into various adult cell types, such as osteocytes, adipocytes, and chondrocytes.76 Furthermore, MSCs possess anti-inflammatory, immunomodulatory, and tissue repair functions.77 MSCs have a wide range of sources and can release a series of biologically active molecules and cytokines through exosomes, facilitating intercellular signaling and regulation.78
Exosomes are nanoscale, double-membrane structures containing proteins, lipids, RNA, metabolites, growth factors, and cytokines that act as multifunctional transporters between cells.29 Exosomes and microvesicles typically carry specific markers such as tetraspanins (CD9, CD63, and CD81), HSP70, MHC, and TSG101.79 Research has shown that all cells can secrete exosomes under normal physiological conditions and pathological processes,29 and exosomes can participate in various diseases, promoting communication as paracrine mediators. Due to the transport properties of exosomes, functional cargoes can be delivered to target cells, making them suitable drug delivery carriers.80,81 Simultaneously, compared to other commonly used drug delivery carriers (eg, liposomes), bioengineered exosomes have advantages such as inherent targeting ability, low immunogenicity, high modification flexibility, and biological barrier permeability.82
Mesenchymal Stem Cell Exosome Biogenesis
Mesenchymal stem cell exosomes (MSC-Exos) secretion can be divided into three key stages: initiation, intermediate formation, and release stages.83
In the initiation stage, MSCs receive various stimuli, including inflammation, stress, and injury, to obtain initiating signals. These stimuli can be conveyed to MSCs through multiple pathways, such as membrane receptor activation and cytokine induction.84 Once MSCs receive the stimulus signals, the signals are transmitted to the interior of the cell through signaling pathways, activating multiple proteins and signaling pathways. These proteins include exosome-associated proteins (such as TSG101, Alix, CD63, etc.) and other proteins and signaling pathways involved in exosome formation. Exosome-associated proteins are essential components of exosome formation. These proteins can be enriched on multivesicular endosomes (MVEs) and participate in the packaging of biomolecules within the cell.85 With the help of the endosomal sorting complex required for transport (ESCRT) and key proteins involved in exosome formation, intraluminal vesicles (ILVs) are formed within MVEs through secondary invagination of the plasma membrane. TSG101 is a common exosome-associated protein that participates in the assembly and disassembly of ESCRT (endosomal sorting complexes required for transport) complexes, promoting exosome formation.86,87 Alix, CD63, and tetraspanins CD81, CD82, and CD9 are also widely considered key proteins involved in exosome formation.88–90 Finally, although some MVEs are degraded through fusion with autophagosomes or lysosomes,91,92 another portion of MVEs fuses with the plasma membrane to release the ILVs contained within through exocytosis, which ultimately form exosomes. These exosomes are released outside MSCs and bind to molecules on the target cells through their surface molecules, realizing signal transmission (Figure 1).
Isolation and Purification of Mesenchymal Stem Cell-Derived Exosomes
Exosomal surfaces comprise an array of distinctive biomolecules, including membrane proteins and glycoproteins, which bestow diverse specificities upon exosomes. The release mechanisms of exosomes may encompass techniques such as differential centrifugation, ultrafiltration, density gradient centrifugation, and immunoaffinity chromatography.93 These methodologies hinge on the analysis and differentiation of the physicochemical properties of exosomes, including size, density, charge, and surface specificity (Table 2).
 |
Table 2 Comparison of Exosome Isolation Methods |
Ultrafiltration, a technique for separating and enriching small molecules utilizing filter membranes, employs membranes with specific pore sizes to filter out macromolecules such as proteins and cell debris, while extracellular exosomes can permeate the membrane pores for concentration and separation.94 However, this approach may result in exosome loss and diminished purity.95
Differential centrifugation, a technique for separating extracellular exosomes from other cellular components (eg, cell debris, large particles) by modulating centrifugation speed,96 achieves separation as distinct cellular components settle in different locations due to their disparate densities and sizes. Nevertheless, this method may lead to mechanical damage, exosome membrane distortion, protein aggregation, lipoprotein contamination, and low purity.97
Density gradient centrifugation separates cellular components of varying densities using layered solutions of different densities to create a gradient. During centrifugation, extracellular exosomes sediment and are separated within the corresponding density gradient layer. Analogous to differential centrifugation, density gradient centrifugation may also inflict mechanical damage on exosomes.95
Immunoaffinity chromatography, a technique leveraging the affinity between specific antibodies and their corresponding antigens for the separation and enrichment of target substances, enables the isolation of extracellular exosomes by employing specific antibodies to recognize and capture surface proteins on exosomes, followed by elution and purification steps to obtain pure exosomes.98,99 However, this method may also cause exosome damage.
Additionally, size exclusion chromatography, polymer precipitation, and microfluidics100,101 may be employed for high-purity preparations, albeit at higher costs or with limited applicability to small samples.
MSC-Exos in Diabetes-Associated Cognitive Impairment
The exosomes in the brain have been demonstrated to participate in normal biological processes, encompassing but not limited to, cellular communication, transcriptional regulation, neurogenesis, neuronal plasticity, and immune responses.102,103 Studies reveal that exosomes derived from bone marrow mesenchymal stem cells (BMSCs) are capable of ameliorating diabetes-induced cognitive dysfunction, amyloid-beta (Aβ) plaques, Aβ deposition area, Aβ1-42, Aβ degradation-associated factors (insulin-degrading enzyme and neprilysin), and levels of pro-inflammatory cytokines.104 Notably, the relatively constant contents within MSC-derived exosomes, such as miRNA and cytokines, play a pivotal role.
Exosomes as Biomarker
In neurodegenerative diseases, certain molecules within MSC-derived exosomes have been identified as potential biomarkers. Particularly, specific microRNAs (miRNAs) and proteins hold significant value in the onset, progression, and diagnosis of these diseases. In neurodegenerative diseases, particular miRNAs have been found to be enriched in MSC-derived exosomes, and numerous miRNAs have been discovered to possess diagnostic capabilities for cerebral or neurodegenerative diseases, such as miR-133b, miR-9,105 miR-134,105 miR-34a,106,107 and miR-125b.108 These miRNAs exhibit significant alterations in cerebrospinal fluid and serum in Alzheimer’s disease (AD) and are highly associated with mesenchymal stem cell origin.109–111 These RNAs exhibit not only significant quantitative differences but also potentially regulate neuronal development, differentiation, apoptosis, and inflammatory response.
Additionally, significant alterations in cargo protein levels may occur in exosomes isolated from the blood and cerebrospinal fluid of AD patients, including remarkable increases in key proteins such as soluble Aβ1-42, Aβ oligomers, and different site-phosphorylated Tau.112–114 These proteins can be detected years before the clinical diagnosis of AD115 and are closely related to blood glucose levels,116 offering the possibility of using exosomes as a diagnostic method for AD. It is noteworthy that the content of neurotrophic factors (eg, brain-derived neurotrophic factor, BDNF) and anti-inflammatory factors (eg, interleukin-10, IL-10) in MSC-derived exosomes also changes during the progression of neurodegenerative diseases, accompanied by alterations in inflammation levels. Recent studies have shown that the levels of mitochondrial proteins NDUFS3 and SDHB within plasma exosomes decrease significantly with the onset of diabetes-induced cognitive impairment.117 With advancements in technology, employing high-throughput sequencing and Olink118 for quantitative analysis of various miRNAs and proteins may provide precise prevention and treatment for neurodegenerative diseases caused by diabetes.
It should be noted that although these molecules hold potential as biomarkers in neurodegenerative disease research, they are still in the early stages. For the clinical analysis of exosomal content as biomarkers, extensive research is required to verify their specificity, sensitivity, and reliability.
Promoting Neuronal Regeneration and Synaptic Plasticity
Cognitive impairments and histological abnormalities similar to those observed with mesenchymal stem cell injections have been found to be reversed. Fluorescently labeled exosomes demonstrated that the injected exosomes were internalized into astrocytes and neurons, subsequently reversing the dysfunction.119 MSC-derived exosomes mediated the transfer of miR-133b to astrocytes and neurons, regulating ras homolog gene family member A,119 and potentially possessing the ability to activate the PI3K/Akt signaling pathway,120 which is beneficial for neural sprouting remodeling and functional recovery. At the same time, miR-132 from MSC-derived exosomes in cognitively impaired mice can target the regulation of GTPase-activating protein RASA1 and increase the phosphorylation of Ras, Akt, and GSK-3β, improving neuronal and synaptic activation.121–123 Additionally, various miRNAs, such as miR-21, which are specifically highly expressed in MSC-derived exosomes, may improve synaptic transmission and plasticity by suppressing inflammation and immunity, but their function and mechanism are currently unclear.124 Recent research has shown that miR-21-5p and miR-486-5p have the highest expression levels in MSC-derived exosomes, and miR-21-5p has been proven to directly target Epha4 and CDKN2C, while miR-486-5p can inhibit FoxO1 in neural stem cells, thereby promoting hippocampal neural stem cell proliferation and neurogenesis in diabetes-induced cognitive impairments.125
Moreover, Bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos) contain various growth factors and neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF).126 BDNF can activate multiple signaling pathways, such as PI3K/Akt, MAPK/ERK, and PLCγ, by binding to its specific receptor TrkB,126–128 while NGF binds to its receptors TrkA and p75NTR, activating signaling pathways like PI3K/Akt, MAPK/ERK, and JAK/STAT.129,130 The activation of these signaling pathways promotes the proliferation, differentiation, and migration of neural stem cells, thereby improving diabetes-induced cognitive impairments through the promotion of neuronal regeneration.130
Anti-Inflammatory Effects
In obesity and T2DM, as well as in elderly and dementia patients, disruptions in brain insulin action can be observed, often accompanied by the occurrence of inflammation, leading to further deterioration of cognitive impairments.131,132 Astrocytes, microglia, and neurons play crucial roles in maintaining tissue homeostasis and promoting inflammatory responses through the secretion of cytokines.133 Exosomal miRNAs regulate inflammatory responses by targeting the expression of various signaling pathways and immune molecules, thus counteracting inflammation.134 BMSC-Exos have been found to have application value in treating diabetes-related cognitive impairments, mainly through the anti-inflammatory effects based on exosomal miRNA content. Intravenous injection of BMSC-Exos can reduce the expression of pro-inflammatory mediators TNF-α, IL-β, and IL-6 in the cortex and hippocampus, but changes in anti-inflammatory factors IL-10 and IL-13 are not obvious.135 However, intracerebroventricular injection of BMSC-Exos can better improve exosomal efficacy, accompanied by significant reductions in BACE, Aβ1-42, and p-Tau protein expression levels, and significant elevations in BDNF expression levels. This greatly reduces the brain inflammatory response caused by diabetic cognitive impairments,126 which may depend on the non-coding RNA content of the exosomes and brain-derived neurotrophic factor (bDNF).
In STZ-induced diabetic rats, an enriched environment can improve the levels of miR-146a in BMSC-Exos, thereby targeting the expression of IRAK1, NF-κB, and TNF-α in astrocytes of diabetic rats and exerting anti-inflammatory effects, inhibiting the onset of diabetes-induced cognitive impairments.136 After the occurrence of diabetic cognitive impairments, exosomes secreted by BMSC enter the cerebrospinal fluid and regulate astrocytes through the exosomal content miR-146a, modulating IRAK1 and NFAT5 and reducing NF-κB signaling pathway levels,137 thereby reducing the proportion of microglia polarizing towards the M1 type and lowering inflammation levels.110,138 However, contradictorily, research has shown that the common MSC-Exos content miR-21-5p seems to promote the polarization of microglia towards the M1 type and is the main source of miR-21-5p in microglia,139,140 but at the same time, miR-21-5p may exert anti-inflammatory effects in other brain tissues,141 for example, intracerebroventricular injection of miR-21-5 significantly inhibits neural defects in rats, repairs cognitive impairments, reduces blood-brain barrier (BBB) permeability, and suppresses the occurrence and apoptosis of neuronal inflammation,142 which may be due to miR-21-5p affecting the PTEN/Akt pathway in the frontal cortex and hippocampal neurons.143 Therefore, further investigation into the tissue- and specificity-dependent effects of miRNAs in MSC-Exos contents is still needed.
Antioxidant Effects
In diabetic patients, elevated blood glucose levels precipitate an escalation of oxidative stress, thereby exacerbating neuronal injury and adversely affecting neuronal function and synapse formation. Although there are relatively few reports on the regulatory role of extracellular vesicles in oxidative stress pertaining to cerebral diseases, it has been demonstrated that exosomes derived from BMSCs can ameliorate cognitive impairment in streptozotocin (STZ)-induced diabetic mice by reducing oxidative stress levels in damaged neurons and astrocytes.119 Furthermore, BMSC-Exos miR-132-3p and miR-126 are postulated to mitigate oxidative stress-induced injury to cerebral endothelial cells by activating the PI3K/Akt/eNOS signaling pathway, inhibiting the production of reactive oxygen species (ROS) in cerebral blood vessels, and attenuating endothelial cell apoptosis,123,144 thereby potentially playing a significant role in diabetes-induced cognitive impairment. Bone marrow-derived miR-146a can suppress the inflammatory response, thereby markedly improving neuronal function and reducing apoptosis and degenerative neurons. Concurrently, exosomes enriched with miR-146a-5p can attenuate oxidative stress associated with the PI3K/Akt/mTOR pathway, thereby reducing neuronal apoptosis.137 Additionally, a study indicates that although the aforementioned literature elucidates the potential mechanisms underlying the regulation of oxidative stress in cognitive impairment by exosomes derived from MSCs and the critical roles played by various miRNAs in cerebral oxidative stress,145,146 further evidence is required to establish the direct link between MSC-Exos and oxidative stress in neurons and microglial cells.
Reducing Beta-Amyloid Protein
Beta-amyloid protein (Aβ) is a polypeptide generated through the cleavage of large precursor protein molecules (Amyloid Precursor Protein, APP) by β- and γ-secretases.147,148 Prolonged hyperglycemia in diabetic patients may promote aberrant Aβ protein aggregation via multiple factors,149 and diabetes may impair the insulin signaling pathway, consequently affecting APP metabolism.150 This could lead to an increase in non-physiological APP cleavage, subsequently elevating Aβ production, adversely impacting neurons, synaptic function, microvasculature, astrocytes and microglia.151 Aβ can induce neuronal apoptosis, synaptic dysfunction, microvascular damage, and inflammatory responses,150,152 culminating in impaired brain function and manifesting as memory and cognitive decline in diabetic patients.
Exosomes’ role in modulating Aβ protein has been frequently reported. Adipose MSC-Exos can reduce Aβ deposition, inhibiting mouse neuronal cell apoptosis.153 Exosomal miR-29b can mitigate Aβ pathogenic effects by targeting β-secretase 1 BACE1 and BIM, effectively improving spatial learning and memory capabilities in Alzheimer’s disease model mice.154 Recent studies reveal that BMSC-Exos can reduce Amyloid Precursor Protein (APP) density,155 alleviate β-amyloid protein1-42 (Aβ1–42) induced cognitive impairments, augment neurogenesis and myelin regeneration in the subventricular zone,155 and enhance mouse learning capabilities.156 Additional research demonstrates that stem cell exosomes, delivered through intravenous injection, can cross the blood-brain barrier, reduce hippocampal Aβ aggregation and neuronal loss, and restore Alzheimer’s disease-related calcium oscillations, dendritic spine alterations, action potential abnormalities, or hippocampal mitochondrial changes.157 Various exosomal components may play a role, including miR-16, miR-107, and miR-124, which can reduce Aβ by targeting BACE1,158–161 although further evidence is needed to confirm whether this regulation occurs in cortical and hippocampal regions via BMSC-Exos.
Furthermore, MSC-Exos can enhance microglial uptake and degradation of Aβ; annexin V deficiency results in impaired exosome uptake accompanied by reduced Aβ uptake. Although comprehensive understanding of exosomes was lacking at the time, this study demonstrated the involvement of MSC-Exos in modulating microglial uptake and clearance of Aβ.162 Subsequent studies confirmed hippocampal Aβ levels, amyloid deposition, and Aβ-mediated synaptic toxicity are associated with brain-derived exosomes, and glycosphingolipids (GSLs) within exosomes can serve as potent Aβ-clearing agents.163,164 Additionally, in vivo studies revealed exosomes can bind and isolate small Aβ oligomers (trimers, tetramers) and mitigate the impact of Aβ on synaptic plasticity.165 Contrarily, some research has shown exosomes may promote amyloid formation, and blocking exosome formation can ameliorate cognitive impairments in mice,166,167 potentially attributable to exosomes’ varying origins.168
Limitations and Future Remark
While the use of MSC-EVs for the treatment of diabetic cognitive impairment currently faces challenges such as unclear mechanisms, unknown long-term effects, limited preparation methods, and undetermined frequency of use. Therefore, more extensive clinical trials are needed to verify these findings and establish a comprehensive database for safety and efficacy. Moreover, by analyzing the surface markers and contents of MSC-EVs from different cellular origins, and with advancements in biotechnology, we can obtain functionally uniform MSC-EVs through in vitro culture, separation, and purification. Personalized adjustments can then be made based on the patient’s clinical phenotype and metabolic characteristics to achieve optimal therapeutic outcomes.
Conclusion
Diabetes-associated cognitive impairment is a prevalent comorbidity in elderly diabetic patients, exhibiting a bidirectional relationship with diabetes. This association often impacts patients’ quality of life, disease prognosis, and increases the risk of other neurological disorders. Although pharmacological interventions controlling blood glucose levels and modulating cerebral chemical messenger concentrations may alleviate the severity of cognitive impairment, these measures typically fail to reverse or halt neuronal and synaptic damage.
MSCs are a heterogeneous population of stromal stem cells that can be isolated from various adult tissues. These cells possess the capacity to suppress the release of pro-inflammatory cytokines and promote the survival of damaged cells. Recent studies have demonstrated that MSC-Exos contain an array of bioactive substances, including cytokines and growth factors, signaling lipids, mRNAs, and regulatory miRNAs, which modulate cellular metabolism in vivo. In comparison to MSC therapy, exosome therapy avoids immune rejection, reduces the risk of tumor formation, and is more readily obtainable, rendering it a safer, more convenient, and efficacious treatment approach.
MSC-Exos, nanoscale extracellular vesicles, have been extensively reported to traverse the blood-brain barrier and exert regulatory effects on the intracerebral milieu. This article summarizes the positive influence of MSC-Exos on neuronal and synaptic regeneration in diabetes-related cognitive impairment and highlights their anti-inflammatory, antioxidative, and amyloid-beta clearance properties, thereby mitigating the generation of detrimental factors in diabetes-associated cognitive impairment (Figure 2). However, due to the complexity of exosomal components, further in-depth investigation is warranted to elucidate their precise effects on neurological disorders. Nevertheless, based on MSC-Exos isolation techniques, the current application of exosome therapy for diabetes-induced cognitive impairment presents novel research directions and potential clinical applications.
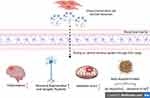 |
Figure 2 Overview of the beneficial effects of mesenchymal stem cell-derived exosomes on diabetes-associated cognitive impairment (created with BioRender.com). |
Data Sharing Statement
The current study was based on the results of relevant published studies.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This review was funded by the Innovation Fund (No.CI2021A04803), Scientific research project of Guangdong Provincial Bureau of Traditional Chinese Medicine and Medicine (No.20231290), Shenzhen Pingshan District Health System Research Project (No.202232) and Natural Science Foundation of Jilin Province (No. YDZJ202301ZYTS475).
Disclosure
The authors declared that they have no competing interests in this work.
References
1. DeFronzo RA, Ferrannini E, Groop L, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. doi:10.1038/nrdp.2015.19
2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. doi:10.1038/nrendo.2017.151
3. Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(10):545–555. doi:10.1038/s41574-020-0381-5
4. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi:10.1038/nature08933
5. Eizirik DL, Pasquali L, Cnop M. Pancreatic beta-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol. 2020;16(7):349–362. doi:10.1038/s41574-020-0355-7
6. Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016;92(1084):63–69. doi:10.1136/postgradmedj-2015-133281
7. Dayan CM, Korah M, Tatovic D, Bundy BN, Herold KC. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet. 2019;394(10205):1286–1296. doi:10.1016/S0140-6736(19)32127-0
8. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. doi:10.1038/nrgastro.2016.147
9. Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vascular disease. Trends Endocrinol Metab. 2001;12(5):225–230. doi:10.1016/s1043-2760(01)00391-5
10. Hill MA, Yang Y, Zhang L, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. doi:10.1016/j.metabol.2021.154766
11. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16(7):377–390. doi:10.1038/s41581-020-0278-5
12. Strain WD, Paldanius PM. Diabetes, cardiovascular disease and the microcirculation. Cardiovasc Diabetol. 2018;17(1):57. doi:10.1186/s12933-018-0703-2
13. Viigimaa M, Sachinidis A, Toumpourleka M, Koutsampasopoulos K, Alliksoo S, Titma T. Macrovascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):110–116. doi:10.2174/1570161117666190405165151
14. Abdel Mageed SS, Doghish AS, Ismail A, et al. The role of miRNAs in insulin resistance and diabetic macrovascular complications - A review. Int J Biol Macromol. 2023;230:123189. doi:10.1016/j.ijbiomac.2023.123189
15. Morsi M, Maher A, Aboelmagd O, Johar D, Bernstein L. A shared comparison of diabetes mellitus and neurodegenerative disorders. J Cell Biochem. 2018;119(2):1249–1256. doi:10.1002/jcb.26261
16. Callisaya ML, Beare R, Moran C, Phan T, Wang W, Srikanth VK. Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: a longitudinal study. Diabetologia. 2019;62(3):448–458. doi:10.1007/s00125-018-4778-9
17. Hadley G, Zhang J, Harris-Skillman E, Alexopoulou Z, DeLuca GC, Pendlebury ST. Cognitive decline and diabetes: a systematic review of the neuropathological correlates accounting for cognition at death. J Neurol Neurosurg Psychiatry. 2022;93(3):246–253. doi:10.1136/jnnp-2021-328158
18. Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14(3):329–340. doi:10.1016/S1474-4422(14)70249-2
19. Zhang J, Chen C, Hua S, et al. An updated meta-analysis of cohort studies: diabetes and risk of Alzheimer’s disease. Diabetes Res Clin Pract. 2017;124:41–47. doi:10.1016/j.diabres.2016.10.024
20. Kahn SE, Cooper ME, Del prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. doi:10.1016/S0140-6736(13)62154-6
21. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75 e2. doi:10.1016/j.jalz.2012.11.007
22. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5(1):64–74. doi:10.1016/S1474-4422(05)70284-2
23. Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591–604. doi:10.1038/s41574-018-0048-7
24. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi:10.1038/nri2395
25. Shi Y, Wang Y, Li Q, et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14(8):493–507. doi:10.1038/s41581-018-0023-5
26. Qi K, Li N, Zhang Z, Melino G. Tissue regeneration: the crosstalk between mesenchymal stem cells and immune response. Cell Immunol. 2018;326:86–93. doi:10.1016/j.cellimm.2017.11.010
27. Qiu G, Zheng G, Ge M, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10(1):359. doi:10.1186/s13287-019-1484-6
28. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi:10.1016/j.semcdb.2015.03.001
29. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
30. Lin Z, Wu Y, Xu Y, Li G, Li Z, Liu T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: recent advances and therapeutic potential. Mol Cancer. 2022;21(1):179. doi:10.1186/s12943-022-01650-5
31. Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111(9):3100–3110. doi:10.1111/cas.14563
32. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi:10.1016/j.jconrel.2015.07.030
33. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
34. Lyu F, Wu D, Wei C, Wu A. Vascular cognitive impairment and dementia in type 2 diabetes mellitus: an overview. Life Sci. 2020;254:117771. doi:10.1016/j.lfs.2020.117771
35. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29(4):494–511. doi:10.1210/er.2007-0034
36. McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291–2299. doi:10.1016/S0140-6736(12)60360-2
37. Mendelson SJ, Prabhakaran S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: a review. JAMA. 2021;325(11):1088–1098. doi:10.1001/jama.2020.26867
38. Maida CD, Daidone M, Pacinella G, Norrito RL, Pinto A, Tuttolomondo A. Diabetes and ischemic stroke: an old and new relationship an overview of the close interaction between these diseases. Int J Mol Sci. 2022;23(4):2397. doi:10.3390/ijms23042397
39. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi:10.1038/nrendo.2014.29
40. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325–336. doi:10.1016/S2213-8587(19)30405-X
41. Janghorbani M, Hu FB, Willett WC, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care. 2007;30(7):1730–1735. doi:10.2337/dc06-2363
42. Sarwar N, Gao P; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
43. Hankey GJ, Jamrozik K, Broadhurst RJ, et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke. 1998;29(12):2491–2500. doi:10.1161/01.str.29.12.2491
44. Rawshani A, Rawshani A, Franzen S, et al. Risk Factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379(7):633–644. doi:10.1056/NEJMoa1800256
45. Joseph JJ, Deedwania P, Acharya T, et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: a scientific statement from the American Heart Association. Circulation. 2022;145(9):e722–e759. doi:10.1161/CIR.0000000000001040
46. Sprafka JM, Virnig BA, Shahar E, McGovern PG. Trends in diabetes prevalence among stroke patients and the effect of diabetes on stroke survival: the Minnesota Heart Survey. Diabet Med. 1994;11(7):678–684. doi:10.1111/j.1464-5491.1994.tb00332.x
47. Policardo L, Seghieri G, Anichini R, et al. Effect of diabetes on hospitalization for ischemic stroke and related in-hospital mortality: a study in Tuscany, Italy, over years 2004–2011. Diabetes Metab Res Rev. 2015;31(3):280–286. doi:10.1002/dmrr.2607
48. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care. 2004;27(1):201–207. doi:10.2337/diacare.27.1.201
49. Sharma M, Hart RG, Connolly SJ, et al. Stroke Outcomes in the COMPASS Trial. Circulation. 2019;139(9):1134–1145. doi:10.1161/CIRCULATIONAHA.118.035864
50. Arboix A, Font A, Garro C, Garcia-Eroles L, Comes E, Massons J. Recurrent lacunar infarction following a previous lacunar stroke: a clinical study of 122 patients. J Neurol Neurosurg Psychiatry. 2007;78(12):1392–1394. doi:10.1136/jnnp.2007.119776
51. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi:10.1101/cshperspect.a020412
52. Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi:10.1016/j.nbd.2009.07.030
53. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17(1):69. doi:10.1186/s12987-020-00230-3
54. Nielsen LB, Wang C, Sorensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression.; Research Support, Non-U.S. Gov’t. Exp Diabetes Res. 2012;2012:896362. doi:10.1155/2012/896362
55. Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol. 2017;12(4):593–601. doi:10.1007/s11481-017-9752-7
56. Montagne A, Barnes SR, Sweeney MD, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85(2):296–302. doi:10.1016/j.neuron.2014.12.032
57. Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1037–1045. doi:10.1016/j.bbadis.2016.04.017
58. Tucsek Z, Toth P, Sosnowska D, et al. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J Gerontol a Biol Sci Med Sci. 2014;69(10):1212–1226. doi:10.1093/gerona/glt177
59. Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8(1):e54514. doi:10.1371/journal.pone.0054514
60. Pallebage-Gamarallage M, Lam V, Takechi R, Galloway S, Clark K, Mamo J. Restoration of dietary-fat induced blood-brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids Health Dis. 2012;11:117. doi:10.1186/1476-511X-11-117
61. van Sloten T, Schram M. Understanding depression in type 2 diabetes: a biological approach in observational studies. F1000Res. 2018;7. doi:10.12688/f1000research.13898.1
62. Najjar S, Pearlman DM, Devinsky O, Najjar A, Zagzag D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi:10.1186/1742-2094-10-142
63. Direk N, Koudstaal PJ, Hofman A, Ikram MA, Hoogendijk WJ, Tiemeier H. Cerebral hemodynamics and incident depression: the Rotterdam Study. Biol Psychiatry. 2012;72(4):318–323. doi:10.1016/j.biopsych.2012.01.019
64. Campayo A, Gomez-Biel CH, Lobo A. Diabetes and depression. Curr Psychiatry Rep. 2011;13(1):26–30. doi:10.1007/s11920-010-0165-z
65. Nouwen A, Adriaanse MC, van Dam K, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36(12):1562–1572. doi:10.1111/dme.14054
66. Srikanth V, Sinclair AJ, Hill-Briggs F, Moran C, Biessels GJ. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8(6):535–545. doi:10.1016/S2213-8587(20)30118-2
67. Rawlings AM, Sharrett AR, Albert MS, et al. The association of late-life diabetes status and hyperglycemia with incident mild cognitive impairment and dementia: the ARIC Study. Diabetes Care. 2019;42(7):1248–1254. doi:10.2337/dc19-0120
68. Esiri MM, Matthews F, Brayne C, et al.; Neuropathology Group. Medical Research Council Cognitive F, Aging S. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet. 2001;357(9251):169–175. doi:10.1016/s0140-6736(00)03589-3
69. Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimers Dement. 2016;12(8):882–889. doi:10.1016/j.jalz.2015.12.006
70. Ojo O, Brooke J. Evaluating the association between diabetes, cognitive decline and dementia. Int J Environ Res Public Health. 2015;12(7):8281–8294. doi:10.3390/ijerph120708281
71. Chan DD, Tsou HH, Chang CB, et al. Integrated care for geriatric frailty and sarcopenia: a randomized control trial. J Cachexia Sarcopenia Muscle. 2017;8(1):78–88. doi:10.1002/jcsm.12132
72. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63(8):747–762. doi:10.1016/j.jacc.2013.09.070
73. Sesti G, Antonelli Incalzi R, Bonora E, et al. Management of diabetes in older adults. Nutr Metab Cardiovasc Dis. 2018;28(3):206–218. doi:10.1016/j.numecd.2017.11.007
74. DeCarlo K, Wallia A. Inpatient management of T2DM and hyperglycemia in older adults. Curr Diab Rep. 2019;19(10):104. doi:10.1007/s11892-019-1209-3
75. American Diabetes Association. 12. Older adults: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S139–S147. doi:10.2337/dc19-S012
76. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi:10.1002/jor.1100090504
77. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells. 2019;8(8):784. doi:10.3390/cells8080784
78. Liu WZ, Ma ZJ, Li JR, Kang XW. Mesenchymal stem cell-derived exosomes: therapeutic opportunities and challenges for spinal cord injury. Stem Cell Res Ther. 2021;12(1):102. doi:10.1186/s13287-021-02153-8
79. Xiao Y, Zheng L, Zou X, Wang J, Zhong J, Zhong T. Extracellular vesicles in type 2 diabetes mellitus: key roles in pathogenesis, complications, and therapy. J Extracell Vesicles. 2019;8(1):1625677. doi:10.1080/20013078.2019.1625677
80. Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells. 2019;8(12):1605. doi:10.3390/cells8121605
81. Zhong J, Xia B, Shan S, et al. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. doi:10.1016/j.biomaterials.2021.121126
82. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int J Nanomedicine. 2020;15:9355–9371. doi:10.2147/IJN.S281890
83. Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13(1):17–24. doi:10.1016/j.gpb.2015.02.001
84. Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi:10.3389/fimmu.2021.749192
85. Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997;110(Pt 16):1867–1877. doi:10.1242/jcs.110.16.1867
86. Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–4151. doi:10.1073/pnas.1200448109
87. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
88. Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219(3). doi:10.1083/jcb.201904113
89. Buschow SI, Nolte-’t Hoen EN, van Niel G, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–1542. doi:10.1111/j.1600-0854.2009.00963.x
90. Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–1091. doi:10.1083/jcb.201002049
91. Babuta M, Furi I, Bala S, et al. Dysregulated autophagy and lysosome function are linked to exosome production by micro-RNA 155 in alcoholic liver disease. Hepatology. 2019;70(6):2123–2141. doi:10.1002/hep.30766
92. Buratta S, Tancini B, Sagini K, et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic- and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21(7):2576. doi:10.3390/ijms21072576
93. Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2. doi:10.3402/jev.v2i0.20360
94. Quintana JF, Makepeace BL, Babayan SA, et al. Extracellular Onchocerca-derived small RNAs in host nodules and blood. Parasit Vectors. 2015;8:58. doi:10.1186/s13071-015-0656-1
95. Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi:10.3402/jev.v4.27031
96. Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. doi:10.1038/srep17319
97. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
98. Shu S, Yang Y, Allen CL, et al. Purity and yield of melanoma exosomes are dependent on isolation method. J Extracell Vesicles. 2020;9(1):1692401. doi:10.1080/20013078.2019.1692401
99. Xu Y, Shen L, Li F, Yang J, Wan X, Ouyang M. microRNA-16-5p-containing exosomes derived from bone marrow-derived mesenchymal stem cells inhibit proliferation, migration, and invasion, while promoting apoptosis of colorectal cancer cells by downregulating ITGA2. J Cell Physiol. 2019;234(11):21380–21394. doi:10.1002/jcp.28747
100. Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015;2015(4):319–323. doi:10.1101/pdb.top074476
101. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. doi:10.3390/cells8070727
102. Saeedi S, Israel S, Nagy C, Turecki G. The emerging role of exosomes in mental disorders. Transl Psychiatry. 2019;9(1):122. doi:10.1038/s41398-019-0459-9
103. Ung TH, Madsen HJ, Hellwinkel JE, Lencioni AM, Graner MW. Exosome proteomics reveals transcriptional regulator proteins with potential to mediate downstream pathways. Cancer Sci. 2014;105(11):1384–1392. doi:10.1111/cas.12534
104. Sha S, Shen X, Cao Y, Qu L. Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer’s disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/beta-catenin pathway. Aging. 2021;13(11):15285–15306. doi:10.18632/aging.203088
105. Riancho J, Vazquez-Higuera JL, Pozueta A, et al. MicroRNA profile in patients with Alzheimer’s disease: analysis of miR-9-5p and miR-598 in raw and exosome enriched cerebrospinal fluid samples. J Alzheimers Dis. 2017;57(2):483–491. doi:10.3233/JAD-161179
106. Sarkar S, Jun S, Rellick S, Quintana DD, Cavendish JZ, Simpkins JW. Expression of microRNA-34a in Alzheimer’s disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Brain Res. 2016;1646:139–151. doi:10.1016/j.brainres.2016.05.026
107. Song Z, Qu Y, Xu Y, et al. Microarray microRNA profiling of urinary exosomes in a 5XFAD mouse model of Alzheimer’s disease. Animal Model Exp Med. 2021;4(3):233–242. doi:10.1002/ame2.12175
108. McKeever PM, Schneider R, Taghdiri F, et al. MicroRNA expression levels are altered in the cerebrospinal fluid of patients with young-onset Alzheimer’s disease. Mol Neurobiol. 2018;55(12):8826–8841. doi:10.1007/s12035-018-1032-x
109. Xu H, Zhao G, Zhang Y, et al. Mesenchymal stem cell-derived exosomal microRNA-133b suppresses glioma progression via Wnt/beta-catenin signaling pathway by targeting EZH2. Stem Cell Res Ther. 2019;10(1):381. doi:10.1186/s13287-019-1446-z
110. Venkat P, Zacharek A, Landschoot-Ward J, et al. Exosomes derived from bone marrow mesenchymal stem cells harvested from type two diabetes rats promotes neurorestorative effects after stroke in type two diabetes rats. Exp Neurol. 2020;334:113456. doi:10.1016/j.expneurol.2020.113456
111. Garnier D, Ratcliffe E, Briand J, Cartron PF, Oliver L, Vallette FM. The activation of mesenchymal stem cells by glioblastoma microvesicles alters their exosomal secretion of miR-100-5p, miR-9-5p and let-7d-5p. Biomedicines. 2022;10(1):112.
112. Abner EL, Jicha GA, Shaw LM, Trojanowski JQ, Goetzl EJ. Plasma neuronal exosomal levels of Alzheimer’s disease biomarkers in normal aging. Ann Clin Transl Neurol. 2016;3(5):399–403. doi:10.1002/acn3.309
113. Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2015;11(6):600–7 e1. doi:10.1016/j.jalz.2014.06.008
114. Goetzl EJ, Mustapic M, Kapogiannis D, et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 2016;30(11):3853–3859. doi:10.1096/fj.201600756R
115. Hamlett ED, Ledreux A, Potter H, et al. Exosomal biomarkers in Down syndrome and Alzheimer’s disease. Free Radic Biol Med. 2018;114:110–121. doi:10.1016/j.freeradbiomed.2017.08.028
116. Ahmad W. Glucose enrichment impair neurotransmission and induce Abeta oligomerization that cannot be reversed by manipulating O-beta-GlcNAcylation in the C. elegans model of Alzheimer’s disease. J Nutr Biochem. 2022;108:109100. doi:10.1016/j.jnutbio.2022.109100
117. Chi H, Yao R, Sun C, et al. Blood neuroexosomal mitochondrial proteins predict Alzheimer disease in diabetes. Diabetes. 2022;71(6):1313–1323. doi:10.2337/db21-0969
118. Pietzner M, Wheeler E, Carrasco-Zanini J, et al. Synergistic insights into human health from aptamer- and antibody-based proteomic profiling. Nat Commun. 2021;12(1):6822. doi:10.1038/s41467-021-27164-0
119. Nakano M, Nagaishi K, Konari N, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805. doi:10.1038/srep24805
120. Lu XC, Zheng JY, Tang LJ, et al. MiR-133b Promotes neurite outgrowth by targeting RhoA expression. Cell Physiol Biochem. 2015;35(1):246–258. doi:10.1159/000369692
121. Ma X, Wang Y, Shi Y, et al. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res Ther. 2022;13(1):315. doi:10.1186/s13287-022-02995-w
122. Hancock ML, Preitner N, Quan J, Flanagan JG. MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J Neurosci. 2014;34(1):66–78. doi:10.1523/JNEUROSCI.3371-13.2014
123. Pan Q, Kuang X, Cai S, et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11(1):260. doi:10.1186/s13287-020-01761-0
124. Malcangio M. Role of the immune system in neuropathic pain. Scand J Pain. 2019;20(1):33–37. doi:10.1515/sjpain-2019-0138
125. Lang HL, Zhao YZ, Xiao RJ, et al. Small extracellular vesicles secreted by induced pluripotent stem cell-derived mesenchymal stem cells improve postoperative cognitive dysfunction in mice with diabetes. Neural Regen Res. 2023;18(3):609–617. doi:10.4103/1673-5374.350205
126. Liu S, Fan M, Xu JX, et al. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J Neuroinflammation. 2022;19(1):35. doi:10.1186/s12974-022-02393-2
127. Yin F, Liu Z, Zhang D, Shen Z, Niu Z, Guo L. Identification of key genes involved in neural regeneration and the repairing effect of BDNF-overexpressed BMSCs on spinal cord ischemia-reperfusion injury in rats. Biomed Pharmacother. 2023;160:114293. doi:10.1016/j.biopha.2023.114293
128. Ma W, Wei X, Gu H, et al. Intra-amniotic transplantation of brain-derived neurotrophic factor-modified mesenchymal stem cells treatment for rat fetuses with spina bifida aperta. Stem Cell Res Ther. 2022;13(1):413. doi:10.1186/s13287-022-03105-6
129. Yu X, Qi Y, Zhao T, et al. NGF increases FGF2 expression and promotes endothelial cell migration and tube formation through PI3K/Akt and ERK/MAPK pathways in human chondrocytes. Osteoarthritis Cartilage. 2019;27(3):526–534. doi:10.1016/j.joca.2018.12.007
130. Li R, Li Y, Wu Y, et al. Heparin-poloxamer thermosensitive hydrogel loaded with bFGF and NGF enhances peripheral nerve regeneration in diabetic rats. Biomaterials. 2018;168:24–37. doi:10.1016/j.biomaterials.2018.03.044
131. Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–766. doi:10.1016/S1474-4422(20)30231-3
132. Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Haring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96(4):1169–1209. doi:10.1152/physrev.00032.2015
133. Liyanagamage D, Martinus RD. Role of mitochondrial stress protein HSP60 in diabetes-induced neuroinflammatIon. Mediators Inflamm. 2020;2020:8073516. doi:10.1155/2020/8073516
134. Lee YS, Olefsky J. Chronic tissue inflammation and metabolic disease. Genes Dev. 2021;35(5–6):307–328. doi:10.1101/gad.346312.120
135. Cui GH, Guo HD, Li H, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun Ageing. 2019;16:10. doi:10.1186/s12979-019-0150-2
136. Kubota K, Nakano M, Kobayashi E, et al. An enriched environment prevents diabetes-induced cognitive impairment in rats by enhancing exosomal miR-146a secretion from endogenous bone marrow-derived mesenchymal stem cells. PLoS One. 2018;13(9):e0204252. doi:10.1371/journal.pone.0204252
137. Duan S, Wang F, Cao J, Wang C. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. 2020;14:3143–3158. doi:10.2147/DDDT.S255828
138. Nakano M, Kubota K, Kobayashi E, et al. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10(1):10772. doi:10.1038/s41598-020-67460-1
139. Yin Z, Han Z, Hu T, et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav Immun. 2020;83:270–282. doi:10.1016/j.bbi.2019.11.004
140. Pandey N, Rastogi M, Singh SK. Chandipura virus dysregulates the expression of hsa-miR-21-5p to activate NF-kappaB in human microglial cells. J Biomed Sci. 2021;28(1):52. doi:10.1186/s12929-021-00748-0
141. Ge X, Huang S, Gao H, et al. miR-21-5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain Res. 2016;1650:31–40. doi:10.1016/j.brainres.2016.07.015
142. Ouyang Y, Li D, Wang H, et al. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell. 2019;18(6):e13022. doi:10.1111/acel.13022
143. Gao X, Xiong Y, Li Q, et al. Extracellular vesicle-mediated transfer of miR-21-5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis. 2020;11(5):363. doi:10.1038/s41419-020-2530-0
144. Pan Q, Wang Y, Lan Q, et al. Exosomes derived from mesenchymal stem cells ameliorate hypoxia/reoxygenation-injured ECs via transferring MicroRNA-126. Stem Cells Int. 2019;2019:2831756. doi:10.1155/2019/2831756
145. Wang X, Zhou Y, Gao Q, et al. The role of exosomal microRNAs and oxidative stress in neurodegenerative diseases. Oxid Med Cell Longev. 2020;2020:3232869. doi:10.1155/2020/3232869
146. Chen P, Chen F, Lei J, Li Q, Zhou B. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates D-galactose-induced brain aging in mice. Neurotherapeutics. 2019;16(4):1269–1282. doi:10.1007/s13311-019-00753-0
147. Thal DR, Walter J, Saido TC, Fandrich M. Neuropathology and biochemistry of Abeta and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015;129(2):167–182. doi:10.1007/s00401-014-1375-y
148. Hur JY. gamma-Secretase in Alzheimer’s disease. Exp Mol Med. 2022;54(4):433–446. doi:10.1038/s12276-022-00754-8
149. Sun Y, Ma C, Sun H, et al. Metabolism: a novel shared link between diabetes mellitus and Alzheimer’s disease. J Diabetes Res. 2020;2020:4981814. doi:10.1155/2020/4981814
150. Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi:10.1016/j.pneurobio.2013.06.004
151. Stukas S, Robert J, Wellington CL. High-density lipoproteins and cerebrovascular integrity in Alzheimer’s disease. Cell Metab. 2014;19(4):574–591. doi:10.1016/j.cmet.2014.01.003
152. Dutta BJ, Singh S, Seksaria S, Das Gupta G, Singh A. Inside the diabetic brain: insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacol Res. 2022;182:106358. doi:10.1016/j.phrs.2022.106358
153. Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces beta-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87–93. doi:10.1016/j.brainres.2018.03.034
154. Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic effects of transplanted exosomes containing miR-29b to a rat model of Alzheimer’s disease. Front Neurosci. 2020;14:564. doi:10.3389/fnins.2020.00564
155. Zhang J, Buller BA, Zhang ZG, et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol. 2022;347:113895. doi:10.1016/j.expneurol.2021.113895
156. Reza-Zaldivar EE, Hernandez-Sapiens MA, Gutierrez-Mercado YK, et al. Mesenchymal stem cell-derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer’s disease. Neural Regen Res. 2019;14(9):1626–1634. doi:10.4103/1673-5374.255978
157. Wang H, Liu Y, Li J, et al. Tail-vein injection of MSC-derived small extracellular vesicles facilitates the restoration of hippocampal neuronal morphology and function in APP/PS1 mice. Cell Death Discov. 2021;7(1):230. doi:10.1038/s41420-021-00620-y
158. Parsi S, Smith PY, Goupil C, Dorval V, Hebert SS. Preclinical evaluation of miR-15/107 family members as multifactorial drug targets for Alzheimer’s disease. Mol Ther Nucleic Acids. 2015;4(10):e256. doi:10.1038/mtna.2015.33
159. Hebert SS, Horre K, Nicolai L, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105(17):6415–6420. doi:10.1073/pnas.0710263105
160. Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28(5):1213–1223. doi:10.1523/JNEUROSCI.5065-07.2008
161. Zhang X, Huang X, Fang C, et al. miR-124 regulates the expression of BACE1 in the hippocampus under chronic cerebral hypoperfusion. Mol Neurobiol. 2017;54(4):2498–2506. doi:10.1007/s12035-016-9845-y
162. Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287(14):10977–10989. doi:10.1074/jbc.M111.324616
163. Yuyama K, Sun H, Sakai S, et al. Decreased amyloid-beta pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem. 2014;289(35):24488–24498. doi:10.1074/jbc.M114.577213
164. Yuyama K, Sun H, Igarashi Y, et al. Immuno-digital invasive cleavage assay for analyzing Alzheimer’s amyloid ss-bound extracellular vesicles. Alzheimers Res Ther. 2022;14(1):140. doi:10.1186/s13195-022-01073-w
165. An K, Klyubin I, Kim Y, et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain. 2013;6:47. doi:10.1186/1756-6606-6-47
166. Perez-Gonzalez R, Kim Y, Miller C, Pacheco-Quinto J, Eckman EA, Levy E. Extracellular vesicles: where the amyloid precursor protein carboxyl-terminal fragments accumulate and amyloid-beta oligomerizes. FASEB J. 2020;34(9):12922–12931. doi:10.1096/fj.202000823R
167. Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35(8):1792–1800. doi:10.1016/j.neurobiolaging.2014.02.012
168. Dinkins MB, Wang G, Bieberich E. Sphingolipid-enriched extracellular vesicles and Alzheimer’s disease: a decade of research. J Alzheimers Dis. 2017;60(3):757–768. doi:10.3233/JAD-160567
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

