Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 8
Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives
Authors Zomer H , Vidane A, Gonçalves N, Ambrosio C
Received 6 May 2015
Accepted for publication 26 June 2015
Published 28 September 2015 Volume 2015:8 Pages 125—134
DOI https://doi.org/10.2147/SCCAA.S88036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Bernard Binetruy
Helena D Zomer,1 Atanásio S Vidane,1 Natalia N Gonçalves,1 Carlos E Ambrósio2
1Department of Surgery, Faculty of Veterinary Medicine and Animal Science, University of São Paulo, São Paulo, SP, Brazil; 2Department of Veterinary Medicine, Faculty of Animal Sciences and Food Engineering, University of São Paulo, Pirassununga, SP, Brazil
Abstract: Mesenchymal stem cells have awakened a great deal of interest in regenerative medicine due to their plasticity, and immunomodulatory and anti-inflammatory properties. They are high-yield and can be acquired through noninvasive methods from adult tissues. Moreover, they are nontumorigenic and are the most widely studied. On the other hand, induced pluripotent stem (iPS) cells can be derived directly from adult cells through gene reprogramming. The new iPS technology avoids the embryo destruction or manipulation to generate pluripotent cells, therefore, are exempt from ethical implication surrounding embryonic stem cell use. The pre-differentiation of iPS cells ensures the safety of future approaches. Both mesenchymal stem cells and iPS cells can be used for autologous cell transplantations without the risk of immune rejection and represent a great opportunity for future alternative therapies. In this review we discussed the therapeutic perspectives using mesenchymal and iPS cells.
Keywords: cell transplantation, cell therapy, iPS, MSC
Introduction
The “stemness” of a stem cell can be defined by two important properties: the ability of self-renewal and the capacity to differentiate into mature cell types.1 The ability of stem cells to differentiate into specific mature lineages is called plasticity and this is the most important property in the content of cell-based therapy.
Various cell types can potentially be used for clinical studies, including embryonic stem cells (ESC), isolated from the inner cell mass of blastocysts;2 stem cells isolated from adult tissues like the mesenchymal stem cells (MSC); and induced pluripotent stem (iPS) cells which are adult somatic cells reprogrammed to pluripotency.3 Several studies have been conducted to identify, characterize, and differentiate stem cells from various sources.4,5 From stem cells’ isolation, quantification, and expansion, their future application in human and animal cell therapy is expected.6
MSC are multipotent stem cells present in adult tissues, such as bone marrow, muscle, liver, and adipose tissue. These cells are highlighted by their abundance and easy collection. iPS cells are the most promising among those classified as pluripotent because of their high plasticity, similar to ESC, without its controversial origin. This review is aimed to discuss and compare the general insights and clinical applications of MSC and iPS cells. The interest in these two distinct cell types comes from their high potential therapeutic associated to the numerous advantages over the other cell lineages, such as easy harvest and high yield, greater proliferation capacity, and high plasticity. Moreover, the iPS cells can be easily differentiated into MSC with similar properties than traditional MSC.
General characteristics of stem cells
In general, a stem cell is defined as a cell with the ability to divide for an indefinite period of time throughout the life of an individual (self-renewal) and, under appropriate conditions and specific signals, can differentiate into a variety of lineages, with different characteristics and specialized functions (differentiation). According to the differentiation potential, stem cells are classified as totipotent, pluripotent, multipotent, oligopotent, and unipotent.1
Totipotent and pluripotent stem cells correspond to ESC. Totipotent cells are found in the zygote in early stage of development (up to 32-cell embryo) and pluripotent cells are found in the inner cell mass of the blastocyst (between 32–64 cells).7 Totipotent cells have the capacity to generate all cell types, including embryonic and extra embryonic tissues. Pluripotent stem cells can give rise to the three germ layer: endoderm, mesoderm and ectoderm, but not the extra embryonic tissues.8,9 Such differentiation can generate, for example, myocytes, hepatocytes, and neurons.8–10
Multipotent stem cells are present in various adult organs and can differentiate into many cell types, usually from the same embryonic germ layer as MSC and hematopoietic stem cells.5 Oligopotent cells have less ability to differentiate and unipotent stem cells can only generate one mature cell type. Therefore, oligopotent and unipotent stem cells are called progenitor cells.7
The ESC are able to form spontaneous multicellular structures in vitro known as embryonic body. These structures have elements of all three germ layers and can give rise to many types of specialized cells such as cardiomyocytes, neurons, and other hematopoietic progenitors.10,11 ESC can be extensively expanded in culture without losing their pluripotency and self-renewing capacity, when factors to prevent their differentiation are used. Therefore, the advantage of using ESC is the ability to proliferate indefinitely and to generate a wide variety of cell groups. These features allow the manipulation in vitro in order to produce specific precursor cell lines for the treatment of various diseases.10,12 Despite the high plasticity, the use of ESC entails ethical implications due to blastocyst destruction for their isolation.
Adult stem cells have lower plasticity than ESC; however, they stand out in terms of their abundance, easy access, and high yield. These cells can be acquired through noninvasive methods from adult tissues and therefore are exempt from the typical ethical limitations.13 In the body, they are tissue specific, and respond to specific stimuli to regulate the homeostasis and replacement of dead cells.5,14
It is known that pluripotent cells express a unique set of factors responsible for the state of pluripotency, and an interconnected network of regulatory genes is responsible for the development and maintenance of pluripotency in embryos.15,16 Recently, Takahashi and Yamanaka generated a new technology to achieve pluripotent stem cells from adult somatic cells. By the integration of pluripotent transcription factors into the genome of the cells, totally differentiated cells can be reprogrammed to acquire an induced pluripotent state. These cells are called iPS cells.17
MSC
MSC are a type of multipotent stem cell and can be isolated from various adult or fetal tissues and membranes,18,19 including fat, bone marrow, umbilical cord blood,20–22 dental pulp,23 placenta, and muscle.24
In vivo, MSC provide structural support in different organs and regulate the flow of some substances. The stromal origin is characterized by their quick adhesion in culture surface as well as their fibroblastic-like morphology. In addition, they present a high and fast proliferation in simple and accessible culture medium and can be maintained in vitro without karyotype alterations for several passages.25
MSC have the ability to differentiate into several cell types such as adipocytes, osteocytes, and chondrocytes, from the mesodermal germ layer.14,26 This plasticity depends on the extra-cellular matrix environment and soluble growth factors.27 Some authors could induce the differentiation of MSC in cells of other embryonic germ layers, such as neurons,28 which are originated in ectoderm, and hepatocytes, derived from endoderm.29 However, the differentiation into nonmesodermal tissues is still controversial due to a lack of in vivo results.22
Due to their plasticity, the MSC are considered the most important cell type for regenerative medicine, and are the most widely studied in preclinical and clinical trials. Their advantages for clinical application include the easy isolation and high yield, high plasticity, and the ability to mediate inflammation and to promote cell growth, cell differentiation, and tissue repair by immunomodulation and immunosuppression, and are exempt from ethical implications.30–32 Besides, MSC do not form teratomas after transplantation, ensuring safety to the host.
The MSC derived from bone marrow have been the most intensively studied; however, invasive procedures are required for their isolation and the quantity and quality of isolated cells vary according to the donor age. Low frequencies of MSC are found in bone marrow aspirates compared to the total cells compounding the bone marrow stroma.33 Due to cell population heterogeneity, their immunogenic properties depend on numerous settings such as isolation methods, surface and culture medium, plating density, and chemical products supplementation.25
Therefore, the identification of alternative sources of MSC has been the focal point of recent researches. Between different sources of MSC, the adipose tissue is highlighted for their accessibility and the abundance of isolated cells.13,26,34–36 Each isolation results in approximately 100-fold more cells than the bone morrow isolation,37 and the process is less invasive.14
MSC are heterogeneous; therefore, their immunophenotypic profile and plasticity varies among species, source, and passage.37 However, MSC positively express a combination of surface markers: CD29, CD73, CD90, CD105, CD44 and CD166 and are negative to CD14, CD31, CD34 and CD45. The expression pattern of some surface markers is controversial, for example, CD34 in humans,14,38 CD44 in ovines,39,40 and CD44 and CD105 in rabbits.41,42
Besides classified as multipotents, MSC express a relatively high level of pluripotent markers related to ESC, such as OCT4, NANOG, and SOX2.14,21,24 These transcription factors are involved in the regulation of the multipotency, self-renewal, and proliferation of MSC.21,24 The OCT4 is evolved in the initial development of mammals and is essential for the formation of embryos’ inner cell mass and ESC maintenance.15 SOX2 regulates the expression of OCT4 and maintains the pluripotent state of ESC, and NANOG is required for the maintenance of nondifferentiated state and self-renewal of stem cells.21 As described eariler, these factors also play a key role in the pluripotency state of iPS cells.
iPS cells
The iPS cells are generated from the induction of expression of transcription factors associated with pluripotency, allowing a differentiated somatic cell to reverse its condition to the embryonic stage. Takahashi and Yamanaka developed this technique where four transcription factors, OCT4, SOX2, KLF4, and C-MYC (shown by the acronym OSKM), were incorporated into the genome of mouse17 and human somatic cells.43 The discovery of such technology was based on the hypothesis that nuclear reprogramming is a process driven by factors that play a critical role in maintaining the pluripotency of ESC.17,44 iPS cells could imply the elimination of ethical issues and problems of rejection after transplantation, as they can be collected from the patient (autologous), expanding the possibilities of research.13,17 It is well known that one or several transcriptional factors can convert one cell to another. Although, the mechanisms whereby exogenous factors change the epigenetic state remains unknown. Although Yamanaka factors are the most used, other combinations of factors were tested successfully, such as the replacement of C-MYC and KLF4 by NANOG and LIN2845 or excluding the factor C-MYC.46
The field of induced pluripotency has been growing exponentially in the last years. The efficiency, reliability, and security are crucial to the success of reprogramming and the method for introduction of transcription factors in the cells is a very significant step. Conventional reprogramming techniques depend on the stable integration of transgenes, but it can introduce the current risk of insertional mutagenesis. Thus, several nonintegrative reprogramming techniques have been developed to improve the quality of the generated MSC.47
The integrative systems consist of viral vectors, such as retroviruses17 and lentiviruses.48 Nonintegrative vectors, such as adenovirus49 or nonviral systems, plasmids,50 proteins,51 and mRNA, do not promote the integration of OSKM factors’ cDNA into the cell genome.50,52,53 Recently, new approaches were tested to induce the pluripotency, by using chemical exogenous molecules54 or episomal vectors.53 Episomal reprogramming seems particularly well-suited for clinical translation because it is integration-free, works reliably with patient fibroblasts and blood cells, and is based on a very simple reagent (plasmid DNA).47 However, it has shown lower efficiency than integrative vectors. 53
In the somatic cells, promoters of pluripotency genes are highly methylated, reflecting a repressed transcriptional state. The generation of iPS cells involves the activation of these genes, and their demethylation is used to determine the success of reprogramming.55 When exogenous pluripotency genes are introduced into the cell, they induce the expression of endogenous pluripotency genes.56 In turn, the upregulation of endogenous factors induces the silencing of exogenous genes by methylation of the promoters.57
The pluripotency state of iPS cells can be attested by the ability to form teratomas in vivo and the formation of embryonic bodies in vitro. Moreover, they have the ESC morphology, such as round shape, large nucleolus, and scarce cytoplasm. The molecular profile of iPS cells is very similar to ESC, expressing the pluripotency markers OCT4, NANOG, SOX2, SSEA1, SSEA3, SSEA4, TRA1-60, TRA1-81, and ALP activity.58–60 Despite these characteristics, Takahashi and Yamanaka17 found that iPS cells are very similar but not identical to ESC.
Many studies have confirmed the repeatability of the iPS cell process in different species such as humans, mice,17,45 rhesus monkeys,61 pigs,62 cattle,63 horses,64 rabbits,65 sheep,66 large cats such as the leopard,67 and canids,68–72 most of them being made from fibroblasts. Honda et al65 were not able to generate iPS cells from rabbit fibroblasts, probably due to the exceptional speed of proliferation of these specific cells, which quickly reach confluence, discontinuing the differentiation. In fact, the high proliferation of donor cells seems to be detrimental to reprogramming.46
As an alternative source, MSC derived from adipose tissue were used to generate iPS cells in mice and humans. Adipose-derived stem cells are naturally multipotent and acquire pluripotency after induction. It is described that the reprogramming of MSC into iPS cells can be achieved 200-fold more efficiently and rapidly than from fibroblasts.73,74
The cellular reprogramming is desired in many different biotechnology areas; therefore, many authors strive to elucidate the mechanisms involved in cell pluripotency. However, the exact mechanism remains unclear and the efficacy is very low. Two issues appear to limit the application of iPS cells: the low efficiency of transgene integration in the somatic genome and the low efficiency of the reprogramming process.75 These factors have imposed significant limitations on their biomedical and therapeutic applications. In this context, considerable effort has been made to identify compounds that can improve the efficiency of reprogramming.76,77
Small molecules able to remodel chromatin and epigenetic control are being actively investigated due to their effect on reprogramming. It has been demonstrated that inhibitors of methyltransferase, histone deacetylase, and histone demethylase may increase the reprogramming efficiency rate.76,78,79 In fact, it is known that inhibitors can induce partial reprogramming colonies to achieve the complete reprogramming state.80,81
Some molecules acting on the signaling pathways involved in self-renewal and pluripotency, such as Wnt, TGFb, and MEK, also increase such rates.80–84 In addition, Esteban et al62 showed that vitamin C, a common nutrient vital to human health, enhances the reprogramming of somatic cells to pluripotent stem cells. The addition of vitamin C to the culture medium resulted in high-quality iPS cells from mouse and human cells. This can be explained by the suppression of reactive oxygen species production, normally accumulated by somatic cells undergoing senescence.
Other strategies to increase efficiency include the reduction of transcription factors, like SOX2 and C-MYC85 or C-MYC and KLF4, and the addition of valproic acid74 or inhibitor of GSK-3 signaling cascade, which is a known facilitator of complete reprogramming in partially reprogrammed colonies.78 Interestingly, Wang et al86 enhanced the generation of iPS cells by the addition of lithium, an antipsychotic drug. This drug interacts metabolically with many pathways and promotes reprogramming by acting on GSK3β. Besides, lithium increases the expression of NANOG and facilitates iPS cell generation with just one (OCT4) or two factors (OCT4 and SOX2 or OCT4 and KLF4).
Even with the advent of new techniques, the transcriptional factor OCT4 remains a key player in the reprogramming process. In fact, OCT4 alone seems to be sufficient to induce pluripotency.87 However, OCT4 could be replaced by nuclear receptors such as NR5a1 and NR5a2 or by a combination of microRNAs such as miR-200c, miR-302s, and miR-369s.88,89 Nevertheless, increasing the efficiency of iPS cell generation is crucial for future therapeutic use.
Clinical perspectives of mesenchymal and iPS cells
Stem cells normally present in the adult organism contribute to the postnatal development by replacement of lost cells due to injury, apoptosis, or physiological programmed turnover.14 When therapeutically applied, stem cells secrete factors and promote physical repair in injured tissues.5
MSC and iPS cells have particular characteristics (Table 1). These features reflect the wide therapeutic potential of both cell types, each possessing its pros and cons. The ideal stem cell for transplantation should be immunologically inert, derived from sources easily accessible, with high and fast expansion in vitro, long-term survival, ability to provide integration into the host site, and able to transfect and express exogenous genes.90 The autologous transplantation, available for MSC and iPS cell strategies, is preferred in regenerative medicine since the rejection risks are avoided.14
  | Table 1 General characteristics of mesenchymal and induced pluripotent stem cells |
The sources of both MSC and iPS cells are diverse. While MSC can be readily isolated from adult tissues and easily expanded in vitro, the iPS cell technology is slightly more complex. However, pluripotency can be achieved from virtually any cell type after several days in vitro, reaching a large amount of iPS cell colonies with great therapeutic potential. Cells can be systematically transplanted by intravenous injection either by direct application at the injury site or by scaffolds, a pre-cultivated structure that keeps the cells attached to the target site. Through tissue engineering, cells cultured in scaffolds can be induced to form tissues before transplantation. There is no agreement on the most effective mode of implementation. Several authors have succeeded using stem cells intravenously91 and through local administration.36,92 In these studies, authors reported a rapid migration and homing of stem cells to the injured tissues,93 attracted by extracellular matrix signals and soluble growth factors.27 However, Lam and Longaker13 argue that injected cells dissipate or die in the body, and the adhesion of cells is directly related to their growth and differentiation. In this context, MSC showed better ability to migrate and engraft more easily than the iPS cells in different biomaterial models.
It is known that pluripotent stem cells require specific culture conditions to maintain an undifferentiated state. iPS cells have been cultured in 2D feeder cells (eg, mouse embryonic fibroblasts); however, these methods require extensive culture time and have high related labor cost.94 The development of biomaterials assembling suitable culture conditions can support large-scale pluripotent cells’ proliferation or differentiation not involving feeder cells. Biomaterials designed for culture or to improve self-renewal capability or cell differentiation for iPS cells and MSC have been investigated.95 Synthetic or natural polymers and hydrogels mimicking specific 2D or 3D extracellular matrix have been used to support guided differentiation of iPS cells into specific cell lineages.94,96–98 These biomaterials are biologically inert and are therefore suitable to prevent allograft rejections and are the key tool for tissue engineering. In addition, the use of biomaterials colonized with pre-differentiated cells accelerates and improves the tissue regeneration.
The wide differentiation potential of the stem cells is essential for their use in multiple applications. MSC are multipotent stem cells with proven capacity to generate mesodermal cells, such as hepatocytes, myocytes, and osteocytes. iPS cells are able to generate cells from the three germ layers. In this context, the iPS cells represent a new possibility of using pluripotent stem cells, exempt from ethical implications surrounding ESC use. The capacity of teratoma formation of iPS cells can be avoided by the pre-differentiation in committed lineages.25
The therapeutic potential of MSC is unquestionably promising as a result of their advantageous effects and safety. These cells have been studied for many human and animal diseases. They exert a paracrine effect by the secretion of growth factors such as BGF, EGF, and BDNF and work by directly differentiating into specific somatic cells.99
In recent years, many preclinical studies have been carried out to investigate the application of stem cells for human disease, especially (neurodegenerative diseases) in animal models.100 Stem cells improved neuron replacement and healing in animal models for Parkinson’s disease,101,102 Alzheimer’s disease,103 epilepsy,104 sclerosis,105 ischemic stroke,106 and spinal cord injury.107 Although promising results were achieved, the mechanisms underlying cell survival, migration, homing, and differentiation in the pathological environment must be investigated before these results can be translated to humans.100
In wound healing, MSC induces the inhibition of the inflammatory response, differentiation into skin cells, stimulation of angiogenesis, and secretion of growth factors.35,108 The beneficial effects of MSC were observed in cancer immunosuppression;109,110 in the formation of new vessels;111 and in cardiac,112 liver,113 and kidney114,115 regeneration. In fact, MSC are extensively studied and tested in various affections, diseases, and even for cosmetic purposes.36
Despite their valuable application for regenerating tissues, the MSC have limitations such as quick loss of plasticity during expansion. Furthermore; the MSC can be isolated from numerous adult or fetal tissues; the isolation procedures are mostly invasive, and the harvested cells are limited in number.116 The iPS cells are obtained through noninvasive methods and can differentiate into all body cell types. Therefore, iPS cells are the most attractive stem cell source for cell therapy.117 Due to rapid growth and high plasticity, direct transplantation of iPS cells can result in in vivo teratoma formation. The differentiation of pluripotent cells into multipotent cells prior to transplantation arises as a promising tool for safe use of iPS cells. Multipotent-like cells derived from pluripotent cells have been investigated as well as effective methods and strategies for iPS cell derived MSC establishment.118
In recent years, the MSC derived from diverse iPS cell lines represent the effective source of multipotent cells, incorporating the advantages of both iPS cells and traditional MSC cells.118,119 The iPS cell-MSC have a greater proliferation capacity in vitro with no time limit.111 They also have immunomodulatory properties similar to traditional MSC lines, and it was reported recently that they are capable of impairing NK-cells’ function to prevent graft rejection.119 Despite their long-term survival after transplantation,111 the iPS cell-MSC are nontumorigenic and are safe and effective for cell-based therapy.
Among the therapeutic progress of iPS cells, Christoforou et al120 generated cardiac progenitors and cardiomyocytes capable of forming biosynthetic tissues and produced an in vitro cellular model of amyotrophic lateral sclerosis.121 Currently, the investigation of pathophysiology, drug development, and toxicology studies are the major applications of these cells.122
Several preclinical trials have been carried out evaluating the in vitro pre-differentiation of iPS cells for regeneration, as in nerve function123 and periodontal regeneration.124 Despite its clinical potential and the possibility to avoid rejection, immunogenic issues were present in previous attempts.45,125 Recent studies have demonstrated that the rejection is related to gene expression and epigenetic inheritance of reprogramming process, and not to specific characteristics of iPS cells. Therefore, increasing the production efficiency and reducing chromosomal and epigenetic alterations, could lead to the use of iPS cells in therapy without rejection issues.126,127
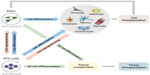
Regarding the clinical approaches, the American government recognizes more clinical trials with MSC, involving neoplasias, immunodeficiency, syndromes and others, however, a few clinical studies are recognized with iPS cells, such as hypertension and fibromuscular dysplasia.128 Clearly, we still have a long way to go regarding iPS cell therapy, but research is advancing rapidly and is heading for satisfactory results. The potential and various possibilities of clinical applications of MSC and iPS cells are summarized in Figure 1.
Conclusion
MSC are easily collected and maintained in culture, show a high proliferation in vitro and are nontumorigenic when transplanted in vivo. They can differentiate into several mesodermal cell types and can be used for cell transplantation or tissue engineering. The therapeutic utilization of MSC is advantageous because they are easy to collect and maintain, and a short period of time is needed between the culture establishment and clinical application. On the other hand, the pluripotency state of iPS cells can mean a wide possibility of disease treatment, and their pre-differentiation in vitro can guarantee the safeness of their utilization. However, iPS cell research is still beginning to reach the preclinical and clinical stage, and much more studies are necessary to determine their therapeutic applications.
Acknowledgment
The authors appreciate the grant support of FAPESP (São Paulo Research Foundation) (grant numbers 2012/04196-4 and 2013/09392-9).
Disclosure
The authors report no conflicts of interest in this work.
References
Malaver-Ortega LF, Sumer H, Liu J, Verma PJ. The state of the art for pluripotent stem cells derivation in domestic ungulates. Theriogenology. 2012;78(8):1749–1762. | |
Keefer CL, Panta D, Blomberg L, Talbot NC. Challenges and prospects for the establishment of embryonic stem cell lines of domesticated ungulates. Anim Reprod Sci. 2007;98(1–2):147–168. | |
Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther. 2013;4(2):23. | |
De Bari C, Dell’Accio F, Tylzanowski P, Luyten F. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. | |
Nardi NB, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;(174):249–282. | |
Barker JN, Wagner JE. Umbilical cord transplantation: current practice and future innovations. Crit Rev Oncol Hematol. 2003;48(1):35–43. | |
Mitalipov S, Wolf D. Totipotency, pluripotency and nuclear reprogramming. Adv Biochem Eng Biotechnol. 2009;114:185–199. | |
Brevini TA, Tosetti V, Crestan M, Antonini S, Gandolfi F. Derivation and characterization of pluripotent cell lines from pig embryos of different origins. Theriogenology. 2007;67(1):54–63. | |
Kuijk EW, Chuva de Sousa Lopes SM, Geijsen N, Macklon N, Roelen BA. The different shades of mammalian pluripotent stem cells. Hum Reprod Update. 2010;17(2):254–227. | |
Verfaillie CM, Pera MF, Lansdorp PM. Stem cells: hype and reality. Hematology Am Soc Hematol Educ Program. 2002;369–391. | |
Evans MJ, Notarianni E, Laurie S, Moor RM. Derivation and preliminary characterization of pluripotent cell lines from porcine and bovine blastocysts. Theriogenology. 1990;33(1):125–128. | |
Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science. 2000;287(5457):1442–1446. | |
Lam MT, Longaker MT. Comparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med. 2012;6 Suppl 3:s80–s86. | |
Sachs PC, Francis MP, Zhao M, et al. Defining essential stem cell characteristics in adipose-derived stromal cells extracted from distinct anatomical sites. Cell Tissue Res. 2012;349(2):505–515. | |
Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95(3):379–391. | |
Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;30(5):631–642. | |
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. | |
Bianchi G, Muraglia A, Daga A, Corte G, Cancedda R, Quarto R. Microenvironment and stem properties of bone marrow-derived mesenchymal cells. Wound Repair Regen. 2001;9(6):460–466. | |
Filioli Uranio M, Valentini L, Lange-Consiglio A, et al. Isolation, proliferation, and pharacterization of mesenchymal stem cells from amniotic fluid, amnion, and umbilical cord matrix in the Dog. Mol Reprod Dev. 2011;78(5):361–373. | |
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. | |
Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;31(8):150. | |
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012;21(14):2724–2752. | |
Xiao L, Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: Stem cells in the core. J Cell Biochem. 2013;114(11):2624–2636. | |
Kisiel AH, McDuffee LA, Masaoud E, Bailey TR, Esparza Gonzalez BP, Nino-Fong R. Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 2012;73(8):1305–1317. | |
Webster RA, Blaber SP, Herbert BR, Wilkins MR, Vesey G. The role of mesenchymal stem cells in veterinary therapeutics – a review. N Z Vet J. 2012;60(5):265–272. | |
Du Y, Roh DS, Funderburgh ML, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis. 2010;16:2680–2689. | |
Vidane AS, Zomer HD, Oliveira BM, et al. Reproductive stem cell differentiation: extracellular matrix, tissue microenvironment, and growth factors direct the mesenchymal stem cell lineage commitment. Reprod Sci. 2013;20(10):1137–1143. | |
Krampera M, Marconi S, Pasini A, et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40(2):382–490. | |
Aurich H, Sgodda M, Kaltwasser P, et al. Hepatocyte differentiation of mesen chymal stem cells from human adipose tissue in vitro promotes hepatic integration in vivo. Gut. 2009;58(4):570–581. | |
Lage-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22(22):3015–3024. | |
Plock JA, Schnider JT, Solari MG, Zheng XX, Gorantla VS. Perspectives on the use of mesenchymal stem cells in vascularized composite allotransplantation. Front Immunol. 2013;4:175. | |
Insausti CL, Blanquer M, García-Hernández AM, Castellanos G, Moraleda JM. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53–63. | |
Bydlowski SP, Debes AA, Maselli LMF, Janz FL. Características biológicas das células-tronco mesenquimais [Biological characteristics of the mesenchymal stem cells]. Rev Bras Hematol Hemoter. 2009;31:25–35. Portuguese. | |
Housman TS, Lawrence N, Mellen BG, et al. The safety of liposuction: results of a national survey. Dermatol Surg. 2002;28(11):971–978. | |
Cherubino M, Rubin JP, Miljkovic N, Kelmendi-Doko A, Marra KG. Adipose-derived stem cells for wound healing applications. Ann Plast Surg. 2011;66(2):210–215. | |
Kim JH, Jung M, Kim HS, Kim YM, Choi EH. Adipose-derived stem cells as a new therapeutic modality for ageing skin. Exp Dermatol. 2011;20(5):383–387. | |
Dey D, Evans GR. Generation of induced pluripotent stem (iPS) cells by nuclear reprogramming. Stem Cells Int. 2011;2011:619583. | |
Yang XF, He X, He J, et al. High efficient isolation and systematic identification of human adipose-derived mesenchymal stem cells. J Biomed Sci. 2011;18:59. | |
Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun. 1999;265(1):134–139. | |
Fadel L, Viana BR, Feitosa ML, et al. Models, Biological Protocols for obtainment and isolation of two mesenchymal stem cell sources in sheep. Acta Cir Bras. 2011;26(4):267–273. | |
Martínez-Lorenzo MJ, Royo-Cañas M, Alegre-Aguarón E, et al. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. J Orthop Res. 2009;27(11):1499–1507. | |
Sunay O, Can G, Cakir Z, et al. Autologous rabbit adipose tissue-derived mesenchymal stromal cells for the treatment of bone injuries with distraction osteogenesis. Cytotherapy. 2013;15(6):690–702. | |
Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131(5):861–872. | |
Yamanaka S. Pluripotency and nuclear reprogramming. Philos Trans R Soc Lond B Biol Sci. 2008;363(1500):2079–2087. | |
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. | |
Xu Y, Wei X, Wang M, et al. Proliferation rate of somatic cells affects reprogramming efficiency. J Biol Chem. 2013;288(14):9767–9778. | |
Diecke S, Jung SM, Lee J, Ju JH. Recent technological updates and clinical applications of induced pluripotent stem cells. Korean J Intern Med. 2014;29(5):547–557. | |
Picanço-Castro V, Russo-Carbolante E, Reis LC, et al. Pluripotent reprogramming of fibroblasts by lentiviral mediated insertion of SOX2, C-MYC, and TCL-1A. Stem Cells Dev. 2011;20(1):169–180. | |
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–949. | |
Okita K, Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Curr Stem Cell Res Ther. 2006; 1(1):103–111. | |
Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. | |
Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. | |
Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. | |
Hou P, Li Y, Zhang X, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compound. Science. 2013; 341(6146):651–654. | |
Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454(7200):49–55. | |
Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132(4):567–582. | |
Hotta A, Ellis J. Retroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent states. J Cell Biochem. 2008;105(4):940–948. | |
Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Curr Opin Biotechnol. 2007;18(5):467–473. | |
Wang Y, Mah N, Prigione A, Wolfrum K, Andrade-Navarro MA, Adjaye J. A transcriptional roadmap to the induction of pluripotency in somatic cells. Stem Cell Rev. 2010;6(2):282–296. | |
Scheper W, Copray S. The molecular mechanism of induced pluripotency: a two-stage switch. Stem Cell Rev. 2009;5(3):204–223. | |
Liu H, Zhu F, Yong J, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3(6):587–590. | |
Esteban MA, Wang T, Qin B, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6(1):71–79. | |
Sumer H, Liu J, Malaver Ortega LF, Lim ML, Khodadadi K, Verma PJ. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J Anim Sci. 2011;89(9):2708–2716. | |
Nagy K, Sung HK, Zhang P, et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 2011;7(3):693–702. | |
Honda A, Hirose M, Hatori M, et al. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Bio Chem. 2010;285(41):31362–31369. | |
Bao L, He L, Chen J, et al. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res. 2011;21(4):600–608. | |
Verma R, Holland MK, Temple-Smitha P, Verma PJ. Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid. Theriogenology. 2012;77(1):220–228. | |
Shimada H, Nakada A, Hashimoto Y, Shigeno K, Shionoya Y, Nakamura T. Generation of canine-induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol Reprod Dev. 2010;77(1):2. | |
Luo J, Suhr ST, Chang EA, et al. Generation of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluripotent stem cells from canine adult somatic cells. Stem Cells Dev. 2011;20(10):1669–1678. | |
Whitworth DJ, Ovchinnikov DA, Wolvetang EJ. Generation and characterization of LIF-dependent canine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 2012;21(12):2288–2297. | |
Gonçalves NJ, Bressan FF, Souza A, et al. Canine fibroblasts expressing human transcription factors: what is in the route for the production of canine induced pluripotent stem cells. Reprod Domest Anim. 2012;47 (Suppl 6):84–87. | |
Koh S, Thomas R, Tsai S, et al. Growth requirements and chromosomal instability of induced pluripotent stem cells generated from adult canine fibroblasts. Stem Cells Dev. 2012;22(6):951–963. | |
Sugii S, Kida Y, Berggren WT, Evans RM. Feeder-independent iPS cell derivation from human and mouse adipose stem cells. Nat Protoc. 2011;6(3):346–358. | |
Sun N, Panetta NJ, Gupta DM, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106(37):15720–15725. | |
Yamanaka S. Strategies and new developments in the generation of patientspecific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. | |
Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–1275. | |
Lyssiotis CA, Foreman RK, Staerk J, et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci U S A. 2009;106(22):8912–8917. | |
Zhu S, Li W, Zhou H, et al. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell. 2010;7(6):651–655. | |
Mali P, Chou BK, Yen J, et al. Butyrate greatly enhances derivation of human induced pluripotent stem cells by promoting epigenetic remodeling and the expression of pluripotency-associated genes. Stem Cells. 2010;28(4):713–720. | |
Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6(10):e253. | |
Lin T, Ambasudhan R, Yuan X, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6(11):805–808. | |
Ichida JK, Blanchard J, Lam K, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5(5):491–503. | |
Marson A, Foreman R, Chevalier B, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3(2):132–135. | |
Maherali N, Hochedlinger K. Tgfβ Signal Inhibition Cooperates in the Induction of iPSCs and Replaces Sox2 and cMyc. Curr Biol. 2009;19(20):1718–1723. | |
Nakagawa M, Koyanagi M, Tanabe K, et al. Generation Of Induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26(1):101–106. | |
Wang Q, Xu X, Li J, et al. Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res. 2011;21(10):1424–1435. | |
Kim D, Kim CH, Moon J, et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell. 20095;4(6):472–476. | |
Heng JC, Feng B, Han J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. | |
Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. | |
Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats – similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95(7):3908–3913. | |
Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. | |
Kamada Y, Yoshida Y, Saji Y, et al. Transplantation of basic fibroblast growth factor-pretreated adipose tissue-derived stromal cells enhances regression of liver fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296(2):157–167. | |
Sohni A, Verfaillie CM. Mesenchymal Stem Cells Migration Homing and Tracking. Stem Cells Int. 2013;2013:130763. | |
Higuchi A, Ling QD, Kumar SS, et al. External stimulus-responsive biomaterials designed for the culture and differentiation of ES, iPS, and adult stem cells. Prog Polym Sci. 2014;39(9):1585–1613. | |
Higuchi A, Ling QD, Kumar SS, et al. Design of polymeric materials for culturing human pluripotent stem cells: Progress toward feeder-free and xeno-free culturing. Prog Polym Sci. 2014;39(7):1348–1374. | |
Xie C, Hu J, Ma H, et al. Three-dimensional growth of iPS cell-derived smooth muscle cells on nanofibrous scaffolds. Biomaterials. 2011; 32(19):4369–4375. | |
Kuo YC, Chung CY. TATVHL peptide-grafted alginate/poly(γ-glutamic acid) scaffolds with inverted colloidal crystal topology for neuronal differentiation of iPS cells. Biomaterials. 2012;33(35):8955–8966. | |
Kuo YC, Chang YH. Differentiation of induced pluripotent stem cells toward neurons in hydrogel biomaterials. Colloids Surf B Biointerfaces. 2013;102:405–411. | |
Bahn JJ, Chung JY, Im W, Kim M, Kim SH. Suitability of autologous serum for expanding rabbit adipose-derived stem cell populations. J Vet Sci. 2012;13(4):413–417. | |
Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders – time for clinical translation? J Clin Invest. 2010;120(1):29–40. | |
Wernig M, Zhao JP, Pruszak J, et al. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci U S A. 2008;105(15):5856–5861. | |
Acquarone M, de Melo TM, Meireles F, et al. Mitomycin-treated undifferentiated embryonic stem cells as a safe and effective therapeutic strategy in a mouse model of Parkinson’s disease. Front Cell Neurosci. 2015;9:97. | |
Kanamaru T, Kamimura N, Yokota T, et al. Intravenous transplantation of bone marrow-derived mononuclear cells prevents memory impairment in transgenic mouse models of Alzheimer’s disease. Brain Res. 2015;1605:49–58. | |
Agadi S, Shetty AK. Prospects of bone marrow mononuclear cells and mesenchymal stem cells for treating status epilepticus and chronic epilepsy. Stem Cells. 2015;33(7):2093–2103. | |
Faghihi F, Mirzaei E, Ai J, et al. Differentiation potential of human chorion-derived mesenchymal stem cells into motor neuron-like cells in two- and three-dimensional culture systems. Mol Neurobiol. Epub 2015 Mar 20. | |
Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265(1–2):73–77. | |
Sareen D, Gowing G, Sahabian A, et al. Human induced pluripotent stem cells are a novel source of neural progenitor cells (iNPCs) that migrate and integrate in the rodent spinal cord. J Comp Neurol. 2014;522(12):2707–2728. | |
Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13(6):1299–1312. | |
Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62(13):3603–3608. | |
Yang C, Lei D, Ouyang W, et al. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int. 2014;2014:109389. | |
Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–1123. | |
Garikipati VN, Jadhav S, Pal L, Prakash P, Dikshit M, Nityanand S. Mesenchymal stem cells from fetal heart attenuate myocardial injury after infarction: an in vivo serial pinhole gated SPECT-CT study in rats. PLoS One. 2014;9(6):e100982. | |
Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499(7459):481–484. | |
Villanueva S, Carreño JE, Salazar L, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond). 2013;125(4):199–210. | |
Wise AF, Williams TM, Kiewiet MB, et al. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2014;306(10):F1222–F1235. | |
Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7(3):e33225. | |
Villa-Diaz LG, Brown SE, Liu Y, et al. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30(6):1174–1181. | |
Hynes K, Menicanin D, Han J, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833–839. | |
Giuliani M, Oudrhiri N, Noman ZM, et al. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118(12):3254–3262. | |
Christoforou N, Liau B, Chakraborty S, Chellapan M, Bursac N, Leong KW. Induced pluripotent stem cell-derived cardiac progenitors differentiate to cardiomyocytes and form biosynthetic tissues. PLoS One. 2013;8(6):e65963. | |
Burkgardt MF, Martinez FJ, Wright S, et al. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2013;56:355–364. | |
Meyer JR. The significance of induced pluripotent stem cells for basic research and clinical therapy. J Med Ethics. 2008;34(12):849–851. | |
Yuan T, Liao W, Feng NH, et al. Human induced pluripotent stem cell-derived neural stem cells survive, migrate, differentiate, and improve neurological function in a rat model of middle cerebral artery occlusion. Stem Cell Res Ther. 2013;4(3):73. | |
Duan X, Tu Q, Zhang J, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011;226(1):150–157. | |
Fairchild PJ. The challenge of immunogenicity in the quest for induced pluripotency. Nat Rev Immunol. 2010;10(12):868–875. | |
Araki R, Uda M, Hoki Y, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. | |
Morizane A, Doi D, Kikuchi T, et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a nonhuman primate. Stem Cell Reports. 2013;1(4):283–292. | |
clinicaltrials.gov [homepage on the Internet]. A service of the US National Institutes of Health. Available from: https://clinicaltrials.gov/. Accessed August 16, 2015. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.