Back to Journals » OncoTargets and Therapy » Volume 12
Merkel cell carcinoma metastatic to cervical lymph node in a patient with rheumatoid arthritis: a case report
Authors Li N, Wang G, Jiang X, Huang M, Tian H, Xuan F, Zhang Y, Lv Y, Hu M, Wang Z, Ren P, Xu M
Received 22 September 2018
Accepted for publication 28 December 2018
Published 19 February 2019 Volume 2019:12 Pages 1395—1400
DOI https://doi.org/10.2147/OTT.S188403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Na Li,1 Guodong Wang,2 Xiaohong Jiang,1 Minguang Huang,2 Huanyong Tian,2 Feng Xuan,2 Yufeng Zhang,2 Yanting Lv,3 Mengjun Hu,3 Zhen Wang,2 Peng Ren,1 Maoyi Xu2
1Department of Pathology, Jiaxing University Afffiliated Women and Children Hospital, Jiaxing Maternity and Child Health Care Hospital, Jiaxing University, Jiaxing, Zhejiang 314051, China; 2Department of Radiotherapy Oncology, Zhuji People’s Hospital of Zhejiang Province, The Zhuji Affiliated Hospital of Wenzhou Medical University, Zhuji, Zhejiang 311800, China; 3Department of Pathology, Zhuji People’s Hospital of Zhejiang Province, The Zhuji Affiliated Hospital of Wenzhou Medical University, Zhuji, Zhejiang 311800, China
Abstract: Merkel cell carcinoma (MCC) is a rare, aggressive skin malignancy that has a propensity for local recurrence and metastasis to the lymph nodes. In this case report, we discuss a 54-year-old female with rheumatoid arthritis (RA) who had received treatment with prednisone (15 mg/day) for symptom relief and management. The patient visited our hospital with complaints of a nodule in right preauricular area. Computed tomography (CT) scans revealed no distant metastasis. The patient underwent surgical resection and histopathological evaluation of the nodule led to the diagnosis of MCC. The patients received post-surgical treatment with 6 MeV electronic wire radiotherapy. Six months later, CT of the head, neck, abdomen and chest demonstrated a right cervical lymph node mass at the C2 level. The patient then underwent cervical lymph node biopsy and pathological diagnosis confirmed metastatic MCC. One month after the lymph node biopsy, the patients received postoperative intensity modulated radiation therapy in the biopsied area. The patient did not experience any adverse effects to the therapy. In conclusion, the MCC patients with RA can tolerate radiation therapy. As MCC is a highly malignant neoplasia, considering the immune checkpoint inhibitors can lead to immune-related adverse events, detection of MCC at earlier stages is associated with better survival. The treatment decisions of MCC patients with RA continues is still challenging.
Keywords: Merkel cell carcinoma, metastasis, cervical lymph node, rheumatoid arthritis
Introduction
Merkel cell carcinoma (MCC), also termed trabecular carcinoma, was initially described in 1972 by Toker.1 The clinical features of symptomatic and asymptomatic MCC include; rapid expansion (<3 months), immune suppression, patient >50 years and UV-exposed site on fair skin.2 MCC cells express neuroendocrine markers such as Synaptophysin (Syn) and Cytokeratin 20 (CK20).3 CK20 is a fairly specific and sensitive marker of MCC, with a characteristic paranuclear dot-like positivity.
MCC has a propensity for widespread metastases and commonly occurs on sun exposed areas of the head and neck. Biologically, MCC is characterized by local recurrence (30%), regional lymph node metastases (65%) and distant metastases (40%). Surgery is the primary treatment strategy for patients with MCC. Wide surgical excision of the primary lesion is the treatment of choice, while the role of prophylactic regional lymphadenectomy is controversial.4 Adjuvant radiotherapy and chemotherapy is frequently associated.
Case report
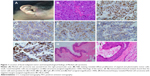
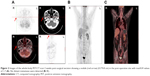
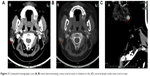
A 54-year-old female patient was admitted for cutaneous and subcutaneous nodule of right preauricular area. She had no history of smoking, alcohol use, blood transfusion, travel abroad or raw meat intake. Twenty-seven years earlier, she had suffered polyarthralgia, morning stiffness in multiple joints and joint swelling of the wrists, knees and feet. Immunology tests revealed that the patient was positive for antinuclear antibodies, rheumatoid factor and cyclic citrullinated peptide at the time of diagnosis. The patient was diagnosed with rheumatoid arthritis (RA) (Steinbrocker classification: stage I, class II), with an unknown Disease Activity Score-28 (DAS28) and received symptomatic treatment (the specific treatment was unknown). Subsequently, she achieved remission of the symptoms, but the symptoms of joint pain and swelling reoccurred, and the patient received treatment with 15 mg/day prednisone. The symptoms and activity of RA were reduced and stable for ten years. A slow-growing cutaneous and subcutaneous nodule was first noted in March 2017, but the patient declined treatment until August 2017. Computed tomography (CT) scans (completed on August 26, 2017) of the head, neck, abdomen and chest were performed. No distant metastases were detected. The patient underwent surgical treatment on the 26 August 2017) (Figure 1). The mass, with a diameter of 1 cm, was excised from the right preauricular area. Pathological examination revealed a diagnosis of MCC (stage I) with negative margins (Figure 1). Immunohistochemical staining showed that tumor cells were positive for CK20, CD56, chromogranin A (CgA) and Syn (Figure 1). The proliferative activity (Ki-67) reached ~ 80% (Figure 1). At the 2-week post operation follow-up, whole body examination with 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT was performed (Figure 2). The 18F-FDG PET/CT scan (September 14, 2017) demonstrated a nodule (0.3×0.8 cm) in the post-operative site and the maximum standardized uptake value was 1.7. Postoperative change was considered. No distant metastases were detected. One-month post-operation (October 09, 2017), the area was treated with 6 MeV electronic wire radiation therapy (50 Gy/25 fractions). The patient did not report any adverse effects.
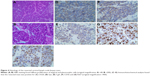
Six months post-surgery, the patient was admitted to hospital for review. The general condition of the patient according to Eastern Cooperative Oncology Group performance status was 1, and a Disease Activity Score-28 (DAS28-CRP) of 2.58. CT scans (March 01, 2018) of the head, neck, abdomen and chest were performed. The scan demonstrated a right cervical lymph node mass at the C2 level (Figure 3). There was no evidence of metastatic disease elsewhere. Then, the patient underwent cervical lymph node biopsy (March 05, 2018) and pathological diagnosis confirmed metastatic MCC (Figure 4). One month after the lymph node biopsy (April 16, 2018), the lymph node biopsy area received intensity modulated radiation therapy (64 Gy/32 fractions). At follow-up, 1 year after the first operation, the patient was in good general health. The patient did not experience any adverse effects of the therapy. The treatment timeline is presented in Figure 5.
  | Figure 3 Computed tomography scan (A, B) neck demonstrating a mass (red arrow) in relation to the (C) cervical lymph node mass (red arrow). |
Discussion
MCC is an uncommon neuroendocrine carcinoma of the skin. MCC has a highly prevalent local recurrence and metastases (including liver, lung, cardiac, bone, pancreatic and other organs).5–9 MCC is more frequent in immunosuppressed patients. Immune surveillance plays a critical role in the control of tumour growth and progression.10 Moreover, immunosuppressive conditions (including HIV infection,11 solid organ transplantation,12 lymphoma,13 and autoimmune disease)14 are associated with an increased risk of MCC development. Chronic inflammatory disorders increase the risk of MCC significantly, and patients with chronic inflammatory disorders are at higher risk for aggressive MCC.14 Indeed, connective tissue diseases are also associated with higher incidence of MCC and show poorer MCC-specific survival.14,15
Connective tissue diseases include RA systemic lupus erythematosus (SLE), systemic sclerosis (SSc), scleroderma, polymyositis-dermatomyositis and vasculitis. MCC in a patient with rheumatoid arthritis is very rarely reported in literature. Furthermore, there is debate about whether patients with connective tissue diseases can tolerate radiation. Many treatment strategies have been considered for patients with connective tissue diseases such as reducing dose prescription, avoiding concomitant treatment and improvement of radiotherapy technology.16
As mentioned previously, MCC has a highly prevalent local recurrence and distant metastasis. The anti-programmed death-1 (anti-PD-1) antibody, pembrolizumab, is a systemic therapy option for patients with advanced stage MCC.4 Furthermore, Avelumab, an anti-programmed death ligand-1 (anti-PD-L1) monoclonal antibody, is a second line therapy for patients with stage IV MCC that progressed following cytotoxic chemotherapy.17 Previous studies have demonstrated that anti-PD-1/PD-L1 can lead to immune-related adverse events, which could be lethal.18–21
T cells are major players involved in the pathogenesis of RA and increasing serum soluble PD-1, which has been reported in RA patient.22,23 Furthermore, soluble PD-1 can enhance T cell responses by interfering with PD-1/PD-L1 pathway in autoimmune and tumor.24–26 Glucocorticoids (eg, prednisone) are anti-inflammatory and immunosuppressive agents that are able to reduce the inflammation and the progression of RA. However, it was reported that a baseline corticosteroid use of ≥10 mg of prednisone or equivalent was associated with poorer outcome in non-small-cell lung cancer patients treated with immunotherapy.27 And prudent use of corticosteroids at the time of initiating immunotherapy is recommended.27 Considering the involvement of activated T cells and the development of immune-related adverse events in anti-PD-1 antibody therapeutics. In this case, clinicians should be aware of the possibility of anti-PD-1/PD-L1-related adverse effects, such as inflammatory arthritis,28 especially in patients with RA. In this case report, the patient with RA tolerated radiation therapy without any adverse side effects. As with MCC of other areas, metastasis or local recurrence can occur after a certain period. Chemotherapy might be a therapeutic option in chemo-sensitive metastatic MCC, and further evaluation is warranted.
Therefore, clinicians should be aware of the use of immune checkpoint inhibitors in MCC patients with RA. We presented a unique case of MCC metastatic to cervical lymph node in a patient with RA. We aim to highlight that the treatment decisions to patients with RA continues to be challenging.
Ethics statement
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. This study was approved by the Institutional Review Board of Zhuji People’s Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. | ||
Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58(3):375–381. | ||
Maricich SM, Wellnitz SA, Nelson AM, et al. Merkel cells are essential for light-touch responses. Science. 2009;324(5934):1580–1582. | ||
Bichakjian CK, Olencki T, Aasi SZ, et al. Basal cell skin cancer, version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(5):574–597. | ||
Shoda K, Ikoma H, Yamamoto Y, et al. A case of long-term survival following hepatectomy for liver metastasis of Merkel cell carcinoma. Surg Case Rep. 2015;1(1):30. | ||
Suttie CF, Hruby G, Horvath L, Thompson J. Cardiac metastasis in Merkel cell carcinoma. J Clin Oncol. 2014;32(13):e52–e53. | ||
Fong LS, Mathur M, Bhindi R, Figtree GA. Right atrial Merkel cell tumour metastasis characterization using a multimodality approach. Eur Heart J. 2012;33(17):2205. | ||
Peloschek P, Novotny C, Mueller-Mang C, et al. Diagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PET. Eur J Radiol. 2010;73(2):317–323. | ||
Maimone A, Bianchi ML, Lorenzini P, de Leone A, De Luca L. Endoscopic ultrasound-guided fine needle biopsy of pancreatic metastasis from Merkel cell carcinoma. Endoscopy. 2016;48(S 01):E199–E200. | ||
Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. | ||
Engels EA, Frisch M, Goedert JJ, Biggar RJ, Miller RW. Merkel cell carcinoma and HIV infection. Lancet. 2002;359(9305):497–498. | ||
Clarke CA, Robbins HA, Tatalovich Z, et al. Risk of Merkel cell carcinoma after solid organ transplantation. J Natl Cancer Inst. 2015;107(2):dju382. | ||
Ziprin P, Smith S, Salerno G, Rosin RD. Two cases of Merkel cell tumour arising in patients with chronic lymphocytic leukaemia. Br J Dermatol. 2000;142(3):525–528. | ||
Sahi H, Sihto H, Artama M, Koljonen V, Böhling T, Pukkala E. History of chronic inflammatory disorders increases the risk of Merkel cell carcinoma, but does not correlate with Merkel cell polyomavirus infection. Br J Cancer. 2017;116(2):260–264. | ||
Paulson KG, Iyer JG, Blom A, et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133(3):642–646. | ||
Giaj-Levra N, Sciascia S, Fiorentino A, et al. Radiotherapy in patients with connective tissue diseases. Lancet Oncol. 2016;17(3):e109–e117. | ||
Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374–1385. | ||
Wang PF, Chen Y, Song SY, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;8:730. | ||
Nishino M, Ramaiya NH, Awad MM, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–6060. | ||
Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1):43–50. | ||
Spallarossa P, Meliota G, Brunelli C, et al. Potential cardiac risk of immune-checkpoint blockade as anticancer treatment: what we know, what we do not know, and what we can do to prevent adverse effects. Med Res Rev. 2018;38(5):1447–1468. | ||
Isaacs JD. Therapeutic T-cell manipulation in rheumatoid arthritis: past, present and future. Rheumatology (Oxford). 2008;47(10):1461–1468. | ||
Guo Y, Walsh AM, Canavan M, et al. Immune checkpoint inhibitor PD-1 pathway is down-regulated in synovium at various stages of rheumatoid arthritis disease progression. PLoS One. 2018;13(2):e0192704. | ||
Shin SP, Seo HH, Shin JH, et al. Adenovirus expressing both thymidine kinase and soluble PD1 enhances antitumor immunity by strengthening CD8 T-cell response. Mol Ther. 2013;21(3):688–695. | ||
Dai S, Jia R, Zhang X, Fang Q, Huang L. The PD-1/PD-Ls pathway and autoimmune diseases. Cell Immunol. 2014;290(1):72–79. | ||
Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97671–97682. | ||
Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–2878. | ||
Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2017;76(1):43–50. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.