Back to Journals » Infection and Drug Resistance » Volume 14
Membranous Nephropathy Complicated with Disseminated Nocardia farcinica Infection: A Case Report and Literature Review
Authors Pan L , Wang XH , Meng FQ, Su XM, Li Y, Xu MT, Su FY , Kong DL, Wang W
Received 30 July 2021
Accepted for publication 25 September 2021
Published 8 October 2021 Volume 2021:14 Pages 4157—4166
DOI https://doi.org/10.2147/IDR.S331737
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lei Pan,1,2 Xu-Hao Wang,1 Fan-Qi Meng,1 Xin-Ming Su,1 Yue Li,1 Ming-Tao Xu,1 Feng-Yuan Su,3 De-Lei Kong,1 Wei Wang1
1Department of Respiratory and Critical Care Medicine, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 2Liaoning Provincial Centers for Disease Control and Prevention, Shenyang, People’s Republic of China; 3China Medical University, Shenyang, People’s Republic of China
Correspondence: De-Lei Kong
Department of Respiratory and Critical Care Medicine, The First Hospital of China Medical University, 155 Nanjing North Street, Heping District, Shenyang, Liaoning, 110001, People’s Republic of China
Email [email protected]
Abstract: Disseminated infection caused by Nocardia farcinica with primary nephrotic syndrome is exceedingly rare. A 66-year-old female visited the outpatient department due to fever and fatigue who had been diagnosed as membranous nephropathy and with a long-term prednisone and immunosuppressive therapy. After lung biopsy for many times, culture from space-occupying lesion of the right lung and species identification by mass spectrometry-based methods (MALDI-TOF) revealed Nocardia farcinica. By imaging examination, space-occupying lesions from the lungs, brain, abdominal cavity and kidney were found. After 2 weeks of meropenem intravenous and up to 6 months of trimethoprim-sulfamethoxazole (TMP-SMX) therapy, our patient has remained relapse-free at that time of writing. Disseminated infection caused by Nocardia farcinica is usually subacute with complex clinical manifestations. In addition, it can be easily confused with diseases such as tumor and mycobacterial infection, and lead to fatal consequences. Therefore, we hope that we can remind clinicians considering by discussing common features of disseminated Nocardia farcinica infection.
Keywords: disseminated nocardiosis, fever, space-occupying lesions, brain abscess, renal insufficiency
Introduction
Nocardia spp. are opportunistic pathogen belonging to aerobic actinomycetes, which can be found in almost all over the world and have been isolated from diverse environments including soil, water, the rhizosphere, insects, fish, as well as from human patients and medical samples.1,2 Since Nocardia spp. had been first described in 1888 by Edmond Nocard, in the United States, it has been estimated that 500–1000 new cases of nocardiosis infection occur every year reported from the centers for disease control and prevention (CDC). With high virulent, Nocardia farcinica have been identified as the source of life-threatening infections especially in immunocompromised persons, who are more likely to develop a disseminated infection.3 According to past literatures, incidence of N. farcinica infection cases presence regional differences. According to an American study, the incidence of N. farcinica made up 14% of all nocardiosis cases.4 Meanwhile, the incidence of Australia study was 3%,5 Spain study was 11.4%,6 and China study was 24.5%.7
The traditional identification of Nocardia spp. is culture but with some limitations. Nocardia spp. are difficult to isolate from clinical samples, and difficult to identification from different species. Over the past two decades, several molecular techniques for identification of Nocardia spp. have been proposed, including 16S rRNA gene sequencing, metagenomic next-generation sequencing (mNGS), as well as mass spectrometry-based methods (MALDI-TOF).8 Following advent of molecular methodologies, a huge number of new Nocardia species have been identified, at the same time, have improved the prognosis of Nocardia treatment.
Almost all Nocardia spp. infection do not result from human to human but typically by inhalation of the organism, presumably from aerosolized dust. Nocardia spp. infection can manifest as single-organ or multifocal diseases. Pulmonary, central nervous system (CNS) and cutaneous or subcutaneous are the most commonly involved sites.9 It’s important to note that disseminated Nocardia spp. infection could mimic tumors, tuberculosis or deep fungal infection, which are difficult to distinguish from. Its high pathogenicity and high mortality have attracted attention from clinicians, especially combining with CNS infection, with a mortality rate as high as 85%.10
Treatment of nocardiosis is individualized. Although there have been a small number of disseminated N. farcinica cases in the past have been reported, but not had been well summarized for treatment before. Currently, trimethoprim-sulfamethoxazole (TMP-SMX) is still regarded as the first-line drug for N. farcinica infection. Other antibiotics such as linezolid,11 amikacin,12 and imipenem13 have also been reported to be effective in treatment combined with TMP-SMX. Conventional intensive antibiotic treatment has improved survival rates, but the overall prognosis of patients with disseminated N. farcinica infection remains unsatisfactory.1
Case Presentation
A 66-year-old woman, a retired hotel caretaker, was admitted to the Respiratory Department of the First Hospital of the China Medical University with the chief complaint of ‘intermittent fever and lung occupation for 2 months’. Six months prior, the patient was diagnosed with primary nephrotic syndrome (idiopathic membranous nephropathy stage I) and chronic renal insufficiency (G5 stage). After tacrolimus administration, the patient received corticosteroid therapy (oral prednisone 50 mg once a day, 5 mg reduced every half month) combined with cyclophosphamide (1.0 g per month), and her kidney function was found to have improved since then (Figure 1C and D).
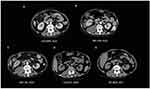
Three months prior, her chest computed tomography (CT) scan showed multiple nodules in both lungs (Figure 2B), which were not observed in prior CT scans (Figure 2A). An enhanced chest CT also revealed irregular nodules around the right lung hilum with mild enhancement, right pleural effusion, and enlarged mediastinal and right hilar lymph nodes (Figure 2C). Initially, these lesions were suspected to be malignant, however, after administration of cephalosporin combined with levofloxacin intravenous for 3 weeks, including three times for bronchoscopies and tissue biopsies and one time for mediastinal lymph nodes inspected by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) were all failed to detect any malignant tissue, aside from benign pathologic changes such as chronic inflammation. To rule out the possibility of tuberculosis, relevant blood tests were performed, all of which had a negative result. Notably, the patient persistently presented with an intermittent fever during hospitalization, and her highest body temperature was reported to reach 39.8°C. As such, the patient was transferred to our department for further diagnosis and treatment, following a multidisciplinary team consultation.
The patient still presented with fever without chills, and the highest body temperature reached 38°C. On physical examination, rough breath sounds were heard in both lungs. Moreover, significantly increased levels of inflammation-related indicators (WBC: 26.72×109 /L, NE: 23.14×109/L, CRP: 57.4 mg/L, PCT: 0.206 ng/L) were seen on laboratory examinations (Figure 1A and B), along with increasing levels of coagulation indicators (Fg: 6.31 g/L, D-D: 4.4 ug/mL), and slightly higher levels of serum tumour markers (CA125: 63 U/mL, CA199: 38.4 U/mL, Cyfra21-1: 3.38U/mL). After testing for penicillin allergy, intravenous piperacillin-tazobactam (2.5 g every 12 hours) was initiated, and prednisone was still used for primary nephrotic syndrome concurrently. For further diagnosis, the patient underwent endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy again, and cultures from pulmonary lesions revealed Nocardia species which was a gram-positive, partial acid-fast positive, rod-shaped bacterium (Figure 3A–C). A further species identification was subjected to MALDI-TOF MS (VITEK® MS, BioMérieux), which was identified as Nocardia farcinica (99.9% confidence).
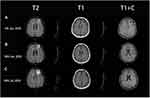
Following pathological diagnosis, the patient underwent brain contrast-enhanced MRI and abdominal enhanced CT scans to exclude other foci. The brain contrast-enhanced magnetic resonance imaging (MRI) images showed mixed solid cystic lesions (Figure 4A), additional diffusion-weighted imaging (DWI) results are showed in Figure 5. Meanwhile, on whole-abdominal-enhanced CT, right abdominal encapsulated effusion and space-occupying lesions were reported (Figure 6B), which were not found in the previous inspection (Figure 6A). We administered to intravenous meropenem (1 g every 12 hours) and oral TMP-SMX (3 pieces every 8 hours, each piece containing 80 mg of trimethoprim and 400mg of sulfamethoxazole). About half month later, another brain contrast-enhanced MRI was performed, revealing the ring-like enhancement lesion was smaller than the previous one (Figure 4B), and a chest-enhanced CT scan also showed lesion size reduction (Figure 2D). Furthermore, laboratory tests revealed that infection-related indicators were all significantly lower than them before (Figure 1A and B). Since treatment was well tolerated with no adverse events, we advised the patient to continue oral TMP-SMX 3 pieces three times a day for 6 months, and to undergo regular follow-ups with blood tests and related imaging examinations. The relevant examination results are presented in Figures 1, 2E–G, 4C, 6C–E and 7. The clinical course is summarized in Figure 8.
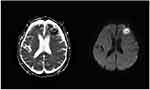 |
Figure 5 DWI: Patchy diffusion restricted lesions with high signal in the left frontal lobe, the corresponding ADC diagram shows uneven low signal changes. |
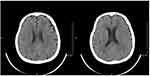 |
Figure 7 Brain CT (13rd Oct 2020): the lesion almost disappeared in the left frontal lobe. |
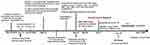 |
Figure 8 The clinical course of this case. |
Discussion and Literature Review
Nocardiosis is a rare infection caused by Nocardia spp., which are environmentally weak, gram-positive, aerobic, non-dynamic, urease-positive, catalase-positive, and partial acid-fast positive filamentous bacteria. Currently, a large number of new Nocardia species have been identified with the development of molecular biology, especially gene sequencing technology,14 in which 119 species of Nocardia have been documented (http://www.bacterio.net/nocardia.html), with more than 40 of them being considered clinically relevant (https://www.cdc.gov/nocardiosis/health-care-workers/index.html).10 Moreover, some cases have reported a hematogenous spread from the primitive site, leading to disseminated nocardiosis when at least two non-contiguous organs are affected.15 Notably, phagocytosis resistance is the main pathogenicity of an Nocardia spp. infection, wherein the filamentous stage has been reported to be more toxic among mouse macrophages by approximately 1000 times.16 Furthermore, N. farcinica is often considered to be the more toxic among the common Nocardia spp. and has inherent resistance to various antibiotics. Thus, infections caused by this specific organism are more prone to disseminated infection involving multiple organs, especially the brain abscess formation, leading to a high fatality rate. As such, we used the Web of Science and PubMed databases to retrieve reports on disseminated infections caused by Nocardia farcinica written in English from 2011 to 2021, and a total of 33 complete cases were included. The 33 cases were summarized and reviewed below, and all cases were shown in the Appendix.
General Demographics and Medical History Associated with Disseminated N. farcinica Infection
As summarized, disseminated N. farcinica infection was more prevalent in men than in women, with most of them occurring in immunocompromised patients (28 cases, 84.85%), and the mean age of the included patients was 58.52 ± 11.72 years. In terms of underlying diseases, SLE was the most frequent (5 cases), and other underlying diseases included but not limited to kidney transplant (3 cases), renal disease (3 cases), inflammatory bowel disease (2 cases), alcohol abuse (2 cases) and hematologic malignancy (2 cases) among others. In immunocompromised patients, steroid combined with immunosuppressive treatment was the highest risk factor for disseminated N. farcinica infection. In addition, chemotherapy, the use of biologics, and targeted therapy were also found to be high-risk factors for disseminated N. farcinica infection (Table 1).
 |
Table 1 Summary of General Demographics and Medical History of Disseminated N. farcinica Infection |
Identification of N. farcinica
In all 33 cases, specimens obtained through biopsy of the lesion was most valuable for positive results (11 cases). Other effective sample included purulent fluid (9 cases), blood (8 cases), pleural fluid (3 cases), bronchoalveolar lavage fluid (2 cases), sputum (1 case), and cerebrospinal fluid (1 case). Of these, 18 cases (54.55%) were confirmed as Nocardia farcinica infection based on molecular methodologies and 15 cases (45.45%) by traditional culture methods. The traditional diagnosis of Nocardiosis is culture but with some limitations. Nocardia spp. culturing usually takes 2–7 days to become positive, sometimes extending to at least two weeks for slow-growing species. Additionally, they are difficult to isolate from clinical samples, especially those including normal flora, such as lung samples (sputum and bronchoalveolar lavage fluid) and difficult to identification from different Nocardia spp. Given these difficulties in culture methods, molecular techniques, such as 16S rRNA gene sequencing, MALDI-TOF, and mNGS have become the most accurate methods for identifying Nocardia to the species level. In our statistics, 16S rRNA gene sequencing was the most commonly used (10 cases), followed by MALDI-TOF (4 cases) and mNGS (2 cases). By far, most of the information available in public databases was for the 16S rRNA gene sequencing. Although it considered to have higher sensitivity and accuracy, a major difficulty with using of 16S rRNA gene sequencing is the high level of sequence similarity among species, only a few base differences separate closely related species. In the past ten years, MALDI-TOF for the identification of Nocardia was widely used because of rapid and cost-efficient. MALDI-TOF MS, as the first commercially available system, has successful identification with common species, including N. farcinica, N. brasiliensis, N. cyriacigeorgica, N. nova/N. nova complex, and N. otitidiscaviarum in an accurate and rapid way.17 With characteristics of high sensitivity, rapid detection and less affected by prior antibiotics usage, mNGS is a new approach to identification for difficult and atypical infectious diseases. But the expensive cost may be a burden to some patients.18 In terms of our statistical data, there were still almost half of the cases using traditional culture methods to identification N. farcinica may be because of the expense and complexity for the above molecular methodologies.
Clinical Manifestations of Disseminated N. farcinica Infection
Clinical manifestations of nocardiosis are complex and varying. Disseminated N. farcinica infection usually has a subacute onset, and manifested as fever, cutaneous or subcutaneous abscess, productive or non-productive cough, dyspnea, chest pain, progressive fatigue, and weight loss. At the same time, metastatic abscesses caused by N. farcinica can also lead to systemic diseases such as septicemia, catheter-related infection, peritonitis, and endocarditis. The clinical features were summarized in Table 2. Among these cases, disseminated N. farcinica was found to invade and usually attack the lungs (28 cases, 84.85%), brain (21 cases, 63.63%), and cutaneous or subcutaneous layers (15 cases, 42.4%) (Table 3). Due to the high toxicity of the organism, we found that 13 cases (45.45%) were critical cases (5 cases were transferred to the intensive care unit, and 8 cases died due to ineffective treatments). Of the 13 critically ill patients, 8 (61.5%) had both the lungs and brain involvement. Therefore, the simultaneous involvement of the lungs and brain should be alarming for clinicians.
 |
Table 2 Summary of Clinical Manifestations About Cases with Disseminated N. farcinica Infection |
 |
Table 3 Summary of Involved Organs and Outcomes |
Treatment of Patients with Disseminated N. farcinica Infection
As previously established, N. farcinica is often the more toxic one among the common Nocardia spp., with inherent resistance to various antibiotics. To avoid the risk of recurrence, the duration of antimicrobial treatment usually needs to be extended in these cases. For patients with normal immune function, 6 months of antibacterial treatment was usually recommended, whereas, for those with a weakened immune system or with CNS disseminated lesions, at least 6 to 12 months were required. In all cured cases of this study, the average treatment time for disseminated infections was 11.25 ± 2.78 months.
For the treatment of N. farcinica, TMP-SMX was first-line therapy. As mentioned above, combined with the lungs and brain infection cases were more likely to develop into critical cases and have a higher mortality rate, thus, a greater importance is attached to the selection of treatment options for these patients. In this study, there were 12 cases with good therapeutic effects in both the lungs and brain involvement. Among the aforementioned cases, 10 cases (83.3%) were treated with TMP-SMX,10 cases (83.3%) were treated with carbapenem, and 8 cases (66.7%) were treated with a combination of TMP-SMX and carbapenem. Therefore, we suggest that the use of TMP-SMX and carbapenem should be considered as a priority in the absence of drug susceptibility tests for the accumulation when infected both lungs and brain. The use of antibiotics in all patients is displayed in Table 4.
 |
Table 4 Summary of Top 8 Effective Disseminated N. farcinica Therapies |
Notably, the increasing recognition of antibiotic resistance highlights the importance of Nocardia identification and sensitivity testing. In all 33 cases, 14 were tested for drug sensitivity, which was summarized for the purpose of experiential treatment and medication guidance for institutions without conditions for sensitivity testing (Table 5).
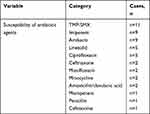 |
Table 5 Summary of Disseminated N. farcinica Antimicrobial Susceptibility Patterns |
Conclusion
As described above, the application of glucocorticoid combined with immunosuppressive treatment for membranous nephropathy was the most important risk factor for N. farcinica infection. We speculated that, due to the immuno-compromised condition of this patient, inhalational N. farcinica could successfully spread to the brain, the abdominal cavity and kidneys from the lungs through blood, thus causing a relatively rare N. farcinica disseminated infection. Due to the difficulty in diagnosis and high virulent, disseminated N. farcinica infection has extremely high mortality especially combining with brain abscesses. In terms of treatment, it could usually be persistent or recurrent due to insensitive antibiotics used and inadequate therapeutic course. This case provided insights for diagnostic methods and treatment strategies of disseminated N. farcinica infection. Meanwhile, we have gained an in-depth understanding of disseminated N. farcinica infection by literature reviewing and summing up relevant clinical cases, which can enable more patients to receive early diagnosis, correct treatment, and obtain a good prognosis.
Abbreviations
Nocardia spp., Nocardia species; N. farcinica, Nocardia farcinica; CDC, Centers for Disease Control and Prevention; MALDI-TOF, mass spectrometry-based methods; metagenomic next-generation sequencing (mNGS), EUS-FNA, endoscopic ultrasonography guided fine needle aspiration; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; CNS, central nervous system; CT, computed tomography; TMP-SMX, trimethoprim-sulfamethoxazole; WBC, white blood count; NE, neutrophils; CRP, C-reactive protein; PCT, procalcitonin; Fg, fibrinogen; D-D, D-dimer; CA-125, cancer antigen 125; CA-199, cancer antigen 199; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; NA, not available; AIDS, acquired immunodeficiency syndrome; SLE, systemic lupus erythematosus.
Acknowledgments
We would like to thank the department of respiratory and critical care medicine, the first hospital of China Medical University for the great health care services, helping and supporting this paper specially in the crisis of the COVID-19.
Disclosure
The authors report no conflict of interest in this work.
References
1. Derungs T, Leo F, Loddenkemper C, et al. Treatment of disseminated nocardiosis: a host-pathogen approach with adjuvant interferon gamma. Lancet Infect Dis. 2021;21(10):e334–e340. doi:10.1016/S1473-3099(20)30920-8
2. Han HJ, Kwak MJ, Ha SM, et al. Genomic characterization of Nocardia seriolae strains isolated from diseased fish. Microbiologyopen. 2019;8(3):e00656. doi:10.1002/mbo3.656
3. Wang A, Xu Q, Wang Y, Liao H. Orbital and intracranial Nocardia farcinica infection caused by trauma to the orbit: a case report. BMC Infect Dis. 2019;19(1):953. doi:10.1186/s12879-019-4605-z
4. Poonwan N, Kusum M, Mikami Y, et al. Antimicrobial-resistant nocardia isolates, United States, 1995–2004. Clin Infect Dis. 2010;51(12):1445–1448. doi:10.1086/657399
5. McGuinness SL, Whiting SE, Baird R, et al. Nocardiosis in the Tropical Northern Territory of Australia, 1997–2014. Open Forum Infect Dis. 2016;3(4):ofw208. doi:10.1093/ofid/ofw208
6. Valdezate S, Garrido N, Carrasco G, et al. Epidemiology and susceptibility to antimicrobial agents of the main Nocardia species in Spain. J Antimicrob Chemother. 2017;72(3):754–761.
7. Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009–2017. Diagn Microbiol Infect Dis. 2019;94(2):165–172. doi:10.1016/j.diagmicrobio.2018.12.007
8. Chhatwal P, Woltemate S, Ziesing S, et al. Molecular characterization and improved diagnostics of Nocardia strains isolated over the last two decades at a German tertiary care center. EXCLI J. 2021;20:851–862.
9. Bagüeste G, Porcel JM. Pleural infection caused by Nocardia farcinica: two cases and review of the literature. Cureus. 2021;13(4):e14697.
10. Mehta HH, Shamoo Y. Pathogenic Nocardia: a diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020;16(3):e1008280. doi:10.1371/journal.ppat.1008280
11. Grond SE, Schaller A, Kalinowski A, et al. Nocardia farcinica brain abscess in an immunocompetent host with pulmonary alveolar proteinosis: a case report and review of the literature. Cureus. 2020;12(11):e11494.
12. Özen Y, Dokuzoguz B, Mumcuoglu I, et al. Disseminated Nocardia farcinica infection presenting as a paravertebral abscess in a patient with systemic lupus erythematosus. Indian J Pathol Microbiol. 2019;62(2):329–331. doi:10.4103/IJPM.IJPM_178_17
13. Lee EK, Kim J, Park DH, et al. Disseminated nocardiosis caused by Nocardia farcinica in a patient with colon cancer: a case report and literature review. Medicine. 2021;100(29):e26682. doi:10.1097/MD.0000000000026682
14. Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ
15. Anagnostou T, Arvanitis M, Kourkoumpetis TK. Nocardiosis of the central nervous system: experience from a general hospital and review of 84 cases from the literature. Medicine. 2014;93(1):19–32. doi:10.1097/MD.0000000000000012
16. Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369–384.
17. Conville PS, Brown-Elliott BA, Smith T, Zelazny AM. The complexities of nocardia taxonomy and identification. J Clin Microbiol. 2017;56(1):e01419–17.
18. Jiao M, Deng X, Yang H, et al. Case report: a severe and multi-site nocardia farcinica infection rapidly and precisely identified by metagenomic next-generation sequencing. Front Med. 2021;8:669552. doi:10.3389/fmed.2021.669552
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.