Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Medications and Food Interfering with the Bioavailability of Levothyroxine: A Systematic Review
Authors Liu H, Lu M, Hu J, Fu G, Feng Q, Sun S , Chen C
Received 27 March 2023
Accepted for publication 19 June 2023
Published 23 June 2023 Volume 2023:19 Pages 503—523
DOI https://doi.org/10.2147/TCRM.S414460
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Hanqing Liu,1,* Man Lu,1,* Jiawei Hu,1 Guangzhao Fu,2 Qinyu Feng,3 Shengrong Sun,1 Chuang Chen1
1Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, People’s Republic of China; 2Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, Hubei, 430060, People’s Republic of China; 3Department of Orthopedics, Zhongnan Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chuang Chen; Shengrong Sun, Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan University, Jiefang Road 238, Wuhan, 430060, People’s Republic of China, Tel/Fax +86 27 88041911, Email [email protected]; [email protected]
Purpose: Levothyroxine is a common prescribed drug. Many medications and food, however, can interfere with its bioavailability. The aim of this review was to summarize the medications, food and beverages that interact with levothyroxine and to assess their effects, mechanisms and treatments.
Methods: A systematic review on interfering substances that interact with levothyroxine was performed. Web of Science, Embase, PubMed, the Cochrane library, grey literature from other sources and the lists of references were searched for human studies comparing the levothyroxine efficacy with and without interfering substances. The patient characteristics, drug classes, effects and mechanism were extracted. The NHLBI study quality assessment tools and the JBI critical appraisal checklist were used to assess the quality of included studies.
Results: A total of 107 articles with 128 studies were included. Drugs interactions were revealed in calcium and iron supplements, proton pump inhibitors, bile acid sequestrants, phosphate binders, sex hormones, anticonvulsants and other drugs. Some food and beverage could also induce malabsorption. Proposed mechanisms included direct complexing, alkalization, alteration of serum thyroxine-binding globulin levels and acceleration of levothyroxine catabolism via deiodination. Dose adjustment, administration separation and discontinuation of interfering substances can eliminate the interactions. Liquid solutions and soft-gel capsules could eliminate the malabsorption due to chelation and alkalization. The qualities of most included studies were moderate.
Conclusion: Lots of medications and food can impair the bioavailability of levothyroxine. Clinicians, patients and pharmaceutical companies should be aware of the possible interactions. Further well-designed studies are needed to provide more solid evidence on treatment and mechanisms.
Keywords: L-T4, drug, interference, malabsorption, side effect, solution
Introduction
Levothyroxine (LT4) is among the most prescribed medications in the world and the mainstay treatment for hypothyroidism.1,2 Generally, a 1.6–1.8 μg/kg/d of oral LT4 is recommended to be ingested on empty stomach early in the morning. Although it is cost-saving, easy-to-take and has many other advantages, its bioavailability has been reported to be altered by lots of medications. The interference can elevate thyrotropin (TSH) and even decrease triiodothyronine (T3) and thyroxine (T4) despite dose elevation (>1.9 μg/kg/d). A retrospective population analysis based on a local database in the UK revealed that 58.9% of LT4-treated patients were concurrently taking ≥1 drug for other medical conditions.3 The most frequently prescribed concomitant drugs were statins (17.6%), proton pump inhibitors (PPIs) (13.6%), and calcium supplements (6.8%). Another database study in Colombia revealed a similar result.4 A percentage of 68.5% of LT4-treated patients took other drugs. The spectrum of concurrent agents was somewhat different from the study in the UK, which may be explained by the differences in the spectrum of diseases and prescription preference. The antiulcer agents (44%) were the leading concurrently taking ones, followed by hypoglycemics (17.4%), and iron supplements (16.1%). Though the majority of patients with co-ingestion were asymptomatic and received constant doses of LT4, it would be up to 12–15% of total LT4-treated patients suffering unsuppressed TSH if 1 in 5 had drug interactions. Besides, some food and beverages could also impair the absorption of oral LT4. Since the concomitant intake of food or certain drugs can either directly bind with LT4 or impair its solubility, a 0.5–2 hours dosing gap is generally required for the empty of stomach.5 The LT4 liquid preparation intrinsically avoid dissolution and can recover impaired bioavailability due to concomitant intake.
The diagnosis of drug interaction depends on comprehensive medical history taking and necessary examinations. Structural abnormalities, such as gastritis, celiac disease and lactose intolerance, should be excluded. Drug withdrawal test (tests for serum thyroid hormones before and after the discontinuation of suspected interfering substance) and LT4 absorption test (after ingesting a certain dose of LT4 on empty stomach with or without suspect interfering substance, serum thyroid hormones are tested continuously for 6 hours) are helpful tools.6
This comprehensive review summarized the drugs, food and beverages that alter the bioavailability of LT4 supplements. The effects, proposed mechanisms and recommended therapy were investigated. The review then discussed the current knowledge of novel formulations on drug-induced malabsorption. The limitations of included articles were also investigated. The systematic review aims to help clinicians, patients and pharmaceutical companies to avoid drug or food interactions with LT4.
Materials and Methods
A systematic review was conducted on Feb. 27th, 2022 according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 checklist (Supplement 1).7 One researcher (HQ. Liu) with meta-analysis experience drafted the search strategy, which was revised and approved by other authors (Supplement 2). The searched databases included Web of Science (WOS), PubMed, Embase, and the Cochrane Library. Other resources such as Drugs.com, Google Scholar, UpToDate and the prescribing information of commercial formulations were also searched. The following keywords were arranged in the search sentence: (levothyroxine OR thyroxine OR L-T4 OR LT4) AND (malabsorption OR interaction). There was no restriction on published date or language. The lists of references were also screened to identify any missed studies. The literature was imported into Endnote X9 for subsequent selection and data extraction.
Two independent reviewers (JW. Hu & GZ. Fu) screened the articles. Disagreements were resolved by a superior reviewer (QY. Feng). Articles meeting the following criteria were included: a) hypothyroid patients or euthyroid individuals concomitantly ingest levothyroxine with other medications, food or beverages, b) thyroid hormone levels or daily LT4 doses were collected, c) the alterations were revealed to or supposed to be induced by drug or food interference. Exclusions were a) review or editorial, b) non-human studies, c) participants did not dose levothyroxine, d) patients on LT4 could not be separated from the total sample. The following items were extracted from the included articles by two other reviewers (HQ. Liu & M. Lu): authors, published year, country, study type, participant number, thyroid state, concomitant disease, LT4 formulation and dosage, interfering drugs or food, effects on thyroid function, and proposed mechanism. The main outcomes were TSH. Additional outcomes included T3, T4, pharmacokinetics properties and LT4 dose. Due to the heterogeneity of included studies, quantitative analysis was not performed. This systematic review has been registered in PROEPERO database (NO. CRD42022314315).
The NHLBI study quality assessment tools were used to assess the quality for randomized controlled trials, pre-post studies, observational cohort studies and case series.8 The revised Cochrane risk-of-bias tool for randomized crossover trials (RoB 2) were used to verified the results.9 The JBI’s critical appraisal tool for case reports was used to evaluate the quality of case reports.10 The study characteristics, such as randomization, blindness, baseline comparability, dropout, outcome measurements, patient selection, were assessed according to guidelines. The assessment was conducted at both outcome and study levels. Studies were not excluded a priori based on quality reporting assessment. Two independent investigators (HQ. Liu & M. Lu) conducted the assessment. Disagreements were resolved by a superior reviewer (QY. Feng).
Results
Literature Selection
A number of 10,748 articles were identified from four databases and 17 from other sources or lists of reference. After duplicate exclusion and reviews on titles and abstracts, 156 articles were processed to full-text review. Of note, three full texts were not available and excluded from subsequent analysis.11–13 Finally, 107 articles were included in the data extraction while the other studies met the exclusions (Figure 1 and Supplement 3). Specifically, the review consists of 15 randomized trials, 40 prospective pre-post studies, 3 prospective cohort studies, 20 retrospective pre-post studies, 7 retrospective cohort studies, and 43 case series or case reports (Table 1). Notably, several studies of different designs may compose one article.
 |
Table 1 Summary of Mechanisms of Interfering Substances and Recommendations for Clinicians |
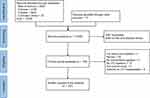 |
Figure 1 The flow chart of literature selection. |
Medications Binding with LT4
Some medications are revealed or supposed to interfere with the absorption of LT4. Certain ingredients or excipients in interfering medications could bind to the LT4 molecules and inhibit their transmembrane transport. Examples include calcium and iron supplements, basic antacids, bile acid sequestrants, etc. The treatments for malabsorption induced by chelation are similar in many ways.
Calcium Supplements
As revealed by a population study in the UK, calcium supplement ranks the third place among the most prescribed concomitant medications in LT4-treated patients.3 A proportion of 6.8% of patients took calcium supplements to address osteoporosis. The earliest case report of drugs interaction between LT4 and calcium is in 1998 by Schneyer.21 The three patients in this report successfully eliminated the malabsorption by either discontinuation of calcium salts, or introducing dosing intervals. In 2000, Singh et al recruited 20 hypothyroid patients.15 On a stable dose of LT4 and regular thyroid hormone levels, each patient was given 1200 mg of calcium to ingest daily with levothyroxine in the morning on an empty stomach. The thyroid functions were tested at two months and three months after dosing calcium. Calcium was then discontinued and thyroid functions were followed up for another two months. Free T4 and total T4 were significantly decreased when taking calcium (1.22±0.05 vs 1.41±0.06 ng/dL, p<0.001, 8.55±0.41 vs 9.31±0.39 µg/dL, p=0.03, respectively), and TSH levels were elevated (2.71±0.43 vs 1.44±0.21 mU/L, p=0.008). They further diluted radioisotope-labeled LT4 into buffer solution with calcium carbonate. Samples were collected from the supernatant after 2-hour incubation and 10-min centrifugation. The percentage of LT4 in the supernatant was less than 60% at pH 2.0. Interestingly, LT4 molecules remained unbound at pH 7.4. The in vitro experiments confirmed the interaction of LT4 and calcium via complexing.
One year later, the interaction was further confirmed by a randomized crossover study by the same group of investigators.14 Singh et al recruited seven healthy individuals and gave them 1000 µg of oral LT4 with or without 2.0 g of calcium carbonate. Two LT4 absorption tests (dosing LT4 on empty stomach and testing thyroid functions for 6 hours) were conducted with a washout period of 1 month. LT4 absorption decreased by 31.0% when simultaneously dosing calcium supplements (579 vs 837 µg). With the same methods, one study suggested that the interaction is not limited to calcium carbonate, but other supplements (eg, calcium citrate and calcium acetate) as well.16 However, another retrospective long-term study revealed that calcium acetate was associated with no alteration on TSH.18 The discrepancy may be explained by study designs and long-term or short-term effects. The interaction between oral LT4 and calcium supplements is also supported by several other observational studies or case reports.3,19,22,23
Several methods can eliminate the malabsorption due to calcium supplements. Given that only a small number of calcium-treated hypothyroid patients have reported TSH elevation of clinical importance,3 we could postulate with reason that most patients remain euthyroid without additional medical interference. In some cases, a dose elevation is sufficient to achieve euthyroidism. In cases with significant TSH elevation or severe malabsorption, the dosing separation or discontinuation of interfering medications is recommended. A switch of calcium supplements to other products of the same class may also work, as suggested by the study of Diskin et al.18 Recently, novel LT4 formulations (liquid solution and soft-gel capsule) have been proved to circumvent the calcium-induced malabsorption since disintegration was not needed for these preparations.17,20 TSH levels were much lower in the solution group in comparison with the tablet group (8.74±7.2 vs 2.15±1.4 mU/L, p<0.001). These studies showed a switch to novel formulations as a promising treatment. Of note, concomitant gastrointestinal diseases could exaggerate the malabsorption24 and eradication of these conditions should also be taken into consideration.
Iron Supplement
Besides, ferrous sulfate is another common drug interfering with LT4. In a Colombian study retrospectively screening ~30 thousand hypothyroid patients, 16.1% of them concomitantly took iron salts.4 The discovery of the interaction between iron and LT4 is even earlier.25 In 1992, Campbell et al recruited 14 hypothyroid patients and ordered them to ingest ferrous sulfate with LT4 for 12 weeks. The serum TSH rose from 1.6±0.4 to 5.4±2.8 mU/L at the end of the observation. An in vitro experiment mixing iron with thyroxine observed a poorly soluble purple complex, which indicated the binding of iron to LT4. Elevated TSH levels were also observed in two female cases receiving ferrous sulfate due to pregnancy and anemia.26,27
The treatment for iron-induced malabsorption is similar to that of calcium supplements. Separation of dosing and discontinuation of iron supplements can eliminate the malabsorption in most cases. Oral solutions can also circumvent the problem. Benvenga et al revealed that when simultaneously ingesting, TSH levels were lower iron in the solution group compared to the tablet group (8.74±7.2 vs 1.68±0.9 mU/L, p<0.001).17 Close monitoring is needed until thyroid hormones return to the target ranges.
Basic Antacids
In 1992, Sperber and Liel reported a male hypothyroid patient presenting with elevated TSH (36 mU/L) due to co-ingestion of aluminum hydroxide.38 The TSH level dropped to 4.63 mU/L after separating dosing times, and furtherly decreased to 1.0 mU/L after the discontinuation of the antacid therapy. Two years later, these investigators recruited five hypothyroid patients and conducted a prospective, one-arm, 4-week study. A significant increase in serum TSH when patients co-ingested aluminum hydroxide was observed (7.19±1.3 vs 2.62±0.8 mU/L, p=0.012). The in vitro experiment revealed a linear relationship of LT4 adsorption to the concentration of aluminum hydroxide. Another study also reported two patients developing hypothyroidism despite high doses of LT4 after the ingestion of magnesium oxide.37 The mechanism was supposed to be the same with aluminum hydroxide. Liquid solutions could ameliorate the antacid-induced malabsorption.118
Sucralfate
Sucralfate is a gastric lumen adherent cytoprotective. It adheres to ulcer sites and protects them from acids and enzymes. In 1992, Havrankova et al reported a case with malabsorption caused by sucralfate for the first time. The in vitro study revealed a very high binding capacity of sucralfate for LT4. However, the responding letter from Khan et al, who conducted a study among 10 hypothyroid patients dosing sucralfate, revealed no positive results on the T4 and TSH levels.43 In 1994, Campbell et al conducted a randomized crossover trial with a washout period of 4 weeks.40 Nine patients with primary hypothyroid received either sucralfate 1 g 4 times daily or placebos. The T4 index was lower with sucralfate (p=0.038) and TSH was borderline higher (p=0.097). Using LT4 absorption tests, the study by Sherman et al revealed 71.7% of peak LT4 absorption reduction in healthy volunteers co-ingesting sucralfate.41
Bile Acid Sequestrants
Cholestyramine is a common bile acid sequestrant for the treatment of hypercholesterolemia. The first case in 1969 was an 18-year-old girl with hypothyroidism and hypercholesterolemia.52 Her basal metabolic rate fell with a constant dose of LT4. Drug withdrawal test and in vitro binding experiment strongly supported the interaction with cholestyramine. A similar case with an in vitro experiment was reported 22 years later.53 Colesevelam was a newer medication approved by the FDA. In 2009. Weitzman et al conducted LT4 absorption tests in 6 healthy volunteers with a 3-week washout period.50 The T4 area under the curve (AUC) in the colesevelam group decreased to 3.8% of that with LT4 alone. An in vitro binding test showed that 67% of LT4 is bound to colesevelam in simulated gastric juice.51
Phosphate Binders
Sevelamer and lanthanum carbonate is used to lower high blood phosphorus levels in those who are on dialysis due to severe kidney disease. The LT4 absorption tests conducted by John-Kalarickal et al showed that the AUC with 10 mg of sevelamer co-ingestion decreased to 50.4% of the area for LT4 alone (p<0.05).49 Lanthanum carbonate could also decrease the T4 AUC to 59.1% of the area of LT4 alone.50 A retrospective study and a case report supported the conclusion.18,61 Although the most probable mechanism of drugs interaction is direct complexing, there was no in vitro experiment supporting the hypothesis yet.
Other Medications
There were some reports of other medications interfering with the absorption of LT4 by direct complexing. In 1993, a female case with hypothyroidism dosed sodium polystyrene sulphonate for the treatment of hyperkalemia and hemodialysis.60 After the daily treatment with 15 g of sodium polystyrene sulphonate, her free T4 decreased to 3.5 pmol/L (reference range 10–25) and TSH increased to 139 mU/L. Subsequent in vitro study showed that the cation-exchange resin reduced the concentration of dissolved thyroxine by ~95%, regardless of pH. Besides, orlistat and simethicone could also impair the bioavailability of LT4 by intraluminal binding.54,55 The malabsorption was resolved by either discontinuation of interfering drugs or separation of dosing times. In addition, some other drugs with different interfering mechanisms, such as H2 antagonists, chromium picolinate and raloxifene may also bind with LT4 molecules in intestinal tracts.
Medications Inducing Alterations in Mucosal Transport Processes
Previous studies have demonstrated LT4 molecule is transported by transmembrane proteins, such as organic anion transporting polypeptide (OATP) family, Na-taurocholate co-transporting polypeptide, L-type amino acid transporter, and monocarboxylate transporter.120–122 Some drugs may alter the distribution or expression of transmembrane proteins and thus affect the absorption of LT4.
Ciprofloxacin has been shown to be transported by OATP1A2.123 In 2005, Cooper et al reported for the first time that two LT4-treated patients had developed malabsorption after dosing ciprofloxacin.71 The TSH levels returned to the reference range after introducing a 6-hour dosing gap. Eight years later, a randomized double-blind crossover trial using the LT4 absorption tests was conducted by Goldberg et al.70 Coadministration of ciprofloxacin significantly decreased the T4 AUC by 39% (p=0.035). The potential mechanism is competing transports of both ciprofloxacin and LT4 by OATP.
Rifampin is a well-known OATP inhibitor.124 Presumptuously, it decreases the intestinal absorption of LT4. Surprisingly, the trial by Goldberg et al showed an opposite result.70 The 6-hour LT4 absorption tests revealed that rifampin significantly increased the T4 AUC by 25% (p=0.003). The result was hard to explain. It is postulated that rifampin could preferentially inhibit the hepatic OATP transporters relative to intestinal ones. The liver catabolism is thus reduced. However, two previous case reports did not support the favorite effect of rifampin on LT4.68,69 Two patients who co-ingested LT4 and rifampin presented with elevated TSH and decreased T4 levels. Thyroid hormones returned to reference range only after the withdrawal of rifampin. Nolan et al postulated the induction of cytochrome P-450 accounted for the alteration.69 The effects of rifampin on LT4 remain unclear up to now. More than one mechanism may play roles and there may be a discrepancy between the long-term and short-term effects of rifampin.
Besides, chromium picolinate is also supposed to reduce LT4 absorption by altering the expression of intestinal transporters,49 although there is currently no evidence supporting the hypothesis.
Medications Altering the Gastric pH
A physiological gastric pH is essential for tablet dissolution, which removes the sodium ion and increases its solubility. Elevated pH due to H. pylori infection and autoimmune gastritis ameliorate the subsequent absorption.28,32,125 The phenomenon is explained by ionization status at different environmental pH.126–128 PPIs and H2 antagonists, which reduce the secretion of H+, are used in the treatment of gastric and duodenal ulcers. These drugs can impair the dissolution of LT4 tablets.
Proton Pump Inhibitors
A percentage of 13.6% of LT4-treated patients receive PPIs concomitantly.3 In 2006, Centanni et al reported the impaired absorption of LT4 due to PPIs for the first time.32 In this retrospective single-center study, a subgroup of 10 patients with nontoxic multinodular goiters concomitantly ingested omeprazole and LT4. TSH levels were significantly higher in patients with omeprazole, as tested during routine follow-ups (1.7 vs 0.1 mU/L, p=0.002). In the same year, another group of investigators recruited 20 volunteers and conducted a randomized, open-labeled, crossover study with a 4-week washout period.34 Participants ingested 4 µg/kg of LT4 with or without 40mg of pantoprazole. Samples were taken every fifteen minutes during the first three hours and every sixty minutes over the next seven hours. The 10-hour LT4 absorption test revealed no significant difference on serum free T4, total T4 and TSH levels. A prospective self-control study in 2008 did not support the drugs interaction either.35 The effects of PPIs on LT4 were conflicting. The discrepancy may be explained by different drugs, and long- or short-term effects. In addition, the healthy volunteers have thyroid function within reference range, which can compensate for the impaired absorption of LT4.
Recent studies provide more information. In 2015, Yue et al conducted a similar randomized, open-labeled, crossover, two-arm trial in 16 healthy individuals using 24-hour LT4 absorption tests.28 These participants received intravenous injections of esomeprazole in either Period One or Period Two. The maximum concentration (Cmax) decreased to 87.32% of that with LT4 alone and AUC0-12h decreased to 83.22% (p<0.1). Besides, several retrospective studies with large samples supported the drugs interaction between PPIs and LT4.3,30,31 However, another prospective study involving 21 hypothyroid patients revealed contradicting results. Patients with either 20 mg or 40 mg of omeprazole showed slightly elevated TSH with no significant difference.36 To conclude, six out of the total nine clinical studies mentioned above supported the drugs interaction. In the remaining three articles, one article studying the long-term effect of omeprazole showed an elevated TSH without statistical significance. The other two studies using LT4 absorption tests reveal no significant difference. Another systematic review also supported the drug interactions.129 Except for alkalization, PPIs may also impair absorption by increasing the biliary clearance of LT4 via induction of UDP- glucuronosyltransferase.32
Interestingly, although there are many studies on the interaction between PPIs and LT4, only one case report has been published.33 The female patients dosed LT4 due to Hashimoto’s thyroiditis. Her TSH level rose above the reference range when she began dosing 40 mg/day of omeprazole. The hypothyroid symptoms resolved and the TSH dropped after she switched LT4 tablets to soft-gel capsules without dose adjustment. Two following articles studied the long-term and short-term effect of PPIs on liquid LT4.28,29 The results suggested that liquid LT4 and soft-gel capsules could eliminate the malabsorption induced by PPIs. Moreover, clinicians can instruct patients to switch to other PPIs or antacids.
Histamine H2-Receptor Antagonists
The indications of H2 antagonists are similar to that of PPIs. In 1992, Jonderko et al conducted 4-hour LT4 absorption tests in 20 individuals randomized assigned to cimetidine/ranitidine or placebo.44 Cimetidine was revealed to decreased AUC significantly (371±72 vs 467±82, p<0.01), while ranitidine did not (477±132 vs 459±109, p>0.05). Sixteen years later, a clinical trial with similar designs and settings evaluated the interaction between famotidine and LT4.35 No significant difference on pharmacokinetics properties was detected. Moreover, a retrospective database study with a large database did not support the interaction either.3 Hence, the interaction of the two drugs remains unknown. If a patient is suspected of malabsorption of LT4 due to cimetidine, a change to other H2-antagonists is helpful.
Vitamin C
Vitamin C is a common over-The-counter drug and can stimulate the secretion of gastric acid. It is assumed to improve the efficacy of LT4 by increasing the gastric dissolution. Antúnez et al conducted a 6-week prospective pre-post study on 28 hypothyroid patients.90 TSH levels were significantly reduced after 1 g/day of vitamin C ingestion (9.01±5.51 vs 2.27±1.61 mU/L, p<0.0001). It is postulated that vitamin C can eliminate the malabsorption in patients with elevated gastric pH, such as gastritis or PPIs. The study of Jubiz et al supported the hypothesis in 2014.91 They recruited 31 patients with hypothyroidism and gastritis, and ordered them to take 500 mg/day of vitamin C for 2 months. The median TSH level decreased from 11.1 to 4.2 mU/L after the co-ingestion of vitamin C (p=0.0001). Hence, vitamin C may be a promising therapy for those with LT4 malabsorption due to elevated gastric pH.
Medications Affecting the Transport LT4 in the Systemic Circulation
A percentage of ~99.8% of T4 and T3 are bound to serum carriers, such as thyroxine-binding globulin (TBG), albumin and transthyretin. The TBG binds more than 80% of thyroid hormones. An elevation in TBG reduces the free T3 and free T4 and leads to hypothyroxinemia. A reduction of TBG works in the opposite way.
Increased need for LT4 was observed in pregnant women and liver cirrhosis.130,131 The possible cause is the elevated serum TBG due to higher estrogen levels. In 2001, Arafah recruited 25 hypothyroid postmenopausal patients with indications for estrogen.66 The thyroid hormones levels were evaluated at baseline and 12 weeks after the initiation of estrogen. The TBG rose from 20.8±3.1 to 30.8±4.0 mg/L at 12 weeks (p<0.001). As a consequence, the free T4 decreased from 1.7±0.4 to 1.4±0.3 ng/dL and TSH rose from 0.9±1.1 to 3.2±3.1 mU/L (p<0.001). Elevated TSH was also observed in 483 LT4-treated patients dosing estrogen.3 Taken together, estrogen supplements can increase the serum TBG, decrease free T4 and T3, and thus induce hypothyroxinemia. Discontinuation of drugs and dose adjustment of LT4 are required to eliminate the impaired bioavailability.
Besides, rifampin, raloxifene and carbamazepine may also elevate serum TBG.63,64,70,76 The elevated TSH levels returned to reference ranges after the separation dosing of raloxifene and LT4 in the two cases. Further studies are needed to verify the mechanisms of drugs interactions.
Presumptuously, on the contrary, androgen decreases the TBG and causes hyperthyroxinemia. The hypothesis was supported by Arafah in 1994.67 Using the same protocol as mentioned above (Arafah, 2001), the study witnessed a decreased TBG by over 50% (p<0.001), increased free T4 and decreased TSH in all included patients. The investigator recommended LT4 doses to be reduced by 25% to 50% to maintain euthyroidism.
Some drugs can compete for the hormone-binding sites. The replacement reduces total T4 and increases renal clearance. In 1970, Larsen et al conducted an in vivo study in five healthy volunteers with radioisotope labeled thyroxine and phenytoin.77 The free T4 decreased and the clearance of T4 increased by 20%. Considering that TBG remained constant and urinary and fecal excretion increased, it is postulated that phenytoin inhibits LT4 absorption and displaces it from serum proteins. Subsequent studies supported the hypothesis.80
Medications Altering the Catabolism of LT4
In livers and other peripheral organs, T4 is degraded via the deiodination route or the non-deiodination route. Consequently, T4 is transformed into bioactive hormone, T3, or other inactive metabolites. The T3:T4 and reverse T3:T4 ratio could indicate alterations of the catabolism of T4.
Carbamazepine is a drug for the treatment of seizures. A retrospective study by Deluca et al in 1986 evaluated the efficacy of oral LT4 in five children with epilepsy. After two months of carbamazepine administration, the T4 decreased from 12.7±1.1 to 7.5±2.3 µg/dL. The T3:T4 ratio elevated significantly (p<0.05), while the change of T3 was not observed. Consequently, persistent hypothyroidism was most likely be caused by the accelerating turnover of T4 to T3 due to carbamazepine.
Fluoxetine and sertraline are selective serotonin reuptake inhibitors for the treatment of depression. In a prospective study by de Carvalho et al, fluoxetine could reduce the T4 over the 90-day period and T3 transiently for 30 days in healthy volunteers.81 Though no significant difference was observed in hypothyroid patients, it was assumed that fluoxetine could activate the deiodinase. Another case series with sertraline also supported the mechanism.82
Sorafenib is a multi-kinase inhibitor for advanced cancers. In a prospective, open-label, 26-week study, Abdulrahman et al evaluated the effect of sorafenib on LT4 and type III deiodinase, which turns T4 to reverse T3 (rT3).87 The thyroid hormones were tested and compared with baseline values. The T3:T4 ratio decreased (p<0.001) and rT3:T3 and rT3:T4 ratios increased (p<0.001, p=0.036) at the end of the study. The results strongly indicate that sorafenib accelerates the deiodination of T4. Elevated TSH due to another multi-kinase inhibitor, motesanib, was supposed to work in the same way.88
Besides, two other studies on propranolol and amiodarone also observed elevated TSH with decreased T3:T4 ratio.83,84 However, the rT3 was not tested in the two studies. Further observational studies are needed to provide more information.
Other drugs, such as mifepristone, capecitabine and rifampin are also supposed to interact with T4 in the same way.65,68,69,86 However, T3:T4 or rT3:T4 ratios were not calculated in these case reports and there was no evidence to support that these drugs could activate deiodinases. T4 could be degraded via the non-deiodinase route. Several drugs, such as rifampin, phenytoin, imatinib, statins and protease inhibitors, are supposed to affect this route.45,46,68,69,72–75,80,89 Although TSH levels were revealed to be elevated and patients had to dose more LT4, no direct evidence supported the hypothesis and the mechanism remains unclear. Drug withdrawal and dose adjustment are generally recommended to eliminate hypothyroid symptoms due to these drugs.
Other Drugs
Metformin
The TSH suppression by metformin was reported in a case series in 2006.59 All of the four patients with diabetes administered LT4 for primary hypothyroidism. TSH levels dropped to lower than 0.50 mU/L after the administration of metformin. Two subsequent observational studies revealed similar results.56,58 Interestingly, metformin suppressed the TSH (2.37±1.17 vs 1.41±1.21 mU/L, p<0.001), whereas the T3 and T4 levels remained unaltered. The mechanisms of drugs interactions are supposed to be a) reducing body weight, b) enhancing the inhibitory modulation of thyroid hormones on central TSH secretion, c) changing the affinity and/or the number of thyroid hormone receptors. The LT4 absorption tests observed no effect of metformin on the absorption of oral LT4.57 In 2019, a meta-analysis including 6 randomized controlled trials and 494 patients revealed that metformin could significantly reduce TSH levels but had no effect on serum free T3 and free T4 levels.132 The exact mechanism remains to be explored.
Medications with No Interference or Lacking Direct Evidence
Randomized crossover trials or pre-post self-control studies have revealed no LT4 interference by some drugs, such as ezetimibe, alendronate and glucocorticoid (Figure 2).3,35,47–49,62 Some other drugs are revealed to alter the thyroid hormone levels in non-LT4-treated patients, animals or in vitro studies.133,134 Examples include colestipol, phenobarbital, nicardipine, chloroquine, tamoxifen, nonsteroidal anti-inflammatory drugs, etc. Further studies are needed to test the effects of these drugs on the efficacy of LT4.
Food and Beverages
It is widely recognized that LT4 tablets should be ingested on empty stomach early in the morning, 30–60 mins before breakfast. The interval enables adequate gastric dissolution and avoidance of LT4-food chelation. Randomized open-label crossover trials in the USA and Brazil have confirmed that TSH concentrations were significantly higher in patients taking LT4 tablets with breakfast for 2–3 months.92,93 The LT4 absorption tests uncovered the acute effects of breakfast on LT4 absorption.94 The Cmax decreased by 40–49%, and the T4 AUC0-48h decreased by 38–40% in the co-ingestion groups. In patients with enteral feeding, co-ingestion also elevated TSH levels.101
One may suppose that the compositions of diet have influences on LT4 absorption. However, studies disapproved the hypothesis. Using LT4 absorption tests, Wenzel et al revealed that both lactose and corn starch could decrease LT4 absorption (with or without lactose: 59.0±9.0% vs.79.9±6.4%, p<0.001; with or without corn starch: 68.2±10.4% vs 78.6±8.6%, p<0.001).96 A retrospective cohort study revealed elevated TSH levels in the soy diet group compared to the non-soy diet group (p=0.02).102 The interaction between LT4 and soy diet was supported by several case reports.103–105 Notably, both soy milk and soy-based formula can impaired the bioavailability of LT4 in congenital hypothyroid infants.104 Since those infants were less able to maintain their thyroid homeostasis, the effect soy supplements in babies was more severe in comparison with adults on LT4. A long duration of soy supplements was associated with higher risk of thyroid dysfunction, as revealed by a systematic review.135 Interestingly, soy isoflavones were revealed to transiently increase reverse levels in a randomized controlled trial.136 The phenomenon could be explained by inhibited deiodinase 1 and/or 2 activities by isoflavone supplements. Besides, Liel et al reported 13 patients developing LT4 malabsorption due to fiber-rich diets.106 The adsorption was proved by a subsequent in vitro experiment. To conclude, the carbohydrate-rich diet, fiber-rich diet and protein-rich diet can impair the absorption of LT4 tablets.
Beverages can also induce LT4 malabsorption. Using LT4 absorption tests, two prospective studies reveal that coffee and milk decreased LT4 absorption by ~32% and ~10% respectively.97,98 A case report also revealed similar results.107 Coffee and milk are supposed to interfere with LT4 via adsorption. Another common beverage, grapefruit juice, may interfere via inhibition of uptake transporters in the intestinal wall. As a randomized, open-label, crossover trial revealed, grapefruit juice can decrease T4 AUC0-4h by 13% (p<0.05).95 In addition, one case report showed that papaya could interfere with the absorption of oral LT4.108 The mechanism is supposed to be the reduction of gastric acid secretion and direct binding by papaya juice. Further experiments are needed to prove the proposed mechanisms.
The standard therapy for food-induced malabsorption is patient education and separation of ingestion. However, the ingestion separation can cause inconvenience and reduce patient compliance. The introduction of liquid solution and soft-gel capsule provides more convenient therapy. In 2016, a randomized, double-blind, placebo-controlled, crossover trial was conducted in 77 hypothyroid patients with liquid LT4.110 No significant difference was detected on T3, T4 and TSH in the two 6-week periods. Recent studies also supported the superiority of solution and soft-gel capsules over tablets when taken with food and coffee.99,100,112–117 From a clinical standpoint, clinicians could recommend novel formulations to those who have difficulty taking drugs on empty stomachs.
Quality Assessment
In the 15 included randomized controlled trials, seven were rated as good quality (Appendix 4 Supplemental Tables 1–6), while the remaining ones were of fair quality. The main factor that affected the quality was non-blindness. Of the 60 pre-port studies, seven were rated as good quality, 48 were of fair quality and 5 were or poor quality. The main affecting factors were patient selection, non-investigator blindness and not multiple outcome measurements. Of the 10 observational cohort studies, three were of good quality and seven of fair quality. Affecting factors included patient selection, outcome assessed prior to exposure measurement and non-investigator blindness. The quality of case series and case reports were also assessed and the results were presented in Appendix 4 Supplement Tables 1–6.
Discussion
The above parts provide a comprehensive discussion on the types, effects, proposed mechanisms and treatments of interfering drugs, food and beverages. There are two other topics worth discussing: a) the malabsorption due to which medications have been proved to be eliminated by novel formulations, and b) which factors reduce the quality of the included articles.
Liquid Solution and Soft-Gel Capsule
Novel formulations (liquid solutions and soft-gel capsules) are the most exciting advance in the levothyroxine field during the past 15 years. Liquid solutions are composed of 96% ethanol, 85% glycerol and other excipients. Ethanol can be totally replaced by glycerol to avoid possible adverse effects on pregnancy and liver. There are two forms of oral solutions in the markets, namely, pre-dosed ampoules and oral drops. The manufacture of capsules is more complex. The LT4 powder is dissolved in glycerin solvent and then encapsulated with a soft gelatin shell. After drying, a translucent flat oval capsule is produced.137 Disintegration and dissolution, which are essential for tablets, are not needed for solutions. Both in vitro and in vivo studies revealed a rather faster dissolution profile of soft-gel capsules compared with tablet one.138,139 Consequently, novel formulations could reach plasmatic Cmax quicker and higher than tablets with a higher gastrointestinal absorption.140 Novel formulations can be considered in subjects with hampered LT4 absorption or who do not allow sufficient time before or after meals for LT4 replacement”.141
As revealed by our review, novel formulations can eliminate the malabsorption due to calcium and iron supplements, basic antacids, sevelamer and PPIs (Figure 2). Another previous review also suggested that liquid formulations could circumvent gastric pH impairment due to PPIs, calcium or iron supplements, sevelamer, aluminum/ magnesium hydroxide and sodium alginate.142 It is easy to notice that these drugs work via direct complexing or alkalization. Presumptuously, novel formulations can ameliorate the LT4 malabsorption due to drugs of the same mechanisms, such as sucralfate, orlistat, phosphate binders, bile acid sequestrants and H2 antagonists. Subsequent trials can prove or disapprove our hypothesis.
Limitations of Included Articles
Although there are some well-designed randomized trials and prospective pre-post studies, the majority of included articles are of moderate or low levels of evidence. Lots of studies are retrospective studies, case series or case reports. Besides, there are some problems in the study designs, such as small sample, no blindness, improper participants (healthy volunteers) and selection outcome report.
Besides, many articles have proposed hypotheses to explain the interactions. Some mechanisms, especially those accelerating the degradation of LT4, remain to be confirmed by in vitro or in vivo experiments.
There were some similar articles on this topic. In 2017, Skelin et al conducted a systematic review on the factors affecting gastrointestinal absorption of LT4.143 A total of 109 original articles were included in the final analysis. This systematic review comprised three major sections, namely, gastrointestinal disorders, food and drugs. In the first section, several diseases, including celiac disease, lactose intolerance, atrophic gastritis, Helicobacter pylori infection, Giardiasis and bariatric surgery, were discovered to cause the malabsorption of LT4 in the digestive tract. The second and third sections also focused on the food and medications which impaired the gastrointestinal absorption of LT4. A similar systematic review by Wiesner et al was published in 2021.144 A number of 63 studies were included in the qualitative synthesis. The interactions of LT4 with a lot of food and dietary supplements were discussed. Another review studied the effect of PPIs on the absorption of LT4.129 Previously published articles focused on the factors which impaired the gastrointestinal absorption of LT4, whereas the current study also analyzed those agents which affected its in vivo deiodination, metabolism and clearance.
Conclusion
Lots of medications, food and beverages have been reported to impair the bioavailability of levothyroxine. The proposed mechanisms of drugs interaction include direct complexing, alkalization, acceleration of catabolism or deiodination, etc. Although the classes of drugs are various, clinical practitioners should be aware that discontinuation or administration separation can suppress TSH in most cases. Liquid formulations (solution and soft-gel capsules) can be used in malabsorption induced by complexing and alkalization. Besides, when encountering patients with refractory hypothyroidism, dose adjustment of LT4, addressing the concomitant diseases, switching to another drug of the same class and close monitoring are also recommended. Some included studies have intrinsic problems and well-designed studies with larger samples are expected to provide more solid evidence on the drugs interaction of LT4. Randomized crossover trials and in vitro experiments are needed to verify the conclusions. Novel formulations of LT4 encapsulated with nanomaterials are expected to circumvent the interference between LT4 and other drugs and prolong its administration interval.
Abbreviations
AUC, area under the curve; Cmax, the maximum concentration; LT4, levothyroxine; OATP, organic anion transporting polypeptide; PPI, proton pump inhibitor; PRISMA, the preferred reporting items for systematic reviews and meta-analyses; rT3, reverse T3; T3, triiodothyronine; T4, thyroxine; TSH, thyrotropin; TBG, thyroxine-binding globulin.
Data Sharing Statement
All data in this article have been displayed in the texts and supplements.
Acknowledgments
We wish to thank all authors who provide data in this field.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the grants from Beijing Xisike Clinical Oncology Research Foundation (Y-SY201901-0189), the Fundamental Research Funds for the Central Universities (2042019kf0229), the Science and Technology Major Project of Hubei Province (Next-Generation AI Technologies) (2019AEA170).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi:10.1089/thy.2014.0028
2. IQVIA. Global medicine spending and usage trends outlook to 2024. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-medicine-spending-and-usage-trends.
3. Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS). Clin Endocrinol (Oxf). 2015;82(1):136–141. doi:10.1111/cen.12559
4. Machado-Alba J, Valencia-Marulanda J, Jimenez-Canizales C, Salazar V, Romero D. Thyroid hormone prescription patterns in a Colombian population. Pan Am J Public Health. 2014;36(2):80–86.
5. Liu H, Li W, Zhang W, Sun S, Chen C. Levothyroxine: conventional and novel drug delivery formulations. Endocr Rev. 2023;44(3):393–416. doi:10.1210/endrev/bnac030
6. Gonzales KM, Stan MN, Morris JC, Bernet V, Castro MR. The levothyroxine absorption test: a four-year experience (2015–2018) at The Mayo Clinic. Thyroid. 2019;29(12):1734–1742. doi:10.1089/thy.2019.0256
7. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372:n71. doi:10.1136/bmj.n71
8. NIH. NHLBI study quality assessment tools. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
9. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Article. Br Med J. 2019;366:
10. Moola S, Munn Z, Tufanaru C, et al. JBI’s critical appraisal tools: checklist for case reports. Available from: https://jbi.global/critical-appraisal-tools.
11. May K, Meng W, Warzok R, et al. Is thyroxine malabsorption caused by autoinduction of intestinal P-glycoprotein? A case report. Naunyn-Schmiedebergs Archiv Pharmacol. 2002;365:R109–R109.
12. Nobre EL, Jorge Z, Anselmo J, et al. A rare case of malabsorption of thyroid hormones. Ma absorcao das hormonas tiroideias. Acta Med Port. 2004;17(6):487–491.
13. Rosenberg R. Malabsorption of thyroid hormone with cholestyramine administration. Conn Med. 1994;58(2):109.
14. Singh N, Weisler SL, Hershman JM. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid. 2001;11(10):967–971. doi:10.1089/105072501753211046
15. Singh N, Singh PN, Hershman JM. Effect of calcium carbonate on the absorption of levothyroxine. JAMA. 2000;283(21):2822–2825. doi:10.1001/jama.283.21.2822
16. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21(5):483–486. doi:10.1089/thy.2010.0296
17. Benvenga S, Di Bari F, Vita R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine. 2017;56(1):138–145. doi:10.1007/s12020-017-1244-2
18. Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB. Effect of phosphate binders upon TSH and L-thyroxine dose in patients on thyroid replacement. Int Urol Nephrol. 2007;39(2):599–602. doi:10.1007/s11255-006-9166-6
19. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. l-thyroxine malabsorption due to calcium carbonate impairs blood pressure, total cholesterolemia, and fasting glycemia. Endocrine. 2019;64(2):284–292. doi:10.1007/s12020-018-1798-7
20. Morini E, Catalano A, Lasco A, Morabito N, Benvenga S. In thyroxine-replaced hypothyroid postmenopausal women under simultaneous calcium supplementation, switch to oral liquid or softgel capsule l-thyroxine ensures lower serum TSH levels and favorable effects on blood pressure, total cholesterolemia and glycemia. Endocrine. 2019;65(3):569–579. doi:10.1007/s12020-019-01908-x
21. Schneyer CR. Calcium carbonate and reduction of levothyroxine efficacy. JAMA. 1998;279(10):750. doi:10.1001/jama.279.10.750-b
22. Butner LE, Fulco PP, Feldman G. Calcium carbonate-induced hypothyroidism. Ann Intern Med. 2000;132(7):595. doi:10.7326/0003-4819-132-7-200004040-00026
23. Mazokopakis EE, Giannakopoulos TG, Starakis IK. Interaction between levothyroxine and calcium carbonate. Can Fam Physician. 2008;54(1):39.
24. Csako G, McGriff NJ, Rotman-Pikielny P, Sarlis NJ, Pucino F. Exaggerated levothyroxine malabsorption due to calcium carbonate supplementation in gastrointestinal disorders. Annals Pharmacother. 2001;35(12):1578–1583. doi:10.1345/aph.1A031
25. Campbell NRC, Hasinoff BB, Stalts H, Rao B, Wong NCW. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117(12):1010–1013. doi:10.7326/0003-4819-117-12-1010
26. Leger CS, Ooi TC. Ferrous fumarate-induced malabsorption of thyroxine. Endocrinologist. 1999;9(6):493–495. doi:10.1097/00019616-199911000-00011
27. Shakir KMM, Chute JP, Aprill BS, Lazarus AA. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. South Med J. 1997;90(6):637–639. doi:10.1097/00007611-199706000-00011
28. Yue CS, Benvenga S, Scarsi C, Loprete L, Ducharme MP. When bioequivalence in healthy volunteers may not translate to bioequivalence in patients: differential effects of increased gastric ph on the pharmacokinetics of levothyroxine capsules and tablets. J Pharm Pharma Sci. 2015;18(5):844–855. doi:10.18433/j36p5m
29. Vita R, Saraceno G, Trimarchi F, Benvenga S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J Clin Endocrinol Metabol. 2014;99(12):4481–4486. doi:10.1210/jc.2014-2684
30. Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13(4):345–349. doi:10.4158/EP.13.4.345
31. Trifiro G, Parrino F, Sultana J, et al. Drug interactions with levothyroxine therapy in patients with hypothyroidism: observational study in general practice. Clin Drug Investig. 2015;35(3):187–195. doi:10.1007/s40261-015-0271-0
32. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354(17):1787–1795. doi:10.1056/NEJMoa043903
33. Vita R, Benvenga S. Tablet levothyroxine (L-T4) malabsorption induced by proton pump inhibitor: a problem that was solved by switching to L-T4 in soft gel capsule. Endocr Pract. 2014;20(3):E38–E41. doi:10.4158/ep13316.Cr
34. Dietrich JW, Gieselbrecht K, Holl RW, Boehm BO. Absorption kinetics of levothyroxine is not altered by proton-pump inhibitor therapy. Hormone Metabol Res. 2006;38(1):57–59. doi:10.1055/s-2006-924980
35. Ananthakrishnan S, Braverman LE, Levin RM, Magnani B, Pearce EN. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine absorption. Thyroid. 2008;18(5):493–498. doi:10.1089/thy.2007.0381
36. Abi-Abib RC, Vaisman M. Is it necessary to increase the dose of levothyroxine in patients with hypothyroidism who use omeprazole? Arq Bras Endocrinol Metabol. 2014;58(7):731–736. doi:10.1590/0004-2730000002997
37. Liel Y, Sperber AD, Shany S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am J Med. 1994;97(4):363–365. doi:10.1016/0002-9343(94)90303-4
38. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152(1):183–184. doi:10.1001/archinte.152.1.183
39. Mersebach H, Rasmussen AK, Kirkegaard L, Feldt-Rasmussen U. Intestinal adsorption of levothyroxine by antacids and laxatives: case stories and in vitro experiments. Pharmacol Toxicol. 1999;84(3):107–109. doi:10.1111/j.1600-0773.1999.tb00883.x
40. Campbell BA, Bantle JP, Schmidt BA. Sucralfate and the absorption of L-thyroxine. Ann Intern Med. 1994;121(2):152. doi:10.7326/0003-4819-121-2-199407150-00024
41. Sherman SI, Tielens ET, Ladenson PW. Sucralfate causes malabsorption of L-thyroxine. Am J Med. 1994;96(6):531–535. doi:10.1016/0002-9343(94)90093-0
42. Havrankova J, Lahaie R. Levothyroxine binding by sucralfate. Ann Intern Med. 1992;117(5):445–446. doi:10.7326/0003-4819-117-5-445_3
43. Khan F, Jeanniton E, Renedo M. Does sucralfate impede levothyroxine therapy? Ann Intern Med. 1993;118(4):317. doi:10.7326/0003-4819-118-4-199302150-00027
44. Jonderko G, Jonderko K, Marcisz C, Kotulska A. Effect of cimetidine and ranitidine on absorption of [125I]levothyroxine administered orally. Acta Pharmacol Sin. 1992;13(5):391–394.
45. Demke DM. Drug-interaction between thyroxine and lovastatin. N Engl J Med. 1989;321(19):1341–1342.
46. Kisch E, Segall HS. Interaction between simvastatin and L-thyroxine. Ann Intern Med. 2005;143(7):547. doi:10.7326/0003-4819-143-7-200510040-00025
47. Abbasinazari M, Nakhjavani M, Gogani S. The effects of simvastatin on the serum concentrations of thyroid stimulating hormone and free thyroxine in hypothyroid patients treated with levothyroxine. Iran J Med Sci. 2011;36(2):80–83.
48. Gormley GJ, Tobert JA. Drug-interaction between thyroxine and lovastatin - reply. N Engl J Med. 1989;321(19):1342.
49. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17(8):763–765. doi:10.1089/thy.2007.0060
50. Weitzman SP, Ginsburg KC, Carlson HE. Colesevelam hydrochloride and lanthanum carbonate interfere with the absorption of levothyroxine. Thyroid. 2009;19(1):77–79. doi:10.1089/thy.2008.0312
51. Brown KS, Armstrong IC, Wang A, et al. Effect of the bile acid sequestrant colesevelam on the pharmacokinetics of pioglitazone, repaglinide, estrogen estradiol, norethindrone, levothyroxine, and glyburide. J Clin Pharmacol. 2010;50(5):554–565. doi:10.1177/0091270009349378
52. Northcutt RC, Stiel JN, Hollifield JW, Stant EG. The influence of cholestyramine on thyroxine absorption. JAMA. 1969;208(10):1857–1861. doi:10.1001/jama.1969.03160100047012
53. Harmon SM, Seifert CF. Levothyroxine-cholestyramine interaction reemphasized. Ann Intern Med. 1991;115(8):658–659. doi:10.7326/0003-4819-115-8-658_2
54. Madhava K, Hartley A. Hypothyroidism in thyroid carcinoma follow-up: orlistat may inhibit the absorption of thyroxine. Clin Oncol. 2005;17(6):492. doi:10.1016/j.clon.2005.05.001
55. Balapatabendi M, Harris D, Shenoy SD. Drug interaction of levothyroxine with infant colic drops. Arch Dis Child. 2011;96(9):888–889. doi:10.1136/archdischild-2011-300333
56. Isidro ML, Penin MA, Nemina R, Cordido F. Metformin reduces thyrotropin levels in obese, diabetic women with primary hypothyroidism on thyroxine replacement therapy. Endocrine. 2007;32(1):79–82. doi:10.1007/s12020-007-9012-3
57. Al-Alusi MA, Du L, Li N, et al. Metformin does not suppress serum thyrotropin by increasing levothyroxine absorption. Thyroid. 2015;25(10):1080–1084. doi:10.1089/thy.2015.0211
58. Cappelli C, Rotondi M, Pirola I, et al. TSH-lowering effect of metformin in type 2 diabetic patients differences between euthyroid, untreated hypothyroid, and euthyroid on L-T4 therapy patients. Diabetes Care. 2009;32(9):1589–1590. doi:10.2337/dc09-0273
59. Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metabol. 2006;91(1):225–227. doi:10.1210/jc.2005-1210
60. McLean M, Kirkwood I, Epstein M, Jones B, Hall C. Cation-exchange resin and inhibition of intestinal absorption of thyroxine. Lancet. 1993;341(8855):1286. doi:10.1016/0140-6736(93)91195-r
61. Iovino M, Iovine N, Petrosino A, et al. Sevelamer carbonate markedly reduces levothyroxine absorption. Endocr Metabol Immune Disord. 2014;14(3):206–209. doi:10.2174/1871530314666140902151804
62. Bone HG, Walter MA, Hurley ME, Epstein S. Pharmacokinetics of coadministration of levothyroxine sodium and alendronate sodium new effervescent formulation. Osteoporos Int. 2017;28(5):1745–1752. doi:10.1007/s00198-017-3941-3
63. Garwood CL, Van Schepen KA, McDonough RP, Sullivan AL. Increased thyroid-stimulating hormone levels associated with concomitant administration of levothyroxine and raloxifene. Pharmacotherapy. 2006;26(6):881–885. doi:10.1592/phco.26.6.881
64. Siraj ES, Gupta MK, Reddy SK. Raloxifene causing malabsorption of levothyroxine. Arch Intern Med. 2003;163(11):1367–1370. doi:10.1001/archinte.163.11.1367
65. Guarda FJ, Findling J, Yuen KCJ, Fleseriu M, Nachtigall LB. Mifepristone increases thyroid hormone requirements in patients with central hypothyroidism: a multicenter study. J Endocr Soc. 2019;3(9):1707–1714. doi:10.1210/js.2019-00188
66. Arafah BM. Increased need for thyroxine in women with hypothyroidism during estrogen therapy. N Engl J Med. 2001;344(23):1743–1749. doi:10.1056/nejm200106073442302
67. Arafah BM. Decreased levothyroxine requirement in women with hypothyroidism during androgen therapy for breast cancer. Ann Intern Med. 1994;121(4):247–251. doi:10.7326/0003-4819-121-4-199408150-00002
68. Isley WL. Effect of rifampin therapy on thyroid function tests in a hypothyroid patient on replacement L-thyroxine. Ann Intern Med. 1987;107(4):517–518. doi:10.7326/0003-4819-107-4-517
69. Nolan SR, Self TH, Norwood JM. Interaction between rifampin and levothyroxine. South Med J. 1999;92(5):529–531. doi:10.1097/00007611-199905000-00018
70. Goldberg AS, Tirona RG, Asher LJ, Kim RB, Van Uum SHM. Ciprofloxacin and rifampin have opposite effects on levothyroxine absorption. Thyroid. 2013;23(11):1374–1378. doi:10.1089/thy.2013.0014
71. Cooper JG, Harboe K, Frost SK, Skadberg O. Ciprofloxacin interacts with thyroid replacement therapy. Br Med J. 2005;330(7498):1002. doi:10.1136/bmj.330.7498.1002
72. Berger J-L, Nguyen Y, Lebrun D, et al. Early neuropsychological adverse events after switching from PI/r to dolutegravir could be related to hyperthyroidism in patients under levothyroxine. Antivir Ther. 2017;22(3):271–272. doi:10.3851/imp3107
73. Sahajpal R, Ahmed RA, Hughes CA, Foisy MM. Probable interaction between levothyroxine and ritonavir: case report and literature review. Am J Health Syst Pharma. 2017;74(8):587–592. doi:10.2146/ajhp160200
74. Touzot M, Le Beller C, Touzot F, Louet AL, Piketty C. Dramatic interaction between levothyroxine and lopinavir/ritonavir in a HIV-infected patient. Aids. 2006;20(8):1210–1212. doi:10.1097/01.aids.0000226969.96880.3c
75. Lanzafame M, Trevenzoli M, Faggian F, et al. Interaction between levothyroxine and indinavir in a patient with HIV infection. Infection. 2002;30(1):54–55. doi:10.1007/s15010-002-2092-3
76. Aanderud S, Myking OL, Strandjord RE. The Influence of carbamazepine on thyroid-hormones and thyroxine binding globulin in hypothyroid patients substituted with thyroxine. Clin Endocrinol (Oxf). 1981;15(3):247–252. doi:10.1111/j.1365-2265.1981.tb00662.x
77. Larsen PR, Atkinson AJ, Wellman HN, Goldsmith RE. The effect of diphenylhydantoin on thyroxine metabolism in man. J Clin Invest. 1970;49(6):1266–1279. doi:10.1172/jci106339
78. Faber J, Lumholtz IB, Kirkegaard C, et al. The effects of phenytoin (diphenylhydantoin) on the extrathyroidal turnover of thyroxine, 3,5,3’-triiodothyronine, 3,3’,5’-triiodothyronine, and 3’,5’-diiodothyronine in man. J Clin Endocrinol Metabol. 1985;61(6):1093–1099. doi:10.1210/jcem-61-6-1093
79. Deluca F, Arrigo T, Pandullo E, Siracusano MF, Benvenga S, Trimarchi F. Changes in thyroid function tests induced by 2 month carbamazepine treatment in L-thyroxine-substituted hypothyroid children. Eur J Pediatr. 1986;145(1–2):77–79. doi:10.1007/bf00441860
80. Blackshear JL, Schultz AL, Napier JS, Stuart DD. Thyroxine replacement requirements in hypothyroid patients receiving phenytoin. Ann Intern Med. 1983;99(3):341–342. doi:10.7326/0003-4819-99-3-341
81. de Carvalho GA, Bahls S-C, Boeving A, Graf H. Effects of selective serotonin reuptake inhibitors on thyroid function in depressed patients with primary hypothyroidism or normal thyroid function. Thyroid. 2009;19(7):691–697. doi:10.1089/thy.2008.0261
82. McCowen KC, Garber JR, Spark R. Elevated serum thyrotropin in thyroxine-treated patients with hypothyroidism given sertraline. N Engl J Med. 1997;337(14):1010–1011.
83. Lumholtz IB, Siersbaek-Nielsen K, Faber J, Kirkegaard C, Friis T. Effect of propranolol on extrathyroidal metabolism of thyroxine and 3,3’,5-triiodothyronine evaluated by noncompartmental kinetics. J Clin Endocrinol Metab. 1978;47(3):587–589. doi:10.1210/jcem-47-3-587
84. Figge J, Dluhy RG. Amiodarone-induced elevation of thyroid stimulating hormone in patients receiving levothyroxine for primary hypothyroidism. Ann Intern Med. 1990;113(7):553–555. doi:10.7326/0003-4819-113-7-553
85. Chiu AC, Sherman SI. Effects of pharmacological fiber supplements on levothyroxine absorption. Thyroid. 1998;8(8):667–671. doi:10.1089/thy.1998.8.667
86. Narula HS, Carlson HE. Capecitabine-induced abnormalities in thyroid function tests. Am J Med. 2004;116(12):855–856. doi:10.1016/j.amjmed.2004.02.025
87. Abdulrahman RM, Verloop H, Hoftijzer H, et al. Sorafenib-induced hypothyroidism is associated with increased type 3 deiodination. J Clin Endocrinol Metabol. 2010;95(8):3758–3762. doi:10.1210/jc.2009-2507
88. Schlumberger MJ, Elisei R, Bastholt L, et al. Phase II study of safety and efficacy of motesanib in patients with progressive or symptomatic, advanced or metastatic medullary thyroid cancer. J Clin Oncol. 2009;27(23):3794–3801. doi:10.1200/jco.2008.18.7815
89. de Groot JWB, Zonnenberg BA, Plukker JTM, van Der Graaf WTA, Links TP. Imatinib induces hypothyroidism in patients receiving levothyroxinc. Clin Pharmacol Ther. 2005;78(4):433–438. doi:10.1016/j.clpt.2005.06.010
90. Antúnez PB, Licht SD. Vitamin C improves the apparent absorption of levothyroxine in a subset of patients receiving this hormone for primary hypothyroidism. La vitamina C mejora la absorción aparente de levotiroxina en ciertos pacientes que reciben esta hormona por hipotiroidismo primario. Revista argentina de endocrinología y metabolismo. 2011;48(1):16–24.
91. Jubiz W, Ramirez M. Effect of vitamin C on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J Clin Endocrinol Metabol. 2014;99(6):E1031–E1034. doi:10.1210/jc.2013-4360
92. Bach-Huynh T-G, Nayak B, Loh J, Soldin S, Jonklaas J. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metabol. 2009;94(10):3905–3912. doi:10.1210/jc.2009-0860
93. Silva Perez CL, Araki FS, Graf H, de Carvalho GA. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid. 2013;23(7):779–784. doi:10.1089/thy.2012.0435
94. Lamson MJ, Pamplin CL, Rolleri RL, Klein I. Quantitation of a substantial reduction in levothyroxine (T4) absorption by food. Thyroid. 2004;14(10):873–876. doi:10.1089/thy.2004.14.873
95. Lilja JJ, Laitinen K, Neuvonen PJ. Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol. 2005;60(3):337–341. doi:10.1111/j.1365-2125.2005.02433.x
96. Wenzel KW, Kirschsieper HE. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism. 1977;26(1):1–8. doi:10.1016/0026-0495(77)90121-4
97. Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid. 2008;18(3):293–301. doi:10.1089/thy.2007.0222
98. Chon DA, Reisman T, Weinreb JE, Hershman JM, Leung AM. Concurrent milk ingestion decreases absorption of levothyroxine. Thyroid. 2018;28(4):454–457. doi:10.1089/thy.2017.0428
99. Cappelli C, Pirola I, Gandossi E, et al. Thyroid hormone profile in patients ingesting soft gel capsule or liquid levothyroxine formulations with breakfast. Int J Endocrinol. 2016;2016:9043450. doi:10.1155/2016/9043450
100. Vita R, Saraceno G, Trimarchi F, Benvenga S. A novel formulation of L-thyroxine (L-T4) reduces the problem of L-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine. 2013;43(1):154–160. doi:10.1007/s12020-012-9772-2
101. Dickerson RN, Maish GO, Minard G, Brown RO. Clinical relevancy of the levothyroxine-continuous enteral nutrition interaction. Nutr Clin Pract. 2010;25(6):646–652. doi:10.1177/0884533610385701
102. Conrad SC, Chiu H, Silverman BL. Soy formula complicates management of congenital hypothyroidism. Arch Dis Child. 2004;89(1):37–40. doi:10.1136/adc.2002.009365
103. Bell DS, Ovalle F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract. 2001;7(3):193–194. doi:10.4158/EP.7.3.193
104. Fruzza AG, Demeterco-Berggren C, Jones KL. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics. 2012;130(3):E699–E702. doi:10.1542/peds.2011-3350
105. Pinchera A, Macgillivray MH, Crawford JD, Freeman AG. Thyroid refractoriness in an athyreotic cretin fed soybean formula. N Engl J Med. 1965;273:83–87. doi:10.1056/nejm196507082730205
106. Liel Y, HarmanBoehm I, Shany S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metabol. 1996;81(2):857–859. doi:10.1210/jc.81.2.857
107. Wegrzyn NM. Malabsorption of L-T4 due to drip coffee: a case report using predictors of causation. J Acad Nutr Diet. 2016;116(7):1073–1076. doi:10.1016/j.jand.2016.02.016
108. Deiana L, Marini S, Mariotti S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr Pract. 2012;18(1):98–100. doi:10.4158/ep11233.Co
109. Mahapatro S, Satapathy AK. Dentifrice reducing levothyroxine efficacy in children. Indian J Pediatr. 2019;86(11):1062. doi:10.1007/s12098-019-02987-4
110. Cappelli C, Pirola I, Daffini L, et al. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: results of the TICO study. Thyroid. 2016;26(2):197–202. doi:10.1089/thy.2015.0422
111. Persiani S, Sala F, Manzotti C, et al. Evaluation of levothyroxine bioavailability after oral administration of a fixed combination of soy isoflavones in post-menopausal female volunteers. Drug Res. 2016;66(3):136–140. doi:10.1055/s-0035-1555784
112. Morelli S, Reboldi G, Moretti S, Menicali E, Avenia N, Puxeddu E. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine. 2016;52(3):571–578. doi:10.1007/s12020-015-0788-2
113. Pirola I, Daffini L, Gandossi E, et al. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J Endocrinol Invest. 2014;37(6):583–587. doi:10.1007/s40618-014-0082-9
114. Cappelli C, Pirola I, Gandossi E, Formenti A, Castellano M. Oral liquid levothyroxine treatment at breakfast: a mistake? Eur J Endocrinol. 2014;170(1):95–99. doi:10.1530/eje-13-0693
115. Marina M, Ceda GP, Aloe R, Gnocchi C, Ceresini G. Circulating concentrations of free thyroxine after an oral intake of liquid LT4 taken either during fasting conditions or at breakfast. Acta bio-medica. 2016;87(3):247–252.
116. Pirola I, Gandossi E, Brancato D, et al. TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30min before breakfast. J Endocrinol Invest. 2018;41(11):1301–1306. doi:10.1007/s40618-018-0867-3
117. Cappelli C, Pirola I, Castellano M. Liquid levothyroxine formulation taken during lunch in Italy: a case report and review of the literature. Case Rep Endocrinol. 2020;2020:8858887. doi:10.1155/2020/8858887
118. Vita R, Di Bari F, Benvenga S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin Drug Deliv. 2017;14(4):467–472. doi:10.1080/17425247.2017.1290604
119. Benvenga S. Liquid and softgel capsules of 1-thyroxine results lower serum thyrotropin levels more than tablet formulations in hypothyroid patients. J Clin Transl Endocrinol. 2019;18:100204. doi:10.1016/j.jcte.2019.100204
120. Virili C, Antonelli A, Santaguida MG, Benvenga S, Centanni M. Gastrointestinal malabsorption of thyroxine. Endocr Rev. 2019;40(1):118–136. doi:10.1210/er.2018-00168
121. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Article. Am J Hum Genet. 2004;74(1):168–175. doi:10.1086/380999
122. Friesema ECH, Grueters A, Biebermann H, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Article. Lancet. 2004;364(9443):1435–1437. doi:10.1016/s0140-6736(04)17226-7
123. Arakawa H, Shirasaka Y, Haga M, Nakanishi T, Tamai I. Active intestinal absorption of fluoroquinolone antibacterial agent ciprofloxacin by organic anion transporting polypeptide, Oatp1a5. Biopharm Drug Dispos. 2012;33(6):332–341. doi:10.1002/bdd.1809
124. Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Therap. 2003;304(1):223–228. doi:10.1124/jpet.102.043026
125. Gupta P, Johnson JT, Soumya SL, Cherian KE, Kapoor N, Paul TV. A case of H. pylori infection presenting as refractory hypothyroidism. J Fam Med Prim Care. 2020;9(7):3770–3772. doi:10.4103/jfmpc.jfmpc_729_20
126. Mazak K, Toth G, Koekoesi J, Noszal B. Thyroxine lipophilicity is dominated by its zwitterionic microspecies. Article. Eur J Pharma Sci. 2012;47(5):921–925. doi:10.1016/j.ejps.2012.09.009
127. Toth G, Mazak K, Hosztafi S, Koekoesi J, Noszal B. Species-specific lipophilicity of thyroid hormones and their precursors in view of their membrane transport properties. Article. J Pharm Biomed Anal. 2013;76:112–118. doi:10.1016/j.jpba.2012.12.010
128. Kaur N, Suryanarayanan R. Levothyroxine sodium pentahydrate tablets - formulation considerations. Review. J Pharm Sci. 2021;110(12):3743–3756. doi:10.1016/j.xphs.2021.08.006
129. Guzman-Prado Y, Vita R, Samson O. Concomitant use of levothyroxine and proton pump inhibitors in patients with primary hypothyroidism: a systematic review. J Gen Intern Med. 2021;36(6):1726–1733. doi:10.1007/s11606-020-06403-y
130. Leung AM. Thyroid function in pregnancy. J Trace Elements Med Biol. 2012;26(2–3):137–140. doi:10.1016/j.jtemb.2012.03.004
131. Benvenga S, Capodicasa G, Perelli S, Ferrari SM, Fallahi P, Antonelli A. Increased requirement of replacement doses of levothyroxine caused by liver cirrhosis. Front Endocrinol (Lausanne). 2018;9:
132. Wang J, Gao J, Fan Q, Li H, Di Y. The effect of metformin on thyroid-associated serum hormone levels and physiological indexes: a meta-analysis. Review. Curr Pharm Des. 2019;25(30):3257–3265. doi:10.2174/1381612825666190918162649
133. IBSA Institut Biochimique SA. Full prescribing information for Tirosint® (levothyroxine sodium) capsules. Available from: https://www.tirosint.com/wp-content/uploads/2019/04/Tirosint-PI.pdf.
134. VistaPharm, Inc. Full prescribing information for THYQUIDITYTM (levothyroxine sodium) oral solution. Available from: https://www.thyquidity.com/pdf/Prescribing-Information.pdf.
135. Otun J, Sahebkar A, Ostlundh L, Atkin SL, Sathyapalan T. Systematic review and meta-analysis on the effect of soy on thyroid function. Article. Sci Rep. 2019;9:
136. Sathyapalan T, Koehrle J, Rijntjes E, et al. The effect of high dose isoflavone supplementation on serum reverse T(3) in euthyroid men with type 2 diabetes and post-menopausal women. Article. Front Endocrinol (Lausanne). 2018;9:
137. Benvenga S. (Soft) capsules of wisdom: preventing myo-inositol malabsorption caused by coffee. Expert Opin Drug Deliv. 2012;9(10):1177–1179. doi:10.1517/17425247.2012.719495
138. Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharma Biopharma. 2009;72(1):105–110. doi:10.1016/j.ejpb.2008.10.008
139. Fiorini G, Ribichini D, Pasquali R, Vaira D. In vivo dissolution of levothyroxine soft gel capsules. Intern Emerg Med. 2016;11(8):1151–1152. doi:10.1007/s11739-016-1526-3
140. Pirola I, Formenti AM, Gandossi E, et al. Oral liquid L-thyroxine (L-T4) may be better absorbed compared to L-T4 tablets following bariatric surgery. Obes Surg. 2013;23(9):1493–1496. doi:10.1007/s11695-013-1015-y
141. Guglielmi R, Frasoldati A, Zini M, et al. Italian Association of Clinical Endocrinologists statement-replacement therapy for primary hypothyroidism: a brief guide for clinical practice. Endocr Pract. 2016;22(11):1319–1326. doi:10.4158/ep161308.Or
142. Gatta E, Bambini F, Buoso C, et al. Liquid levothyroxine formulations in patients taking drugs interfering with L-T4 absorption. Review. Front Endocrinol (Lausanne). 2022;13:1080108. doi:10.3389/fendo.2022.1080108
143. Skelin M, Lucijanic T, Klaric DA, et al. Factors affecting gastrointestinal absorption of levothyroxine: a review. Clin Ther. 2017;39(2):378–403. doi:10.1016/j.clinthera.2017.01.005
144. Wiesner A, Gajewska D, Pasko P. Levothyroxine interactions with food and dietary supplements-a systematic review. Pharmaceuticals. 2021;14(3):
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

