Back to Journals » Infection and Drug Resistance » Volume 16
Medical Applications of Hydrogels in Skin Infections: A Review
Authors Teng Y , Li S, Tang H, Tao X, Fan Y, Huang Y
Received 10 November 2022
Accepted for publication 29 December 2022
Published 23 January 2023 Volume 2023:16 Pages 391—401
DOI https://doi.org/10.2147/IDR.S396990
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Yan Teng,1 Sujing Li,2 Hui Tang,2 Xiaohua Tao,1 Yibin Fan,1,* Youming Huang1,*
1Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Hangzhou, 310014, People’s Republic of China; 2Graduate School of Clinical Medicine, Bengbu Medical College, Bengbu, 233030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Youming Huang; Yibin Fan, Center for Plastic & Reconstructive Surgery, Department of Dermatology, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital of Hangzhou Medical College, Tel +86-18368023136 ; +86-18806538451, Email [email protected]; [email protected]
Abstract: Skin infections are common diseases for which patients seek inpatient and outpatient medical care. Globally, an increasing number of people are affected by skin infections that could lead to physical and psychological damage. Skin infections always have a broad spectrum of clinical presentations that require physicians to make an aggressive and accurate diagnosis for prescribing the proper symptomatic antimicrobials. In most instances, the treatment for skin infections mainly includes oral or topical anti-infective drugs. However, some of the classical anti-infective drugs have limitations, such as poor water solubility, low bioavailability, and poor targeting efficiency, which can lead to poor efficacy, adverse effects, and drug resistance. Therefore, research priorities should focus on the development of more effective drug delivery systems with new materials. Hydrogels are a highly multifunctional class of medical materials with potential applications in dermatology. Several hydrogel dressings with anti-infective functions have been formulated and demonstrated to improve the efficacy and tolerance of oral or topical classical anti-infective drugs to a certain degree. In this study, the medical applications of hydrogels for the treatment of various skin infections are systematically reviewed to provide an important theoretical reference for future research studies on the treatment options for skin infections.
Keywords: hydrogel, dermatology, skin infections, anti-infective agent, drug delivery systems
Introduction
An increasing proportion of the global population is affected by skin infections, primarily bacterial, viral, fungal, and parasitic skin infections. The major bacteria causing skin infections include Propionibacterium acnes, Staphylococcus epidermidis, Staphylococcus aureus, and Streptococcus pyogenes. These major pathogens lead to complications such as acne, impetigo, erysipelas, cellulites, etc. The major viruses involved in skin infections include herpes simplex virus (HSV), human papillomavirus, molluscum contagiosum virus, etc. leading to herpes simplex, warts, molluscum contagiosum, etc. Dermatophytes and Candida are the major fungi involved in skin infections which could lead to different types of dermatophytosis. The major pathogens leading to parasitic skin infections include Sarcoptes scabiei mites, Leishmania sp., etc. which are the causative agents of scabies, cutaneous leishmaniasis, etc. In the absence of prompt and effective treatment, these conditions can eventually cause severe damage to the physical and psychological health of the patients. Some of the currently available oral or topical anti-infective therapeutic approaches have major shortcomings that can lead to repeated and prolonged skin infections. The major challenges associated with these approaches are (1) poor water solubility, low transdermal capacity, and low drug bioavailability that can contribute to poor therapeutic efficacy, (2) dose-responsive adverse effects, systemic toxicity, and high drug resistance, and (3) inconvenience, high costs, raw material shortages, and manufacturing quality concerns that can affect patient compliance. For example, the topical application of drugs containing acyclovir (ACV) is clinically restricted by its low transdermal penetration capacity and poor water solubility. These properties make it difficult for medical practitioners for recommending these drugs to patients, as they need to be applied multiple times a day. Similarly, topical nitric oxide (NO) is considered an effective and targeted therapy against warts and molluscum contagiosum; however, its application is always limited by its inconvenience and lack of safe methods of delivery to the target skin lesions. The examples discussed above emphasize the need for new developments in drug delivery for the treatment of skin infections and indicate the great market potential of these drug delivery systems. The use of hydrogels may offer several advantages that can overcome the limitations mentioned above.1,2 For example, the high-water content of hydrogels could enhance the transdermal penetration capacity of anti-infective agents to increase the drug bioavailability. Additionally, hydrogels have exhibited good biocompatibility, quick absorption of wound exudate, protection of the delicate skin, and are relatively easy and painless to remove, all of which are attributes of an ideal wound dressing.3–7 The hydrogel-based anti-infective therapy combined with other drugs or technology for other systems is a promising strategy. By adding sensitive agents or making specific modifications to the hydrogel-based therapy, the reaction to hydrogel could be triggered by pH value,8 temperature,9 ultrasound,10 visible light,11 etc. For example, pH-responsive dapsone-loaded hydrogels could respond to the changes in alkalinity/acidity in the gastrointestinal tract, thereby effectively reducing the required dapsone dosage and controlling its release after oral administration to reduce the possible adverse events.12 The microemulsion (ME) system is a transparent mixture of two immiscible liquids, such as water and oil, which is stabilized by the surfactant molecules. By introduction of the ME system, AzA- and TTO-loaded ME hydrogels could have a better zone of inhibition and low minimum inhibitory concentration (MIC) values against pathogens such as S. aureus, S. epidermidis, and P. acnes.13 In addition, chitosan-based hydrogel might be a potential approach for acne treatment in combination with photodynamic therapy (PDT).14 Figure 1 shows the detailed mechanisms underlying the possible medical applications of hydrogels in skin infections. Figure 2 indicates the common skin infections treated by hydrogels. In this study, the medical applications of hydrogel dressings in the treatment of skin infections are critically reviewed to highlight their immense market potential. The various hydrogels and their applications in skin infections are shown in Table 1. The types and composition of hydrogels as well as major reports on the clinical applications of hydrogels, and their outcomes for skin infections are shown in Table 2.
 |
Table 1 A Summary of Major Pathogens Causing Skin Infections and Their Optimized Hydrogels |
 |
Table 2 The Hydrogels Types, Main Reports, and Outcomes for Skin Infections |
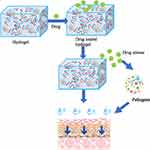 |
Figure 1 Detailed mechanisms underlying the medical application of hydrogels in skin infections. |
 |
Figure 2 Common skin infections treated by hydrogels. |
Bacterial Skin Infections
Acne Vulgaris-A Chronic Inflammatory Disorder Associated with P. acnes, S. aureus, and S. epidermis
Acne vulgaris is a common cutaneous inflammatory disorder of pilosebaceous units, which comprises hair follicles and their accompanying sebaceous glands. The formation of acne vulgaris involves multiple factors including follicular hyper-keratinization, hormonal dysregulation, and increased sebum production by the sebaceous glands.15,16 The trapped sebum in the pilosebaceous glands present in healthy skin produces a substrate that promotes the growth of bacterial flora such as S. aureus, S. epidermidis, and P. acnes, which causes comedones and inflammatory lesions17,18 (Figure 3). Therefore, topical antibiotics, such as clindamycin, erythromycin, etc., and systemic antimicrobials, such as minocycline and doxycycline, are applied for the clinical treatment of these bacterial infections. However, they can lead to severe adverse effects and bacterial resistance, indicating the need for alternative topical therapies. Azelaic acid (AZA) and essential oils such as tea tree oil (TTO) inhibit S. aureus and have been used for the treatment of acne vulgaris for a long time. They can lead to a few adverse effects like local skin sensitization and irritation or incidences of bacterial resistance.19,20 It is essential to co-administer AZA and TTO for reducing the risk of dose-responsive adverse effects and achieving synergistic effects. Bisht et al13 combined AZA and TTO for co-delivery in the form of a microemulsion (ME) system with hydrogel. As drug delivery systems, MEs offer unique advantages such as a better drug-release profile and increased skin permeation and targeting. Hence, ME hydrogel composite formulations demonstrated reduced side effects and better skin permeation and retention characteristics. Additionally, the studies also indicated that they had a better zone of inhibition and low minimum inhibitory concentration (MIC) values against pathogens such as S. aureus, S. epidermidis, and P. acnes. Chitosan has been reported to possess antimicrobial activity that targets cell membrane permeability, thereby leading to the death of microorganisms, including fungi, algae, and bacteria. Moreover, chitosan was demonstrated to form a hydrogel for drug delivery due to its bioadhesion and permeability properties. Frade et al14 demonstrated the antimicrobial activity of chitosan against P. acnes suspension and films. The delivery of topical chitosan hydrogel with methylene blue (MB) not only exhibited excellent activity against inflammatory and infectious skin disorders but also enhanced the elasticity of hydrogel, thus delaying the release of the photosensitizer.
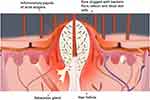 |
Figure 3 An illustration showing the formation of the inflammatory papule in acne. |
Impetigo-A Superficial Bacterial Infection Induced by S. aureus and Streptococcus pyogenes
Impetigo is a common and superficial contagious bacterial infection that mainly affects children aged 2–5 years, although adults may also be affected.21,22 S. aureus and Streptococcus pyogenes, which heavily colonize the epidermis, are major pathogens causing impetigo. Cephalexin, a first-generation semi-synthetic cephalosporin antibiotic, exhibits antimicrobial activity against both gram-positive and gram-negative bacteria by inhibiting bacterial cell wall synthesis. Therefore, it is commercially used in tablets, capsules, and suspension dosage forms.23 Additionally, cephalexin nanoparticles (NPs), generally used as first-line topical treatments of impetigo, may be used for enhancing patient compliance, controlling adverse effects, reducing systemic toxicity, and alleviating the first-pass effect.24,25 Sara et al26 formulated a PF-127-chitosan hydrogel embedded with cephalexin NPs, for use as a topical antibacterial delivery system for impetigo. The incorporation of polymeric NPs within the thermosensitive hydrogel led to improved tissue targeting, diminished burst release, and controlled drug release rate. Moreover, chitosan was used for its antibacterial and bioadhesive properties. In particular, the novel thermosensitive and bioadhesive cephalexin nanohydrogel has become a promising topical antibacterial delivery system that can not only reduce therapy costs but also diminish antibiotic-associated adverse effects. Liposomal polyvinylpyrrolidone (PVP)-iodine (3%) hydrogel has been demonstrated to be well-tolerated, effective against biofilm formation, and have better wound healing properties. Augustin et al27 conducted a preliminary pilot study to evaluate the possible applications of liposomal PVP-iodine hydrogels for impetigo treatment. They reported that a liposomal PVP-iodine hydrogel improved the disease-related symptoms and global clinical severity scores of impetigo contagiosa. Thus, this hydrogel represents a promising alternative therapy for impetigo.
Leprosy-A Chronic Contagious Disease Caused by Mycobacterium leprae
Leprosy (also known as Hansen’s disease) is an infectious disease caused by Mycobacterium leprae that affects both the skin and peripheral nerves and is an important global health issue. Early and accurate diagnosis and treatment are essential for reducing the risks of eye, hand, and foot disabilities due to neuropathy, as these conditions are usually irreversible and require lifelong care. First-line treatments for leprosy include dapsone and rifampin, and lepromatous disease include clofazimine.54 Dapsone has low solubility, which leads to its poor therapeutic efficacy and high bacterial resistance. To address this limitation, Chaves et al12 developed an innovative and optimized pH-responsive chitosan-based hydrogel formulation for reducing the required dapsone dosage and controlling its release after oral administration to avoid any possible adverse effects. The pH-responsive hydrogels respond to the changes in alkalinity/acidity levels in the gastrointestinal tract. Therefore, pH-responsive dapsone-loaded hydrogels represent a novel approach to the development of promising formulations for leprosy treatment.
Fungal Skin Infections
Fungal Skin Diseases-Superficial Fungal Infections Caused by Dermatophyte Invasion of the Epidermis
Fungal skin diseases are a type of infection caused by fungi, mainly affecting the superficial skin layers, mucous membranes, hair, and skin appendages. Dermatophytosis or superficial skin fungal infections have been shown to impact 20–25% of the global population.28 The common characteristics of these diseases include high incidence, infectiousness, and frequent relapse or re-infection, and Dermatophytes and Candida are the major causative agents. Fungi prefer a warm and humid environment, and dermatomycosis mostly occurs when the environmental conditions are suitable for fungal growth and reproduction on human skin. During the past few years, several studies were conducted for the application of solid lipid nanoparticles (SLNs) as topical drug carriers. When compared to classical carriers, such as creams and emulsions, SLNs offer controlled release, good patient tolerance, and protection of active components. Additionally, SLNs were reported to favor drug penetration into the skin while maintaining a sustained drug release to avoid systemic absorption. Miconazole nitrate (MN) is a broad-spectrum antifungal agent usually employed as a topical treatment for dermatophytosis and superficial mycoses and as an oral gel for the treatment of Candida infections. However, its poor water solubility reduces its efficacy in therapeutic applications. Jain et al30 formulated MN-loaded SLN and successfully incorporated them into hydrogels for topical applications. They demonstrated that MN-loaded SLN-bearing hydrogel provided a sustained MN release. Itraconazole (ITR), also a broad-spectrum antifungal agent, is used to treat a broad spectrum of dermatophytes, eg, Microsporum, Epidermophyton, Trichophyton spp. The conventional oral ITR undergoes erratic, dose-dependent, and mostly incomplete absorption, and leads to frequent adverse events such as constipation, abdominal pain, and headaches. Therefore, it is necessary to investigate and explore topical formulations for the administration of ITR rather than oral administration. When compared to liposomes, nonionic surfactant vesicles (NSVs) are stable, robust, and inexpensive colloidal carriers. The topical NSV preparation loaded with ITR could lead to instantaneous delivery of the drug specifically to infected superficial skin layers, which can overcome the limitations of classical oral administration. The treatment of fungal skin infections with an optimized NSV hydrogel demonstrated better antifungal activity when compared to the commercial therapies generally prescribed for tinea pedis.29 Sertaconazole (STZL) is an imidazole antifungal agent used for the treatment of dermatophytosis and applied as topical formulations, such as creams, gels, etc. Similar to the other anti-fungal agents, the major limitation of commercial STZL is its poor permeability. Sahoo et al33 formulated a topical ME-based STZL hydrogel for the effective treatment of fungal skin diseases. The hydrogel of STZL microemulsion (HSM) comprised of 2.5% w/w STZL-containing MEs in 0.75% w/w Carbopol 940. Carbopol is hydrophilic, thereby making it a suitable candidate for the preparation of gel-type formulations. The results highlighted that HSM-4 significantly enhanced skin permeation of STZL due to its high permeability and anti-fungal ability of STZL in HSM-4, thereby reducing the STZL concentration required for the treatment of fungal skin diseases.
Viral Skin Infections
Herpes Simplex-A Recurrent Viral Infectious Disease Induced by HSV
HSV, a typical representative herpes virus species, can be categorized into two serotypes: HSV-1 and HSV-2 based on the differences in its antigenicity. The most common clinical manifestation of HSV is clusters of herpes lesions on the skin and mucous membranes.31,32 When compared with oral drugs, topical antiviral drugs have targeted therapeutic efficacy but as they need to be applied multiple times a day, they are more difficult to use. A majority of topical drugs contain ACV or related components as active ingredients. ACV, a guanosine antiviral drug, is one of the most commonly used treatments for HSV infection. However, the topical application of ACV is limited by its low transdermal penetration capacity and poor water solubility. The hydrogels are classified under various classes based on their mechanical and structural characteristics, the nature of the polymer, and the physical structure of the polymer network. Nanoemulsions (NE) are emulsions having droplet sizes typically in the range of 5 to 200 nm and a transparent appearance. NE formulations could be used to enhance ACV bioavailability and overcome the difficulties associated with its clinical use. Al-Subaie et al37 formulated an optimized ACV NE hydrogel to overcome the deficiencies of ACV creams, which are presently available in the market. The optimized hydrogels were incorporated into NE using chitosan as the gelling agent and Eugenol as a skin permeation enhancer. When compared with the raw ACV hydrogel and commercial ACV creams, the optimized ACV NE hydrogel exhibited excellent drug bioavailability. The elastic liposomes or flexible membrane vesicles (FMVs) have proven to be useful in overcoming the limitations of commercially available topical ACV creams, such as their poor skin permeation. The application of ACV-loaded FMVs incorporated into a hydrogel significantly led to a reduction in the frequency of required ACV dosage.34 However, current information on their efficacy is based on a preclinical study, and further intensive clinical studies are necessary to investigate their antiviral therapeutic potential. Tannic acid-modified silver nanoparticles (TA-AgNPs) have been demonstrated the ability to inhibit virus attachment, penetration, and cell-to-cell transmission. Moreover, they have been shown to exhibit anti-inflammatory effects on HSV-induced inflammatory mediators.35 However, the liquid state of TA-AgNPs limits their local application against HSV infections. Szymanska et al41 formulated a class of multifunctional mucosal-adhesion hydrogels by incorporating TA-AgNPs into a Carbopol 974P gel that appeared to significantly inhibit HSV-1 and block the attachment and penetration of HSV-2. Houston et al demonstrated the potential virucidal activity of pomegranate rind extract (PRE) and ZnSO4 against HSV-1, HSV-2, and ACV-resistant HSV-1. Hence, they formulated a simple hydrogel comprising 2.5% HPMC, 1.25 mg/mL PRE, and 0.25 M ZnSO4 in a phthalate buffer of pH 4.5 for facilitating the topical delivery of major bioactive compounds within PRE and Zn (II) from hydrogels across mucus membranes to clustered vesicles of HSV while maintaining its potent virucidal and anti-inflammatory effects.
Molluscum Contagiosum-A Viral Contagious Skin Disease Caused by Molluscum Contagiosum Virus and Transmitted Primarily by Direct Contact
Molluscum contagiosum virus (MCV) is a poxvirus leading to chronic local infections characterized by flesh-colored and dome-shaped papules on the skin. The treatment methods for MCV mainly include topical therapy, curettage, cryotherapy, and laser or chemical destruction.36 However, the frequent severe pain experienced during curettage or cryotherapy treatment is unacceptable to patients, particularly children. Therefore, a topical eutectic mixture of local anesthetics is often applied to minimize discomfort, but it takes more than 30 minutes for achieving the desired result.38 Nanorap, a hydrogel composed of 2.5% lidocaine and 2.5% prilocaine and 50% of its active products formulated as nanocapsules, takes a maximum of 10 minutes for achieving satisfactory analgesia in healthy adults.39 Gobbato et al47 conducted a study investigating the tolerability of Nanorap in children affected with molluscum contagiosum (MC). The study results demonstrated that a single 1 g dose of 2.5% lidocaine/prilocaine hydrogel applied to MC lesions was well-tolerated and effective in the management of MC in healthy children. So far, no in-depth studies have been conducted for evaluating the efficacy of topical therapies in the management of MC. NO is an effective antiviral agent that provides localized immunity against foreign pathogens.40 The application of topical NO therapy is limited by its inconvenience and lack of safe methods of delivery to the target sites of infection or inflammation. SB206 consists of two components, namely, berdazimer sodium and a hydrogel, and the former has been demonstrated to exhibit antiviral effects. After application, SB206 could enhance NO release from the macromolecules.43 Hebert et al48 conducted a randomized clinical trial for evaluating the efficacy and tolerability of the NO-releasing topical gel and found that the application of 12% SB206 once a day provided the best balance between MC lesion clearance and tolerability.
Parasitic Skin Infections
Scabies-A Common Contagious Skin Disorder Caused by the Mite Sarcoptes scabiei
Scabies is a global public health concern, affecting individuals of all ages, genders, and races, particularly in developing countries, and is characterized by pruritus which aggravates at night, and scabietic nodules. The most typical symptom of scabies is intense itching. Owing to their adverse effects, oral therapies are poor treatment options for scabies. Hence, topical acaricides, such as crotamiton (CRT) 10% cream, permethrin 5% cream, lindane 1% cream, etc., comprise the major treatment options. Although CRT 10% lotion or cream is approved by Food and Drug Administration (FDA) for the treatment of adults, its poor solubility, low bioavailability, and frequency of adverse events limit its clinical applicability.44 TTO has shown acaricidal effects; however, its application is limited by the tendency to evaporate rapidly from the skin surface, thereby limiting its penetration into the deeper dermal layers.45 Therefore, Chen et al46 formulated a CRT-loaded TTO ME-based hydrogel having a huge potential to overcome the limitations of CRT and TTO. When compared with classical CRT formulations, the optimized CRT-loaded ME-based hydrogel enhanced the epidermal deposition of CRT with minimal adverse effects. Benzyl benzoate (BB) is used as an alternative classical agent for the treatment of scabies, but the application of concentrated lotion (25%) in topical BB therapy is generally associated with an initial acute burning sensation. After repeated topical application, further side effects like blister information, itching, crusting, reddening, and scaling of skin could occur. Sharma et al55 developed an optimized formulation of BB-loaded ME hydrogel, and the results demonstrated that the optimized formulation offered threefold higher percutaneous BB permeation across the stratum corneum and improved skin targeting properties when compared to the conventional formulation. Moreover, it demonstrated the desired characteristic of slow drug release without irritation, thus providing an attractive alternative treatment for scabies.
Cutaneous Leishmaniasis-A Zoonotic Disease Caused by Leishmania
Leishmaniasis is one of the most neglected tropical diseases, mainly due to a lack of adequate treatment options. The cutaneous form of leishmaniasis is the most prevalent, with an annual incidence of 1–1.5 million cases. Cutaneous leishmaniasis (CL) mainly causes primary skin ulcers and generally does not lead to visceral lesions. It is primarily caused by Leishmania tropicalis and L. major, which are prevalent in some countries of the Middle East and North Africa. Although the skin lesions may heal within 2 to 12 months, patients are at a high risk of infections caused by bacteria and other pathogens during the recovery phase, resulting in long-lasting ulcers. The CL treatment is expected to accelerate healing, reduce the risk of scarring, prevent parasite dissemination, and reduce the chance of relapse. The World Health Organization has recommended local treatment options to manage L. tropica infections, including intralesional injection of antimonials. However, this strategy has some constraints such as costs, risk of adverse effects, development of drug resistance, raw material shortages, and manufacturing quality concerns. Jebran et al42 conducted a clinical trial consisting of L. tropical CL patients treated with bipolar high-frequency electrocauterization (EC) followed by daily moist wound therapy (MWT) using a polyacrylate hydrogel infused with or without pharmaceutical sodium chlorite (DAC N-055). The clinical trial results revealed that EC plus MWT using a polyacrylate hydrogel induced rapid wound closure, compared to the classical intralesional antimony injection, and represents a simple and highly efficient method for the clinical treatment of CL skin lesions. Additionally, buparvaquone (BPQ), a hydroxynaphthoquinone with in vitro activity in the nanomolar range against Leishmania sp., has limited application as a viable clinical treatment due to its poor oral bioavailability induced by its poor aqueous solubility. Both the hydrous gel and the water-in-oil emulsion of BPQ were demonstrated to significantly reduce cutaneous parasite burdens.49,50 Therefore, Lalatsa et al51 developed an optimized topical self-nanoemulsifying drug delivery system (SNEDDS) loaded with BPQ for CL treatment. As a non-invasive topical CL treatment option, the BPQ-SNEDDS hydrogel is readily scalable, cost-effective, and exhibits potential clinical applicability. Ivermectin (IVM) is another antiparasitic drug that has been evaluated for the treatment of leishmaniasis in several studies.51,52 Romero et al53 developed a new method of administering IVM for CL treatment using thermosensitive hydrogels based on poloxamers. This formulation allowed the controlled release of IVM and is considered a promising and improved treatment for CL. The application of Amphotericin B (Amph-B), an antifungal drug used intravenously for the treatment of leishmaniasis, is clinically restricted due to the risk of adverse effects, such as arrhythmia and renal dysfunctions. Therefore, Oliveira et al54 applied Cobalt-60 gamma irradiation for crosslinking a polymeric hydrogel before the incorporation of Amph-B into the gel to effectively avoid adverse effects induced by the systemic administration of the drug.
Concluding Marks and Future Perspectives
In this review, we have summarized the recent advances in the applications of different hydrogel dressings for the treatment of several skin infections. We also highlighted a new trend of hydrogel-based therapies applied to skin infections, which might prove to be useful for clinical physicians and researchers. The hydrogel dressings can be used both as drug carriers for the treatment of infectious skin diseases and as auxiliary methods for the alleviation of pain during the treatment process. For example, 2.5% lidocaine/prilocaine hydrogel is used for rapid pain alleviation during the freezing treatment of MC. Several classical anti-infective drugs, such as BB, ACV, etc., can be incorporated into hydrogels to significantly improve their efficacy and tolerability. Some drugs, eg, dapsone and itraconazole, that previously could only be administered orally or intravenously, can be infused into hydrogel dressings to significantly reduce the adverse reactions caused by their systematic administration. At present, hydrogels are widely used with good efficacy for skin wound healing, but their clinical applications in the treatment of skin infections are less frequently reported. For example, physical therapies such as freezing, are preferred treatments for recalcitrant plantar warts, while topical agents are rarely used in clinical medicine due to their poor efficacy and irritability. Therefore, it can be beneficial to formulate a type of hydrogel dressing that can be applied for the effective treatment of plantar warts. Moreover, rare dermatological infections, such as non-tuberculous mycobacterium infections and certain fungal infection-related skin diseases have always been difficult to treat, and the application of hydrogels might provide a breakthrough in the management of such infections. However, a large number of hydrogel-based therapies against skin infections mentioned in this review are still in the stage of clinical trials. An increasing number of practical questions should be discussed and solved further. For instance, an appropriate time and interval are significant for hydrogel-based administration especially in the early stages of skin infections to possibly shorten the disease course. Additionally, the majority of previous studies have not evaluated the important properties, such as the long-term efficacy and tolerability, biodegradability, and possible routes of excretion of the applied hydrogels. Therefore, it is important to overcome these issues in future studies, which can eventually optimize the existing hydrogels and contribute to the clinical approval of these products. In conclusion, the applications of hydrogels for the treatment of skin infections need to be further investigated and evaluated, as they have the potential to be a powerful treatment and diagnosis strategy in this field.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khan MUA, Razak SIA, Haider S, Mannan HA, Hussain J, Hasan A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int J Biol Macromol. 2022;208:475–485. doi:10.1016/j.ijbiomac.2022.03.091
2. Khan MUA, Yaqoob Z, Ansari MNM, et al. Chitosan/poly vinyl alcohol/graphene oxide based pH-responsive composite hydrogel films: drug release, anti-microbial and cell viability studies. Polymers. 2021;13(18):3124. doi:10.3390/polym13183124
3. Peppas NA, Bures P, Leobandung W, Ichikawa HE. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50(1):27–46. doi:10.1016/S0939-6411(00)00090-4
4. Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products - ScienceDirect. Eur Polym J. 2015;65:252–267. doi:10.1016/j.eurpolymj.2014.11.024
5. Hubbell JA. Hydrogel systems for barriers and local drug delivery in the control of wound healing. J Control Release. 1996;39(2–3):305–313. doi:10.1016/0168-3659(95)00162-X
6. Khan MUA, Razaq SIA, Mehboob H, Rehman S, Al-Arjan WS, Amin R. Antibacterial and hemocompatible pH-responsive hydrogel for skin wound healing application: in vitro drug release. Polymers. 2021;13(21):3703. doi:10.3390/polym13213703
7. Khan MUA, Iqbal I, Ansari MNM, et al. Development of antibacterial, degradable and pH-responsive chitosan/guar gum/polyvinyl alcohol blended hydrogels for wound dressing. Molecules. 2021;26(19):5937. doi:10.3390/molecules26195937
8. Rizwan M, Yahya R, Hassan A, et al. pH sensitive hydrogels in drug delivery: brief history, properties, swelling, and release mechanism, material selection and applications. Polymers. 2017;9(4):137.
9. Koga T, Tomimori K, Higashi N. Transparent, high-strength, and shape memory hydrogels from thermo-responsive amino acid-derived vinyl polymer networks. Macromol Rapid Commun. 2020;41(7):e1900650. doi:10.1002/marc.201900650
10. Fisher DR, Fidel J, Maitz CA. Direct interstitial treatment of solid tumors using an injectable yttrium-90-polymer composite. Cancer Biother Radiopharm. 2020;35(1):1–9. doi:10.1089/cbr.2019.2947
11. Raman R, Hua T, Gwynne D, et al. Light-degradable hydrogels as dynamic triggers for gastrointestinal applications. Sci Adv. 2020;6(3):eaay0065. doi:10.1126/sciadv.aay0065
12. Chaves LL, Silveri A, Vieira ACC, et al. pH-responsive chitosan based hydrogels affect the release of dapsone: design, set-up, and physicochemical characterization. Int J Biol Macromol. 2019;133:1268–1279. doi:10.1016/j.ijbiomac.2019.04.178
13. Bisht A, Hemrajani C, Rathore C, et al. Hydrogel composite containing azelaic acid and tea tree essential oil as a therapeutic strategy for Propionibacterium and testosterone-induced acne. Drug Deliv Transl Res. 2022;12(10):2501–2517. doi:10.1007/s13346-021-01092-4
14. Frade ML, de Annunzio SR, Calixto GMF, Victorelli FD, Chorilli M, Fontana CR. Assessment of chitosan-based hydrogel and photodynamic inactivation against Propionibacterium acnes. Molecules. 2018;23(2):473. doi:10.3390/molecules23020473
15. Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi:10.1016/S0140-6736(11)60321-8
16. Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2010;18(10):821–832.
17. O’Neill A, Gallo R. Host-microbiome interactions and recent progress into understanding the biology of acne vulgaris. Microbiome. 2018;6. doi:10.1186/s40168-018-0558-5
18. Blaskovich M, Elliott AG, Kavanagh AM, Ramu S, Cooper MA. In vitro antimicrobial activity of acne drugs against skin-associated bacteria. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-50746-4
19. Pazyar N, Yaghoobi R, Bagherani N, Kazerouni A. A review of applications of tea tree oil in dermatology. Int J Dermatol. 2013;52(7):784–790. doi:10.1111/j.1365-4632.2012.05654.x
20. Sevimli Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992. doi:10.1177/0300060519847367
21. Bowen AC, Antoine M, Hay RJ, et al. The global epidemiology of impetigo: a systematic review of the population prevalence of impetigo and pyoderma. PLoS One. 2015;10(8):e0136789. doi:10.1371/journal.pone.0136789
22. Romani L, Steer AC, Whitfeld MJ, Kaldor JM. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015;15(8):960–967. doi:10.1016/S1473-3099(15)00132-2
23. Salarian AA, Mollamahale YB, Hami Z, Soltani-Rezaee-Rad M. Cephalexin nanoparticles: synthesis, cytotoxicity and their synergistic antibacterial study in combination with silver nanoparticles. Mater Chem Phys. 2017;198:125–130. doi:10.1016/j.matchemphys.2017.05.059
24. Poulikakos P, Falagas ME. Aminoglycoside therapy in infectious diseases. Expert Opin Pharmacother. 2013;14(12):1585–1597. doi:10.1517/14656566.2013.806486
25. Brown J, Shriner DL, Schwartz RA, Janniger CK. Impetigo: an update. Int J Dermatol. 2010;42(4):251–255. doi:10.1046/j.1365-4362.2003.01647.x
26. Salatin S, Lotfipour F, Jelvehgari M. Preparation and characterization of a novel thermosensitive and bioadhesive cephalexin nanohydrogel: a promising platform for topical antibacterial delivery. Expert Opin Drug Deliv. 2020;17(6):1–13. doi:10.1080/17425247.2020.1764530
27. Augustin M, Goepel L, Jacobi A, Bosse B, Mueller S, Hopp M. (2017). Efficacy and tolerability of liposomal polyvinylpyrrolidone-iodine hydrogel for the localized treatment of chronic infective, inflammatory, dermatoses: an uncontrolled pilot study. Clin Cosmet Investig Dermatol, 10 373–384. doi: 10.2147/CCID.S141887
28. Aggarwal N, Goindi S. Preparation and evaluation of antifungal efficacy of griseofulvin loaded deformable membrane vesicles in optimized Guinea pig model of Microsporum canis--dermatophytosis. Int J Pharm. 2012;437(1–2):277–287. doi:10.1016/j.ijpharm.2012.08.015
29. Kumar N, Goindi S. Statistically designed nonionic surfactant vesicles for dermal delivery of itraconazole: characterization and in vivo evaluation using a standardized Tinea pedis infection model. Int J Pharm. 2014;472(1–2):224–240. doi:10.1016/j.ijpharm.2014.06.030
30. Jain S, Jain S, Khare P, Gulbake A, Bansal D, Jain S K. (2010). Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv, 17(6), 443–51. doi: 10.3109/10717544.2010.483252
31. Looker KJ, Magaret AS, May MT, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10(10):e0140765. doi:10.1371/journal.pone.0140765
32. Looker KJ, Magaret AS, Turner K, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10:e114989.
33. Sahoo S, Pani N Ranjan, Sahoo S Kumar. (2016). Effect of microemulsion in topical sertaconazole hydrogel: in vitro and in vivo study. Drug Deliv, 23(1), 338–45. doi: 10.3109/10717544.2014.914601
34. Sharma G, Thakur K, Setia A, et al. Fabrication of Acyclovir-loaded flexible membrane vesicles (FMVs): evidence of preclinical efficacy of antiviral activity in murine model of cutaneous HSV-1 infection. Drug Deliv Transl Res. 2017;7(5):683–694. doi:10.1007/s13346-017-0417-0
35. Piotr O, Emilia T, Marianna G, et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS One. 2014;9(8):e104113. doi:10.1371/journal.pone.0104113
36. Nguyen HP, Franz E, Stiegel KR, Hsu S, Tyring SK. Treatment of molluscum contagiosum in adult, pediatric, and immunodeficient populations. J Cutan Med Surg. 2014;18(5):1–8.
37. Al-Subaie M M, Hosny K M, El-Say K Mohamed, Ahmed T A, Aljaeid B M. (2015). Utilization of nanotechnology to enhance percutaneous absorption of acyclovir in the treatment of herpes simplex viral infections. Int J Nanomedicine, 10 3973–85. doi: 10.2147/IJN.S83962
38. Jessica FMD, Carl-Fredrik WMD. EMLA cream provides rapid pain relief for the curettage of molluscum contagiosum in children with atopic dermatitis without causing serious application‐site reactions. Pediatr Dermatol. 2010;15(4):309–312.
39. Gagliano-Jucá T, Castelli MR, Mendes GD, Arruda A, Nucci GD. Pharmacokinetic and pharmacodynamic evaluation of a nanotechnological topical formulation of lidocaine/prilocaine (Nanorap) in healthy volunteers. Ther Drug Monit. 2015;37(3):362. doi:10.1097/FTD.0000000000000156
40. Stasko N, Mchale K, Hollenbach SJ, Martin M, Doxey R. Nitric oxide-releasing macromolecule exhibits broad-spectrum anti-fungal activity and utility as a topical treatment for superficial fungal infections. Antimicrob Agents Chemother. 2018;62(7):e01026–e01017.
41. Szymańska E, Orłowski P, Winnicka K, Tomaszewska E, Bąska P, Celichowski G, Grobelny J, Basa A, Krzyżowska M. (2018). Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int J Mol Sci, 19(2). doi: 10.3390/ijms19020387
42. Jebran AF, Schleicher U, Steiner R, et al. Rapid healing of cutaneous leishmaniasis by high-frequency electrocauterization and hydrogel wound care with or without DAC N-055: a randomized controlled phase iia trial in Kabul. PLoS Negl Trop Dis. 2014;8(2):e2694. doi:10.1371/journal.pntd.0002694
43. Tyring SK, Rosen T, Berman B, Stasko N, Maeda-Chubachi T, Phase A. 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17(10):1100–1105.
44. Mila-Kierzenkowska C, Woźniak A, Krzyżyńska-Malinowska E, Kałużna L, Owcarz M. Comparative efficacy of topical pertmehrin, crotamiton and sulfur ointment in treatment of scabies. J Arthropod Borne Dis. 2017;11(1):1–9.
45. Hada E, Derda M, Cholewiński M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol Res. 2017;116(3):997–1001. doi:10.1007/s00436-017-5377-2
46. Chen L, Alrobaian M, Afzal O, et al. Crotamiton-loaded tea tree oil containing phospholipid-based microemulsion hydrogel for scabies treatment: in vitro, in vivo evaluation, and dermatokinetic studies. Drug Deliv. 2021;28(1):1972–1981. doi:10.1080/10717544.2021.1979131
47. Gobbato A A, Babadópulos T, Gobbato C A, Moreno R A, Gagliano-Jucá T, De Nucci G. (2016). Tolerability of 2.5% Lidocaine/Prilocaine Hydrogel in Children Undergoing Cryotherapy for Molluscum Contagiosum. Pediatr Dermatol, 33(3), e214–5. doi: 10.1111/pde.12842
48. Hebert A A. Siegfried E C, Durham T, de León E N, Reams T, Messersmith E, Maeda-Chubachi T. (2020)Efficacy and tolerability of an investigational nitric oxidereleasing topical gel in patients with molluscum contagiosum: A randomized clinical trial. J Am Acad Dermatol;)Efficacy and tolerability of an investigational nitric oxidereleasing topical gel in patients with molluscum contagiosum: A randomized clinical trial. J Am Acad Dermatol;82(4):887–894. doi:10.1016/j.jaad.2019.09.064
49. Smith LA, Serrano DR, Mauger M, Bolás-Fernández F, Dea-Ayuela MA, Lalatsa A. Orally bioavailable and effective buparvaquone lipid-based nanomedicines for visceral leishmaniasis. Mol Pharm. 2018;15(7):2570–2583. doi:10.1021/acs.molpharmaceut.8b00097
50. Venkatesh G, Majid M, Mansor SM, Nair NK, Croft SL, Navaratnam V. In vitro and in vivo evaluation of self-microemulsifying drug delivery system of buparvaquone. Drug Dev Ind Pharm. 2010;36(6):735. doi:10.3109/03639040903460446
51. Lalatsa A, Statts L, Adriana de Jesus J, et al. Topical buparvaquone nano-enabled hydrogels for cutaneous leishmaniasis. Int J Pharm. 2020;588:119734. doi:10.1016/j.ijpharm.2020.119734
52. Crump A. Ivermectin: enigmatic multifaceted ‘wonder’ drug continues to Surprise and exceed expectations. J Antibiot. 2017;70(5):495–505. doi:10.1038/ja.2017.11
53. Romero AI, Cid AG, Minetti NE, et al. Sustained-release hydrogels of ivermectin as alternative systems to improve the treatment of cutaneous leishmaniasis. Ther Deliv. 2020;11(12):779–790. doi:10.4155/tde-2020-0090
54. Oliveira MJA, Villegas GME, Motta FD, et al. Influence of gamma radiation on Amphotericin B incorporated in PVP hydrogel as an alternative treatment for cutaneous leishmaniosis. Acta Trop. 2021;215:105805. doi:10.1016/j.actatropica.2020.105805
55. Sharma G, Dhankar G, Thakur K, Raza K, Katare O P. (2016). Benzyl Benzoate-Loaded Microemulsion for Topical Applications: Enhanced Dermatokinetic Profile and Better Delivery Promises. AAPS PharmSciTech, 17(5), 1221–31. 10.1208/s12249-015-0464-0
56. Scollard DM. Infection with Mycobacterium lepromatosis. Am J Trop Med Hyg. 2016;95(3):500–501. doi:10.4269/ajtmh.16-0473
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
