Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Mechanistic Regulation of Wnt Pathway-Related Progression of Chronic Obstructive Pulmonary Disease Airway Lesions
Received 27 September 2022
Accepted for publication 28 April 2023
Published 15 May 2023 Volume 2023:18 Pages 871—880
DOI https://doi.org/10.2147/COPD.S391487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Minrong Liu,* Yating Huo,* Yuanxiong Cheng
Department of Respiratory and Critical Care Medicine, The Third Affiliated Hospital, Southern Medical University, Guangzhou, Guangdong, 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanxiong Cheng, Department of Respiratory and Critical Care Medicine, The Third Affiliated Hospital, Southern Medical University, 183 Zhongshan Dadao West, Tianhe District, Guangzhou, Guangdong, 510630, People’s Republic of China, Tel +86 137 2985 2986, Fax +86 020 62784382, Email [email protected]
Abstract: Chronic obstructive pulmonary disease (COPD) is a chronic disease associated with inflammation and structural changes in the airways and lungs, resulting from a combination of genetic and environmental factors. This interaction highlights significant genes in early life, particularly those involved in lung development, such as the Wnt signaling pathway. The Wnt signaling pathway plays an important role in cell homeostasis, and its abnormal activation can lead to the occurrence of related diseases such as asthma, COPD, and lung cancer. Due to the fact that the Wnt pathway is mechanically sensitive, abnormal activation of the Wnt pathway by mechanical stress contributes to the progression of chronic diseases. But in the context of COPD, it has received little attention. In this review, we aim to summarize the important current evidence on mechanical stress through the Wnt pathway in airway inflammation and structural changes in COPD and to provide potential targets for COPD treatment strategies.
Keywords: mechanical signal, Wnt signaling pathway, COPD, airway remodeling, β-catenin
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic disease associated with inflammation and structural changes in the airways and lungs, characterized by irreversible airflow limitation caused by abnormal airway (broncho inflammation) and lung parenchyma (emphysema).1,2 Airway remodeling is an essential cause of irreversible airflow limitation in COPD, which is closely related to the severity of the disease.3 A recent study based on family and population shows that environmental factors such as smoking cannot fully explain the susceptibility and heterogeneity of COPD, and a large part of the risk of COPD is related to genetic variation.4 In recent years, the incidence of COPD has been increasing globally, and it has surpassed cancer to become the third leading cause of death.5 As a fatal chronic lung disease, COPD imposes a heavy health and economic burden around the world.6 At present, the clinical treatment for COPD patients with dyspnea and shortness of breath after activity caused by pathological changes such as airway remodeling is still very limited. Therefore, it is crucial and urgent to understand the mechanisms underlying COPD airway remodeling and other pathological changes in order to find potential therapeutic targets to overcome the shortcomings of current therapies.
Wnt-dependent effects were discovered more than 30 years ago, mainly during embryogenesis in organisms such as sea urchins, Drosophila melanogaster, and Xenopus laevis.1 The Wnt signaling pathway plays a vital role in the processes of stem cell differentiation, embryonic development, and homeostasis, and its abnormal activation can lead to the occurrence of related diseases,7 such as asthma, COPD, cancer, idiopathic pulmonary fibrosis, and other diseases.8–10 According to research, the Wnt signaling pathway not only regulates airway inflammation11 but also plays an crucial role in the process of airway structural changes such as remodeling, goblet cell metaplasia, and airway smooth muscle (ASM) proliferation.1
Mechanical regulation is defined as the cell’s perception of extracellular mechanical signals via the integrin and actomyosin cytoskeleton, followed by a mechanical regulatory process that leads to the expression of related genes.12 Mechanical signaling influences multiple biological processes during development and in adult organisms, including cell fate transition, cell migration, morphogenesis, and immune response.13–16 Mechanical signals direct cell function and fate, which are ultimately coordinated by intracellular signaling pathways such as Wnt, whose guiding signaling pathway is mechanically sensitive.17 A study published in the New England Journal of Medicine showed that non-inflammatory bronchoconstriction (methacholine) can cause airway remodeling in asthmatic patients.18 The research discovered that Wnt3A not only induced phosphorylation of GSK-3β and accumulation of β-catenin, but also RhoA activation in RAW264.7 and HEK293 cells. Notably, sh-RhoA abrogated GSK-3B phosphorylation and β-catenin accumulation.19
More and more studies have shown that mechanical signaling is highly related to the role of the Wnt pathway in the occurrence and development of airway diseases, involving airway inflammation, airway remodeling, and other changes. These and other studies have led us to have considerable interest in how Wnt signaling is coupled to mechanical signaling and plays a regulatory role in the progression of COPD airway disease. In this review, we highlight and discuss the current molecular mechanisms by which mechanical signals are involved in airway inflammation and structural alterations in COPD through the Wnt pathway, as well as how they may influence pharmacological approaches for COPD.
Overview of COPD
COPD is a common and treat incompletely reversible disease characterized by persistent respiratory symptoms and airflow limitation due to respiratory and or alveolar abnormalities. The chronic airflow limitation that characterizes COPD is caused by a combination of small airway disease (eg, obstructive bronchiolitis) and parenchymal destruction (emphysema).20,21 Increased numbers of different cells, such as neutrophils, macrophages, CD8+ T cells, and various inflammatory mediators, have been reported.22 Airway remodeling, as a significant feature of chronic inflammatory airway diseases such as chronic obstructive pulmonary disease, is closely related to irreversible airflow limitation. Airway remodeling is the term used to describe structural changes in the airway wall caused by repeated injury and repair processes, such as ASMC proliferation and migration, goblet cell metaplasia, basement membrane thickening, subepithelial fibrosis, and airway neovascularization.3
It is commonly believed that the development of COPD is caused by heavy exposure to harmful particles or gases, especially smoking. However, research in recent years has discovered that host factors, like genetic abnormalities, aberrant lung development, and accelerated aging, also predispose individuals to developing COPD. Overall, COPD is the end result of a series of dynamic, interactive, and cumulative gene-environment interactions from pregnancy to death.21
Overview of the Wnt Signaling Pathway
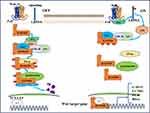
Since the first member of the Wnt family was first identified 35 years ago,23 the study of Wnt signaling has been increasingly extensive, ranging from cancer and development to early animal evolution. Wnt signaling pathway is a tightly controlled and highly conserved pathway.24 In mammals, there are 19 different Wnt family members involved in regulating embryogenesis and controlling various processes in the later stages of life, including embryonic development, cell proliferation, survival, migration, polarity, cell fate determination, and stem cell self-renewal.25,26 Not surprisingly, Wnt ligand levels or the activity of their downstream effectors can alter normally preferred pathways, induce developmental defects, and contribute to the etiology of the disease.27 As a key signaling molecule of the Wnt pathway, β-catenin exists in three different intracellular pools: membranous, cytoplasmic and nuclear.28 β-catenin acts as both an adaptor protein and a transcription coregulator.29 On the one hand, beta-catenin interacts with E-cadherin on the cell membrane and plays an important structural role in cell-to-cell adhesion connection.More specifically, actin - α-catenin - beta-catenin - E-cadherin Interactions throughout the structure facilitate adhesion connexin aggregates, thus stabilizing cell adhesion, and through the interaction between the N-terminus of beta-catenin and a-catenin, beta-catenin is connected to the actin cytoskeleton.30,31 On the other hand, when involved in intracellular Wnt signaling pathway transduction, β-catenin, which is not captured by the destruction complex for degradation, accumulates in cytoplasm and translocates into the nucleus to participate in the transcriptional regulation of downstream genes. However, the spatial separation of β-catenin at plasma membrane, cytoplasm and nucleus is regulated by specific phosphorylation mechanisms.32 According to the downstream involvement of beta-catenin, the Wnt signaling pathway is divided into canonical and noncanonical, and the latter is further divided into Wnt/PCP (planar cell polarity) signaling pathway and Wnt-cGMP/Ca2+ signaling pathway based on phenotypic responses.33,34 The key switch in the canonical Wnt pathway is the cytoplasmic protein beta-catenin, whose stability is controlled by the destruction complex (DC), structurally composed of the Axin as a scaffold with tumor-suppressor protein APC, and two constructively active serine-threonine kinases (CK1 and GSK3β) Interaction composition.35 In the absence of Wnt ligand, beta-catenin is sequestrated in the cytoplasm and captured by the destruction complex for phosphorylation. The phosphorylated beta-catenin serves as a docking site for the f-box-containing protein E3 ubiquitin ligase b-Trcp due to its “degron” -motif, inducing ubiquitination and subsequent proteasomal degradation of beta-catenin (Figure 1).36,37
The Canonical Wnt Signaling Pathway
In the canonical Wnt signaling pathway, Wnt ligands (eg, Wnt1 and Wnt3a ligands) and their LDL receptor-related protein 5 (LRP5/6) bind to act as activators of the Wnt/β-catenin pathway.38 This interaction recruited Dishevelled and phosphorylated the cytosolic domain of LRP5/6, which resulted in β-catenin disruption complex translocation to the cytosolic side of the plasma membrane39 and inhibition of glycogen synthase kinase 3 (GSK-3). This leads to the formation of a so-called signaling body, which is internalized and targeted to multivesicular bodies.40–43 Therefore, the β-catenin destruction complex cannot target the newly synthesized β-catenin for degradation. Stable β-catenin accumulates in the cytoplasm and translocates to the nucleus, where it binds to TCF/LEF transcription factors and activates gene transcription by binding to specific regulatory sequences.44
The Noncanonical Wnt Signaling Pathway
Noncanonical Wnt signaling is triggered by the binding of Wnt ligands to Frizzled receptor and various coreceptors, such as receptor tyrosine kinase-like orphan receptor 1/2 (ROR1/2) or receptor tyrosine kinase (Ryk).45 Noncanonical Wnt ligands such as Wnt4, Wnt5A, Wnt7A, Wnt7B, and Wnt11, through other intracellular effectors such as calcium, JNK, and small GTPases Rho and Rac, lead to a “β-catenin”-independent signaling pathway, also known as the “noncanonical Wnt pathway”.46–48 According to the phenotypic response, the noncanonical Wnt signaling pathway is divided into the Wnt/planar cell polarity (PCP) signaling pathway and the Wnt/Ca2+ signaling pathway. The PCP pathway is involved in the regulation of cell polarity, movement, orientation, and other developmental processes. Signaling through small GTPases such as RhoA and Rac1, the PCP pathway activates Rho kinase (ROCK), leading to actin polymerization, and at the same time activates downstream Jun-N-terminal kinases, leading to Jun-N-terminal kinase-dependent transcription factors such as activation of the transcriptional programs of ATF2, activator protein-1, and activated t-cell nuclear factor.49 Noncanonical Wnt ligands such as Wnt5A interact with ROR family orphan receptor tyrosine kinases such as ROR2 to activate JNK and RhoA. This has been shown to inhibit the transcriptional activation potential of the β-catenin /TCF complex by modulating the tyrosine phosphorylation of β-catenin and the translocation of β-catenin from the cytoplasm to the nucleus to inhibit the canonical Wnt signaling pathway, thereby reducing the expression of the downstream target gene CyclinD1.50,51 Wnt/Ca2+ signaling regulates various developmental processes, such as cytoskeletal rearrangement, cell adhesion, and tissue segregation.52 Through Wnt-fz-induced phospholipase C (PLC) activation, it stimulates the production of diacylglycerol and inositol-1,4, 5-triphosphate Ins (1,4,5) P3. Ins (1,4,5) P3 triggers the release of calcium from intracellular stores, which increases cytoplasmic Ca2+ levels and subsequently activates calcium-dependent factors such as calmodulin-dependent kinase II (CAMKII), calcineurin, and certain isoforms of protein kinase C (PKC). These factors promote gene transcription by acting on T-cell-associated transcriptional regulatory nuclear factor (NFAT), TGF-B-activated kinase (TAK1), and neMO-like kinase (NLK).53
Correlation Between Wnt Signaling Pathway and Pulmonary Disease
The Wnt signaling cascade is a master regulator of development throughout the animal kingdom, and Wnts are also key drivers of most types of tissue stem cells in adult mammals.34 In mammals, the Wnt/ β-catenin signaling pathway mainly consists of three steps, including Wnt signal transduction in the cell membrane, regulation of β-catenin stabilization in the cytoplasm and activation of Wnt target genes in the nucleus.24 Obviously, abnormal activation or destruction of the Wnt/β-catenin pathway is closely related to a variety of lung diseases, including asthma, IPF, cancer, etc.54–56
Regulation of Wnt Signaling Pathway in Airway Inflammation and Remodeling in COPD
In chronic obstructive pulmonary disease, the Wnt signaling pathway plays an important immunomodulatory role in inflammation and airway remodeling in COPD patients. In COPD, airway inflammation is mainly caused by inhaled cigarette smoke or other harmful particles. Pathological changes were seen in the airways, lung parenchyma, and pulmonary vasculature.
Cigarette smoke induces epithelial-mesenchymal transformation (EMT) by activating the Wnt/beta-catenin signaling pathway.57 During acute inflammation, Wnt5a is involved in inducing the anti-inflammatory effects of macrophages to prevent excessive and harmful immune responses.58,59 Studies have shown that miR-149-3p regulates the expression of Wnt1, beta-catenin, RhoA, and Wnt5a, affects the signaling of Wnt pathway, and leads to changes in the expression of alveolar inflammatory factors. Ultimately, it affects the progression of COPD.60
The study found that serum SFRP1 levels were higher in COPD patients than in healthy volunteers, and higher in AECOPD patients than in STCOPD patients. It is suggested that SFRP1, as a member of the largest family of Wnt signaling pathway inhibitors, is involved in the occurrence and development of COPD by competitively binding to Frizzled receptor on the cell membrane to inhibit the Wnt pathway.10,61 By analyzing the transcriptome of wild-type and mutant lungs, Wnt/RYK signaling was observed to regulate chronic obstructive pulmonary disease by up-regulating inflammatory genes in vitro.62 These data suggest that Wnt signaling is important to avoid an overwhelming and harmful inflammatory response during acute infection.
Wnt signaling pathway also plays a key role in airway changes, such as hyperplastic goblet cells with excessive mucus secretion, small airway wall thickening.22 After nicotine exposure, activation of both Wnt3a and the canonical Wnt/ β-catenin pathway is up-regulated in epithelial cells.63,64 Activation of β-catenin was accompanied by an increase in mesenchymal markers such as α-SMA, vimentin, and type I collagen, as well as a downregulation of E-cadherin. Knockdown of Wnt3a prevented the observed effect.65 In fibroblasts, hyperplasia suppressor gene (HSG) is found to inhibit airway remodeling in COPD by inhibiting the Wnt pathway and reducing beta-catenin expression to reduce cell viability.66 Reduced levels of DKK1, an endogenous inhibitor of Wnt, are seen as more positive changes in lung function in COPD patients.67 Studies show that in primary human airway smooth muscle cells, activation of Wnt/beta-catenin signaling pathway participates in TGF-beta-induced ECM protein deposition, including increased expressions of collagen I, fibronectin, multifunctional proteoglycan, laminin, and core proteoglycan, providing new insights for the occurrence and development of airway remodeling in lung diseases.68
An Overview of Mechanical Regulation
Mechanical regulation has profound implications for biology through cooperation between mechanical inputs, cell-surface mechanics, and intracellular signals. Mechanical inputs include outside-in mechanical signals, such as mechanical sensing of substrate properties or shear stresses, and mechanical signals regulated by the physical properties of the cell surface itself.
Mechanical signals are sensed by cells via integrins on the cell membrane and the intracellular actomyosin cytoskeleton, which activates effector molecules downstream of the pathway.17 RhoA is generally regarded as a major regulator of cellular actin organization, mechanics, and is a member of the Rho family of small GTPases.69,70 Rho-associated protein kinase (ROCK) is one of the effector molecules downstream of RhoA with serine-threonine kinase activity.71 RhoA/ROCK plays a non-negligible role in the mechanical regulation of cell fate selection by regulating actin-related mechanisms.17 However, mechanistic changes in cell function and fate cannot be guided without the coordination of intracellular signaling pathways.
Close Coordination of Mechanical Regulation and Wnt
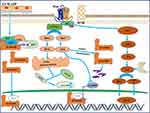
In recent years, the relationship between mechanics and the Wnt signaling pathway has become more and more close (Figure 2). In the noncanonical signaling pathway of Wnt, Dishevelled protein and activated Daam1 bind to RhoGEF (a weakly similar GEF) to promote RhoAGTP and induce ROCK activation and dynamic cytoskeletal rearrangement.72 It has also been confirmed that Wnt signaling regulates the structural changes of actin through noncanonical pathways, thereby regulating the transcription of corresponding cellular genes, while the actin remodeling process related to cell polarization and motility is controlled by signals sent by RAC and RHO activation.31 Kuldeep Kumawat et al showed that Wnt-11 regulates ASM cells by activating RhoA and subsequent actin cytoskeleton remodeling in response to TGF-β1-induced upregulation of SM-A actin expression in airway smooth muscle cells, namely the Wnt-11-dependent Rho-kinase-actin-MRTF-A signaling axis expression of SM-A actin.73 In recent years, new target proteins of Rho-associated kinases (ROCK) have been discovered, including glycogen synthase kinase (GSK), an important effector molecule of the Wnt signaling pathway.74 Studies in transgenic mice showed that mechanical signals from extracellular matrix stiffness increase actomyosin-mediated cell tone through activation of the Rho/ROCK pathway, thereby promoting beta-catenin stabilization and nuclear translocation, leading to excessive proliferation and epidermal hyperplasia, and eventually papilloma progression to carcinoma. TCF/LEF target gene transcripts indicated that transgenic mice with enhanced ROCK activity exhibited higher collagen deposition and matrix hardness, which correlated with enhanced β-catenin activity.75
The Role of Mechanical and Wnt Coupling in the Development of COPD
The regulatory role of mechano mechanical signals in airway inflammation and structural changes in COPD by coupling with the Wnt pathway is becoming more and more interesting. The RhoA/ROCK signaling pathway is known to play an important role in airway remodeling, partly by regulating the proliferation and differentiation of airway smooth muscle cells.76 The study found that in the asthma model in mice, acetyl choline a repeated stimulation induced bronchoconstriction can maintain eosinophilic inflammation and airway remodeling, further illustrate the bronchoconstriction caused by mechanical stress to the epithelium and airway smooth muscle cells and is considered to maintain an important factor of induced allergic airway inflammation and remodeling.18 The research showed that the addition of a muscarinic receptor antagonist, Luotoacil, to primary human airway smooth muscle cells could inhibit methacholine induced airway remodeling, such as the deposition of extracellular matrix, by inhibiting the Wnt-β catenin signaling pathway.68 Research also demonstrated that RhoA/ROCK signaling is involved in allergen-induced airway remodeling by upregulating Wnt signaling effector lymphoenhancer binding factor 1 (Lef1).77 Moreover, studies showed that miR-149-3p regulates the expression of Wnt1, beta-catenin, RhoA, and Wnt5a, affects the signaling of Wnt pathway, and leads to changes in the expression of alveolar inflammatory factors. Ultimately, it influences the progression of COPD.60 The research found that during lung hyperexpansion, the myogenic response of human airways to acute mechanical stress is dependent on Rho kinase and Wnt signaling pathways, thus generating the release of leukotriene through Nitric Oxide mediated after stretching the bronchus.78 After exposure to mechanical stretching, the expression of Wnt-induced signaling protein 1 (WYP-1) in alveolar type 1 epithelial cells is significantly upregulated in a hyaluronic acid and innate immune adapter molecule Myd88-dependent manner to induce epithelial-mesenchymal transition (EMT), and the blocking of WYP-1 prevents EMT in stretched cells.79 Mechanical stress reduces the expression of crimp-associated protein SFRPs secreted in vivo and in vitro and promotes subtemporal collar joint osteoarthritis (TMJOA) through the Wnt/beta-catenin signaling pathway. Inhibition of Wnt/beta-catenin signaling pathway can save mechanical stress-induced cartilage degeneration.80 These studies suggest that Wnt signaling and bronchoconstriction play an important role in the pathogenesis of airway remodeling in COPD and that stabilizing the expression of key molecules in this pathway in the future may be a novel pharmacological approach for airway remodeling-related respiratory diseases.
Conclusion and Future Development Direction
COPD is a disease characterized by persistent airflow limitation. Our understanding of the pathogenesis of COPD is still lacking and growing slowly. Chronic inflammation in the airways and difficult to reverse airway remodeling are both major features of the disease. Common treatments for these two key symptoms include inhaled glucocorticoids and bronchodilators to improve airflow and symptoms and deterioration. A growing body of literature now shows that the effect of mechanical stress caused by bronchial constriction on the biological behavior of cells through downstream signaling effector molecules activated by coupling with the Wnt signaling pathway is sufficient to cause changes in pathophysiology leading to airway remodeling. That is, bronchial constriction itself turns on pathological signals that lead to COPD progression. Therefore, we must develop preemptive treatment strategies, rather than reactive treatment strategies. In support of this view, we discuss recent evidence that mechanical stress acting on the airway epithelium is involved in the molecular mechanisms of airway inflammation and structural changes in COPD via Wnt signaling. Targeting mechanical sensors and targeting gene products of Wnt signaling pathways to improve COPD treatment may be a potential therapeutic strategy.
In addition, on the one hand, abnormal activation of Wnt is associated with the development of airway inflammation in COPD, but on the other hand, Wnt has received a lot of attention in the treatment of COPD regenerative repair in recent years. The research has pointed out that activation specificity of Wnt/beta-catenin leads to an increase in the number of alveolar-like organs, thus saving the distal lung epithelial progenitor cells from the mouse model of emphysema induced by protease to show decreased organogenesis ability.81 And studies have found that the Wnt/planar cell polarity (PCP) signaling pathway controls the formation of alveoli, and the decreased Wnt5A level in COPD patients may indicate the loss of the potential for emphysema to be recoverable.82
Interestingly, study also found that Wnt/beta-catenin signaling is inactivated in COPD tissues, and its reactivation leads to improved structural recovery of alveolar epithelium. Moreover, Wnt/beta-catenin signal is activated in IPF, and inhibition of this signal can reduce lung inflammation and fibrosis.10
In the future, more studies are needed to confirm whether these different mechanisms of effect are related to the different types of cells isolated from the bronchus by the Wnt signaling pathway. Therefore, the role of Wnt coupled to mechanical signaling in airway disease in COPD needs to be considered in the context of different conditions. Overall, we are now at the point in the history of COPD treatment where the impact of mechanical stress coupled with Wnt signaling on understanding the disease is coming into focus. Efforts to find a way to interfere are still in their early stages, but there are promising leads that could soon be translated into real treatments.
Acknowledgments
This work was supported by National Natural Science Foundation of China (grant no. 82100049).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Reuter S, Beckert H, Taube C. Take the Wnt out of the inflammatory sails: modulatory effects of Wnt in airway diseases. Lab Invest. 2016;96(2):177–185. doi:10.1038/labinvest.2015.143
2. Huertas A, Palange P. COPD: a multifactorial systemic disease. Ther Adv Respir Dis. 2011;5(3):217–224. doi:10.1177/1753465811400490
3. Hirota N, Martin JG. Mechanisms of airway remodeling. Chest. 2013;144(3):1026–1032. doi:10.1378/chest.12-3073
4. Cho MH, Hobbs BD, Silverman EK. Genetics of chronic obstructive pulmonary disease: understanding the pathobiology and heterogeneity of a complex disorder. Lancet Respir Med. 2022;10(5):485–496. doi:10.1016/S2213-2600(21)00510-5
5. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
6. Mannino DM. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest. 2002;121(5 Suppl):121S–126S. doi:10.1378/chest.121.5_suppl.121S
7. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi:10.1146/annurev.cellbio.20.010403.113126
8. Kim HT, Yin W, Nakamichi Y, et al. WNT/RYK signaling restricts goblet cell differentiation during lung development and repair. Proc Natl Acad Sci U S A. 2019;116(51):25697–25706. doi:10.1073/pnas.1911071116
9. Hiremath IS, Goel A, Warrier S, Kumar AP, Sethi G, Garg M. The multidimensional role of the Wnt/β-catenin signaling pathway in human malignancies. J Cell Physiol. 2022;237(1):199–238. doi:10.1002/jcp.30561
10. Shi J, Li F, Luo M, Wei J, Liu X. Distinct roles of Wnt/β-catenin signaling in the pathogenesis of chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Mediators Inflamm. 2017;2017:3520581. doi:10.1155/2017/3520581
11. Reuter S, Martin H, Beckert H, et al. The Wnt/β-catenin pathway attenuates experimental allergic airway disease. J Immunol. 2014;193(2):485–495. doi:10.4049/jimmunol.1400013
12. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi:10.1038/nrm3896
13. Le HQ, Ghatak S, Yeung CY, et al. Mechanical regulation of transcription controls Polycomb-mediated gene silencing during lineage commitment. Nat Cell Biol. 2016;18(8):864–875. doi:10.1038/ncb3387
14. Przybyla L, Lakins JN, Weaver VM. Tissue mechanics orchestrate Wnt-dependent human embryonic stem cell differentiation. Cell Stem Cell. 2016;19(4):462–475. doi:10.1016/j.stem.2016.06.018
15. Totaro A, Castellan M, Battilana G, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:15206. doi:10.1038/ncomms15206
16. Solis AG, Bielecki P, Steach HR, et al. Author correction: mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature. 2019;575(7784):E7. doi:10.1038/s41586-019-1755-5
17. De Belly H, Paluch EK, Chalut KJ. Interplay between mechanics and signalling in regulating cell fate. Nat Rev Mol Cell Biol. 2022;23(7):465–480. doi:10.1038/s41580-022-00472-z
18. Grainge CL, Lau LC, Ward JA, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011;364(21):2006–2015. doi:10.1056/NEJMoa1014350
19. Kim JG, Kim MJ, Choi WJ, et al. Wnt3A induces GSK-3β phosphorylation and β-catenin accumulation through RhoA/ROCK. J Cell Physiol. 2017;232(5):1104–1113. doi:10.1002/jcp.25572
20. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017
21. Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi:10.1056/NEJMra1900475
22. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi:10.1146/annurev.pathol.4.110807.092145
23. Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi:10.1016/0092-8674(82)90409-3
24. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi:10.1016/j.cell.2012.05.012
25. Parsons MJ, Tammela T, Dow LE. WNT as a driver and dependency in cancer. Cancer Discov. 2021;11(10):2413–2429. doi:10.1158/2159-8290.CD-21-0190
26. Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi:10.1038/sj.cr.7290260
27. Kikuchi A, Yamamoto H, Sato A, Matsumoto S. New insights into the mechanism of Wnt signaling pathway activation. Int Rev Cell Mol Biol. 2011;291:21–71.
28. Kumar R, Bashyam MD. Multiple oncogenic roles of nuclear beta-catenin. J Biosci. 2017;42(4):695–707. doi:10.1007/s12038-017-9710-9
29. Masuda T, Ishitani T. Context-dependent regulation of the β-catenin transcriptional complex supports diverse functions of Wnt/β-catenin signaling. J Biochem. 2017;161(1):9–17. doi:10.1093/jb/mvw072
30. Wang B, Li X, Liu L, Wang M. β-Catenin: oncogenic role and therapeutic target in cervical cancer. Biol Res. 2020;53(1):33. doi:10.1186/s40659-020-00301-7
31. Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9(9):317–321. doi:10.1016/0168-9525(93)90250-L
32. Shah K, Kazi JU. Phosphorylation-dependent regulation of WNT/beta-catenin signaling. Front Oncol. 2022;12:858782. doi:10.3389/fonc.2022.858782
33. Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18(11):564–571. doi:10.1016/S0168-9525(02)02770-1
34. Xiao Q, Chen Z, Jin X, Mao R, Chen Z. The many postures of noncanonical Wnt signaling in development and diseases. Bio Pharm. 2017;93:359–369. doi:10.1016/j.biopha.2017.06.061
35. Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16(13):3797–3804. doi:10.1093/emboj/16.13.3797
36. Kitagawa M, Hatakeyama S, Shirane M, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 1999;18(9):2401–2410. doi:10.1093/emboj/18.9.2401
37. Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(6):985–999. doi:10.1016/j.cell.2017.05.016
38. Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–593. doi:10.1038/nri2360
39. Stamos JL, Chu ML, Enos MD, Shah N, Weis WI. Structural basis of GSK-3 inhibition by N-terminal phosphorylation and by the Wnt receptor LRP6. Elife. 2014;3:e1998. doi:10.7554/eLife.01998
40. Zeng X, Tamai K, Doble B, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438(7069):873–877. doi:10.1038/nature04185
41. Davidson G, Wu W, Shen J, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438(7069):867–872. doi:10.1038/nature04170
42. Gammons M, Bienz M. Multiprotein complexes governing Wnt signal transduction. Curr Opin Cell Biol. 2018;51:42–49. doi:10.1016/j.ceb.2017.10.008
43. Taelman VF, Dobrowolski R, Plouhinec JL, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi:10.1016/j.cell.2010.11.034
44. Li VS, Ng SS, Boersema PJ, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149(6):1245–1256. doi:10.1016/j.cell.2012.05.002
45. Oliva CA, Montecinos-Oliva C, Inestrosa NC. Wnt signaling in the central nervous system: new insights in health and disease. Prog Mol Biol Transl Sci. 2018;153:81–130.
46. Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5(3):367–377. doi:10.1016/S1534-5807(03)00266-1
47. Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23(3):265–277. doi:10.1101/gad.1760809
48. Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131(7):1378. doi:10.1016/j.cell.2007.12.011
49. Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi:10.1038/nrc3419
50. Yan L, Du Q, Yao J, Liu R. ROR2 inhibits the proliferation of gastric carcinoma cells via activation of non-canonical Wnt signaling. Exp Ther Med. 2016;12(6):4128–4134. doi:10.3892/etm.2016.3883
51. Yuan Y, Niu CC, Deng G, et al. The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. Int J Mol Med. 2011;27(1):63–69. doi:10.3892/ijmm.2010.560
52. Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi:10.1016/j.ceca.2005.06.022
53. De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin. 2011;43(10):745–756. doi:10.1093/abbs/gmr079
54. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi:10.1016/j.cell.2006.10.018
55. Vladar EK, Königshoff M. Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem Soc Trans. 2020;48(1):231–243. doi:10.1042/BST20190597
56. Koopmans T, Gosens R. Revisiting asthma therapeutics: focus on WNT signal transduction. Drug Discov Today. 2018;23(1):49–62. doi:10.1016/j.drudis.2017.09.001
57. Su X, Chen J, Lin X, et al. FERMT3 mediates cigarette smoke-induced epithelial-mesenchymal transition through Wnt/β-catenin signaling. Respir Res. 2021;22(1):286. doi:10.1186/s12931-021-01881-y
58. Naskar D, Maiti G, Chakraborty A, Roy A, Chattopadhyay D, Sen M. Wnt5a-Rac1-NF-κB homeostatic circuitry sustains innate immune functions in macrophages. J Immunol. 2014;192(9):4386–4397. doi:10.4049/jimmunol.1302817
59. Neumann J, Schaale K, Farhat K, et al. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010;24(11):4599–4612. doi:10.1096/fj.10-160994
60. Zhang X, Wang Y, He X, Sun Z, Shi X. Diagnosis of chronic obstructive pulmonary disease and regulatory mechanism of miR-149-3p on alveolar inflammatory factors and expression of surfactant proteins A (SP-A) and D (SP-D) on lung surface mediated by Wnt pathway. Comput Intell Neurosci. 2022;2022:7205016.
61. He Y, Yu J, Zhu D, Gao J. Predictive value of serum markers SFRP1 and CC16 in acute exacerbation of chronic obstructive pulmonary disease. Evid Based Complement Alternat Med. 2022;2022:6488935. doi:10.1155/2022/6488935
62. Kim HT, Panza P, Kikhi K, et al. WNT/RYK signaling functions as an antiinflammatory modulator in the lung mesenchyme. Proc Natl Acad Sci U S A. 2022;119(24):e2093260177. doi:10.1073/pnas.2201707119
63. Krebs M, Sakurai R, Torday JS, Rehan VK. Evidence for in vivo nicotine-induced alveolar interstitial fibroblast-to-myofibroblast transdifferentiation. Exp Lung Res. 2010;36(7):390–398. doi:10.3109/01902141003714023
64. Sakurai R, Cerny LM, Torday JS, Rehan VK. Mechanism for nicotine-induced up-regulation of Wnt signaling in human alveolar interstitial fibroblasts. Exp Lung Res. 2011;37(3):144–154. doi:10.3109/01902148.2010.490288
65. Zou W, Zou Y, Zhao Z, Li B, Ran P. Nicotine-induced epithelial-mesenchymal transition via Wnt/β-catenin signaling in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2013;304(4):L199–L209. doi:10.1152/ajplung.00094.2012
66. Ge Z, Yang Y, Zhou X, et al. Overexpression of the hyperplasia suppressor gene inactivates airway fibroblasts obtained from a rat model of chronic obstructive pulmonary disease by inhibiting the Wnt signaling pathway. Mol Med Rep. 2019;20(3):2754–2762. doi:10.3892/mmr.2019.10504
67. Dai L, Xu D, Wan C, Liu L, Wen F. DKK1 positively correlates with lung function in COPD patients and reduces airway inflammation. Int J Chron Obstruct Pulmon Dis. 2022;17:93–100. doi:10.2147/COPD.S341249
68. Huo Y, Guan L, Xu J, Zhou L, Chen R. Tiotropium inhibits methacholine-induced extracellular matrix production via β-catenin signaling in human airway smooth muscle cells. Int J Chron Obstruct Pulmon Dis. 2018;13:1469–1481. doi:10.2147/COPD.S158552
69. Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22(5):690–696. doi:10.1016/j.ceb.2010.08.020
70. Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24(1):203–216. doi:10.1093/molbev/msl145
71. Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi:10.1038/nrm1128
72. Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27(4):606–617. doi:10.1038/emboj.2008.9
73. Kumawat K, Koopmans T, Menzen MH, et al. Cooperative signaling by TGF-β1 and WNT-11 drives sm-α-actin expression in smooth muscle via Rho kinase-actin-MRTF-A signaling. Am J Physiol Lung Cell Mol Physiol. 2016;311(3):L529–L537. doi:10.1152/ajplung.00387.2015
74. Choi EK, Kim JG, Kim HJ, et al. Regulation of RhoA GTPase and novel target proteins for ROCK. Small GTPases. 2020;11(2):95–102. doi:10.1080/21541248.2017.1364831
75. Samuel MS, Lopez JI, McGhee EJ, et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell. 2011;19(6):776–791. doi:10.1016/j.ccr.2011.05.008
76. Yang Q, Shi W. Rho/ROCK-MYOCD in regulating airway smooth muscle growth and remodeling. Am J Physiol Lung Cell Mol Physiol. 2021;321(1):L1–L5. doi:10.1152/ajplung.00034.2021
77. Ke X, Do DC, Li C, et al. Ras homolog family member A/Rho-associated protein kinase 1 signaling modulates lineage commitment of mesenchymal stem cells in asthmatic patients through lymphoid enhancer-binding factor 1. J Allergy Clin Immunol. 2019;143(4):1560–1574. doi:10.1016/j.jaci.2018.08.023
78. Faisy C, Pinto FM, Le Guen M, et al. Airway response to acute mechanical stress in a human bronchial model of stretch. Crit Care. 2011;15(5):R208. doi:10.1186/cc10443
79. Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. J Biol Chem. 2011;286(20):17435–17444. doi:10.1074/jbc.M110.137273
80. Cai S, Zou Y, Zhao Y, et al. Mechanical stress reduces secreted frizzled-related protein expression and promotes temporomandibular joint osteoarthritis via Wnt/β-catenin signaling. Bone. 2022;161:116445. doi:10.1016/j.bone.2022.116445
81. Hu Y, Ng-Blichfeldt JP, Ota C, et al. Wnt/β-catenin signaling is critical for regenerative potential of distal lung epithelial progenitor cells in homeostasis and emphysema. Stem Cells. 2020;38(11):1467–1478. doi:10.1002/stem.3241
82. Zhang K, Yao E, Wang SA, et al. A functional circuit formed by the autonomic nerves and myofibroblasts controls mammalian alveolar formation for gas exchange. Dev Cell. 2022;57(13):1566–1581. doi:10.1016/j.devcel.2022.05.021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.