Back to Journals » Degenerative Neurological and Neuromuscular Disease » Volume 12
Mechanisms of Action of Semen Ziziphi spinosae in the Treatment of Tourette Syndrome
Received 11 April 2022
Accepted for publication 9 July 2022
Published 15 July 2022 Volume 2022:12 Pages 85—96
DOI https://doi.org/10.2147/DNND.S370278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Thomas Müller
Fei Fan,1 Fei Han,1 Long Hao2
1Department of Pediatrics, Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2Department of Paediatrics, Beijing Fangshan District Liangxiang Hospital, Beijing, People’s Republic of China
Correspondence: Long Hao, Department of Paediatrics, Beijing Fangshan District Liangxiang Hospital, No. 45 Gongchen Street, Fangshan District, Beijing, People’s Republic of China, Tel +86 10-813560000, Email [email protected]
Background: Semen Ziziphi spinosae, known as Suanzaoren (SZR) in Chinese, is a Chinese herbal medicine widely used in sedatives and tranquilizers. Although SZR is important for the clinical treatment of Tourette syndrome (TS), its mechanism of action remains unclear. Therefore, we investigated the pharmacological mechanisms of SZR in TS treatment using network pharmacology and systems biology approaches.
Methods: The bioactive components and potential targets of SZR were screened using the TCMSP database. UniProt was used to identify targets by mapping the known genes related to SZR. The known genes related to TS were identified by GeneCards and OMIM databases. A protein-protein interaction network was constructed using information from STRING 11.0 database. Cytoscape 3.8.0 software and Bioinformatics online platform were used for plotting this network. Gene ontology and KEGG enrichment analyses were performed using Metascape. Finally, AutoDock was used to verify the molecular docking.
Results: We found that SZR had 10 active compounds. There were 30 overlapping target genes between TS and SZR. These genes were associated with several signaling and metabolic pathways. AChE, SLC6A4, and HTR3A were the top three hub genes. The active components in SZR had a high binding affinity for the key targets.
Conclusion: SZR therapy for TS could achieve network regulation through the action of various active components of Chinese medicine on different targets and generate a complex regulatory relationship via interaction with potential targets, thereby playing a therapeutic role. Thus, SZR is a potential candidate for treating TS because it regulates nervous system functions.
Keywords: Tourette syndrome, tics, Semen Ziziphi spinosae, suanzaoren, network pharmacology, molecular docking
Introduction
Tourette syndrome (TS) is a common childhood-onset neurodevelopmental condition characterized by the presence of multiple sudden, involuntary, rapid, recurrent, and non-rhythmic motor tics and at least one vocal tic that persists for at least a year.1 The prevalence of TS in school children ranges from 0.3% to 0.9%,2 while that of other chronic tic disorders ranges from 0.9% to 2.8%.3 It is widely believed that the occurrence of TS is causally related to the interactions of genetic, neurobiochemical, inflammation-related, immunological, and environmental factors. However, our understanding of the pathophysiological mechanisms of TS remains limited. Current research suggests that abnormalities of neurotransmitters, including dopamine, glutamate, serotonin, and acetylcholine, which participate in the cortico–striato–thalamo–cortical (CSTC) circuit are the primary reason for the development of TS.4
Patients with TS may experience subjective discomfort, sustained social problems, and emotional problems.5,6 The lifetime prevalence of comorbid behavioral disorders is estimated to be 85.7%.7 Moreover, attention-deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and anxiety disorders are commonly associated with TS.7,8 About 5% of the patients with TS have life-threatening symptoms, including mild self-injurious behaviors and borderline personality disorder, especially in severe cases.9 Medications and behavioral treatments are the primary means of treating TS.10,11 Comprehensive behavioral intervention for tics is the main method used in behavioral treatments. However, this kind of treatment cannot be carried out on a large scale and is suitable only for patients aged 9 years and older with mild symptoms. The efficacy of the medications used to treat TS, such as haloperidol, aripiprazole, tiapride, clonidine, and risperidone, is not satisfactory.10,11 Furthermore, the medications have many side effects, including fatigue, somnolence, and extrapyramidal symptoms.12,13 Consequently, patients with TS are increasingly using complementary and alternative medicine.10
Semen Ziziphi spinosae, also known as spine date seed or suanzaoren (SZR), consists of the dried seeds of Ziziphus jujuba and is a widely known Chinese herbal medicine.14 According to the available literature on traditional Chinese medicine (TCM), SZR is widely used in sedatives and tranquilizers in the treatment of insomnia, anxiety, and other neuropsychiatric diseases.15,16 Recently, SZR has also shown significant neuroprotective activities in animals with dementia by exerting antioxidant and anti-inflammatory effects.16 Although previous research has shown that SZR may play an important role in clinical TS treatment, the underlying mechanism is still unclear.17 Therefore, in this study, systems biology approaches were used to screen the active compounds of SZR, identify its target genes, and explore the potential molecular mechanism of its therapeutic anti-tic effect.
Materials and Methods
Identification of the Active Compounds of SZR
The relevant data for identifying SZR constituents were retrieved from the database of the traditional Chinese medicine systems pharmacology database and analysis platform (TCMSP) database (http://lsp.nwu.edu.cn).18 The active compounds were screened based on absorption, distribution, metabolism, and excretion (known as ADME screening). Following this, we examined whether the active compounds of SZR met the criteria of oral bioavailability (OB) ≥ 25%, drug-likeness (DL) ≥ 0.18, and blood-brain barrier (BBB) ≥ −0.3, as recommended in the TCMSP database. OB may represent the percentage of an oral dose of a drug that enters the systemic circulation unchanged.19 DL is a qualitative term that refers to the optimized pharmacokinetics of a drug and its properties.20 The BBB restricts proteins and potential diagnostic and therapeutic drugs from entering the brain parenchyma.21,22 The detailed calculations of these three parameters have been reported by Wang et al.22
Identification of Targets Related to Active Compounds of SZR
The targets related to active compounds of SZR were further predicted based on the computer targeting technology developed by the TCMSP. UniProt (http://www.uniprot.org/) is the protein database with the most abundant resources and the most extensive categories.23 It is composed of data from Swiss-Prot, TrEMBL, and PIR-PSD. Using the UniProt KB search function of UniProt database, SZR target proteins were searched and limited to “human” studies, with the UniProt codes of retrieved active compounds of SZR converted into gene symbols.
Identification of Known Genes Related to TS
Known TS genes were acquired from two existing resources: (1) GeneCards database (https://www.genecards.org/) and (2) Online Mendelian Inheritance in Man (OMIM) database (https://omim.org). The GeneCards database helps in screening correlations between genes and diseases and provides an algorithm to retrieve more relevant targets.24 The OMIM database includes information on human diseases that describes the categories and names of new diseases as well as the relationship between phenotypes and related etiological genes.25 The keywords “Tourette syndrome” or “Tic disorder” were entered, and the repeated genes retrieved were removed from the search results and matched with the genes related to the active compounds of SZR to obtain their potential target genes in the treatment of TS.
Protein-Protein Interaction Network of Targets of TS and SZR
Using STRING 11.0, an online software (http://string-db.org),26 the genes included in the intersection of target genes related to active compounds of SZR and TS were entered, the organism “Homo Sapiens” was selected, the relevant information on the interactions and relationships between proteins were obtained, and a protein-protein interaction (PPI) network was constructed. Cytoscape 3.8.0 software (The Cytoscape Consortium, San Diego, CA, USA) was used for plotting the network between SZR, active compounds, target genes, and TS.
Correlation Pathway and Annotation Analysis
Gene ontology (GO) terms belong to three categories, namely, biological processes, molecular functions, and cellular components.27 The Kyoto Encyclopaedia of Genes and Genome (KEGG) can consign selected sets of genes to the most relevant signaling pathways.28 In this study, Metascape (http://metascape.org)29 was used to conduct GO term and KEGG pathway enrichment analyses of the gene sets to explore the molecular mechanism underlying anti-TS effects of SZR, with the threshold significance value set at the default P < 0.01. The enrichment GO term histogram and enrichment KEGG dot bubble were plotted using Bioinformatics (http://www.bioinformatics.com.cn), an online data visualization platform.
Molecular Docking
The crystal structures of the verified components of SZR were obtained from the RCSB Protein Data Bank (PDB, https://www.rcsb.org/).30 The crystal structure of components were stored as a docking ligand in MOL2 format, and the iGEMDOCK software wa. Finally, AutoDock was used to verify the molecular docking.
Results
Compounds of SZR
Using TCMSP, it was found that SZR includes 33 kinds of traditional ingredients. Among these ingredients, a total of 10 active compounds met the criteria of OB ≥ 25%, DL ≥ 0.18, and BBB ≥ −0.3 (Table 1).
 |
Table 1 Detailed Information on the Active Compounds of SZR |
Target Gene Prediction
Target sites of SZR were retrieved from TCMSP, and corresponding targets were selected according to the selected chemical components. Among these components, one candidate did not have any targets, and a total of 42 non-repeating targets were obtained. Moreover, 2624 target genes corresponding to TS were identified from GeneCards and OMIM. There were 30 overlapping target genes between TS and SZR (Figure 1).
 |
Figure 1 Venn diagram of the target genes for TS and SZR active compounds. TS has 2624 target genes, while SZR has 42 target genes. There are 30 overlapping target genes between the two sets. |
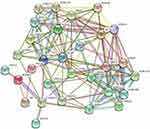
Protein-Protein Interaction Network
A PPI network of the 30 overlapping target genes in SZR and TS was constructed using the STRING database (Figure 2). In this study, the genes with high connectivity (≥ 5) were defined as hub genes. Acetylcholinesterase (AChE), sodium-dependent serotonin transporter (SLC6A4), and 5-hydroxytryptamine receptor 3A (HTR3A) were the top three hub genes among the 25 hub genes (Figure 3).
Network Among SZR, Compounds, Target Genes, and TS
The active compounds of SZR and TS-related overlapping target genes were imported into the Cytoscape 3.8.0 software to construct the network between SZR-compounds-target genes-TS (Figure 4). The results showed that SZR mainly acted on 30 targets through five active compounds, which may affect the occurrence of TS.
 |
Figure 4 Network of SZR, compounds, target genes, and Tourette syndrome (TS). Red rectangle, TS; yellow diamond, SZR; yellow triangle, compounds; blue octagon, target genes. |
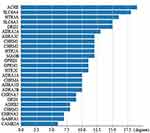
GO Term and KEGG Pathway Enrichment Analysis
In each major category of the GO terms (Figure 5), the most significantly enriched terms were “chemical synaptic transmission”, “postsynaptic membrane”, and “G protein-coupled amine receptor activity.” The overlapping target genes were closely related to synapses, receptors, and metabolic signaling pathways (Figures 6 and 7).
 |
Figure 5 GO term enrichment analysis. |
 |
Figure 6 KEGG pathway enrichment analysis. |
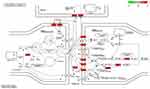 |
Figure 7 KEGG pathway enrichment analysis demonstrating the interactions among various pathways. |
Verification of Molecular Docking
When the main active components of a therapeutic agent are docked with a core target, the more stable the binding conformation, the lower the binding energy required. In this study, binding energy < −9.0 kcal/mol was selected as the screening condition, as shown in Table 2. We selected AChE docking with the verified SZR active molecules. (S)-Coclaurine, Sanjoinine B and Zizyphusine had the three lowest energy value, with binding energies of −10.03, −9.76, and −9.7 kcal/mol. Moreover, Pymol 2.1 software was used to create the schematic diagram for the docking result with the lowest binding energy (Figure 8). This shows that (S)-coclaurine has a good binding ability to AChE protein.
 |
Table 2 AChE Docking Results for the Compounds of SZR |
 |
Figure 8 Binding pattern of (S)-coclaurine) and AChE protein. |
Discussion
TS is a neurodevelopmental condition characterized by the presence of tics and associated behavioral problems.1 Some Chinese herbs may play an important role in clinical TS treatment, Bombyx Batryticatus have been used to treat convulsions.31 Os Draconis, Concha Ostreae, and Radix Bupleuri have been used to relieve epileptic symptoms.32 SZR is a Chinese herbal medicine that has been widely used in sedatives and tranquilizers in traditional clinics.17 Based on the current clinical and experimental research status of SZR treatment for TS, the present study further explored the mechanism of action of SZR by using a systems biology approach.
In this study, SZR was found to have 10 compounds and 42 non-repeating targets. Among the compounds, there were 5 active components: (S)-coclaurine, dl-nuciferine, sanjoinine B, sanjoinenine, and zizyphusine. DL-Nuciferine is an isoquinoline derivative that acts as a D-2-like receptor agonist.33 Sanjoinine B is a cyclic alkaloid peptide, while zizyphusine, sanjoinenine, and (S)-coclaurine are alkaloids. They all exert diverse effects on nerve cells, neurotransmitters, receptors, and sleep parameters.34,35 These findings suggest that they may be the key compounds of SZR that play a role in TS treatment. The active amino acid residues bound by (S)-coclaurine and AChE include Glu-199, His-440, Phe-330, Ser-122, and Tyr-334. Hydrogen bonds are formed between the hydroxyl and hydrogen atoms of (S)-coclaurine and the active amino groups of Glu-199, His-440, Ser-122, and Tyr-334. For example, strong hydrogen bonds are formed between hydroxyl protons and amino protons of Glu-199, His-440, Ser-122, and Tyr-334 with distances of 2.1 Å, 2.7 Å, 2.0 Å, and 2.6 Å, respectively. The bond length of a conventional hydrogen bond is markedly less than 3.5 Å. In addition, the benzene ring of (S)-coclaurine can form π-π conjugated interactions with the benzene ring of Phe-330 residue. These hydrogen bonds and π-π conjugate interactions indicate that (S)-coclaurine binds strongly to AChE protein and can match the active pocket of AChE protein suitably.
There were 30 overlapping target genes between TS and SZR. Twenty-five hub genes were identified using PPI network analysis. AChE, SLC6A4, and HTR3A were the top 3 hub genes among them; they are all closely related to neuropsychiatric diseases. AChE can termination of neurotransmission at cholinergic synapses by rapid hydrolysis of the ACh,36 and exerts beneficial effects in several neurological disorders such as Alzheimer disease and Creutzfeldt-Jakob disease through its critical role in termination of cholinergic neurotransmission.37,38 The corpus striatum of the basal ganglia possesses the highest density of AChE markers, thereby highlighting the importance of AChE in this structure. Accumulating evidence from several animal experiments has indicated that the nicotinic-cholinergic system is involved in physiological motor control. Therefore, the development of AChE drugs can play a pivotal role in treating movement disorders.39
Numerous studies have shown that SLC6A4 is associated with the etiology of TS.40 Increased blood SLC6A4 mRNA levels in the striatum of TS38 rats has been found to be positively correlated with the severity of the tic.41,42 The serotonin transporter (SERT)-linked polymorphic region (5-HTTLPR) in the promoter region directly upstream of SLC6A4 has been implicated in the etiology of OCD and TS.43,44 5-HTTLPR is a 43-base pair repeat with a long (L) and a short (S) allele. The L allele is associated with increased SLC6A4 mRNA expression in blood, resulting in reduced serotonergic neurotransmission through the increased serotonin clearance mediated by SERT.40 SLC6A4 has also been implicated in other neuropsychiatric disorders, such as ADHD and OCD, which are important and common complications of TS.41 HTR3A is one of the serotonin receptor subtypes. Serotonin has been implicated in various neuropsychiatric disorders; therefore, it may affect the detected variants contributing to the severity of these diseases. Serotonergic dysfunction is thought to be involved in the pathophysiology of TS.45 These candidate genes offer vast potential benefits for application in gene-targeted therapy of TS.
To further understand the mechanism of action, GO and KEGG enrichment analyses were performed on these target genes. GO enrichment analysis demonstrated that SZR plays a regulatory role in chemical synaptic transmission, postsynaptic membrane, G protein-coupled amine receptor activity, and other biological processes. According to the enrichment results of the KEGG pathway, SZR plays a role in treating TS through neuroactive ligand-receptor interaction, cholinergic synapse, cGMP-PKG signaling, serotonergic synapse, cocaine addiction, morphine addiction, and retrograde endocannabinoid signaling. These research results are consistent with the holistic view of TCM and the principle of treatment based on ZHENG differentiation.46 Therefore, it is speculated that SZR may not only through the action of various active components on different targets, but also through the interaction among potential targets, thereby playing a therapeutic role in TS. Moreover, the present findings can facilitate the isolation of active compounds in SZR to optimize therapy.
Previous studies had shown the therapeutic potential of SZR in treating TS.15,16 SZR had also been used in a randomized, double blind, double dummy, parallel controlled trial with TS children.17 Further studies could be designed to subsequent cell and animal experiments to reveal the underlying pharmacological and therapeutic effects of SZR for the treatment of TS.
Limitations
However, the present study had some limitations. First, because we could not validate these results experimentally in this study, the specific mechanism of action is still unclear and needs to be studied further. Second, we chose the criteria of oral bioavailability (OB) ≥ 25%, drug-likeness (DL) ≥ 0.18, and blood-brain barrier (BBB) ≥ −0.3, as recommended in the TCMSP database, to identified the active compounds of SZR in this study. Thus, the chooses of different criterias may lead to different results.
Conclusions
In general, the present study revealed the active compounds of SZR and possible targets and mechanism of action in the treatment of TS from the perspective of network pharmacology. The key molecules identified in this study are candidate drug targets, and the critical status of these molecules indicates that they may be the key targets of disease control.43 Network pharmacology is categorized as data mining research, and the research results are mainly based on existing studies and prediction system analysis of active ingredients and targets; therefore, there are some differences between the current results and those of actual experiments and clinical trials, which need to be further verified in the future.
Abbreviations
SZR, Suanzaoren; TS, Tourette syndrome; CSTC, cortico–striato–thalamo–cortical; ADHD, attention-deficit hyperactivity disorder; OCD, obsessive-compulsive disorder; TCM, traditional Chinese medicine; TCMSP, traditional Chinese medicine systems pharmacology database and analysis platform; OB, oral bioavailability; DL, drug-likeness; BBB, blood-brain barrier; OMIM, Online Mendelian Inheritance in Man; PPI, protein-protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genome; Ach, acetylcholine.
Data Sharing Statement
The data supporting the findings of the study are obtained from the following databases: TCMSP (http://lsp.nwu.edu.cn), UniProt (http://www.uniprot.org/), GeneCards database (https://www.genecards.org/), Online Mendelian Inheritance in Man (OMIM) database (https://omim.org), STRING 11.0 online software (http://string-db.org), Bioinformatics (http://www.bioinformatics.com.cn), Metascape (http://metascape.org/gp/index.html#/main/step1), and RCSB Protein Data Bank (PDB, https://www.rcsb.org/).
Ethical Approval and Informed Consent
Not applicable – Not required for this study.
Author Contributions
FF was the primary contributor to writing the manuscript. LH and FH participated in research design. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by a grants from the Fundamental Research Funds for the Central Public Welfare Research Institutes, Beijing, China [grant numbers ZZ13-024-5 and ZZ15-XY-PT-03].
Disclosure
The authors declare no conflicts of interest in relation to this work.
References
1. Robertson MM, Eapen V, Singer HS, et al. Gilles de la Tourette syndrome. Nat Rev Dis Primers. 2017;3(1):16097. doi:10.1038/nrdp.2016.97
2. Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221–228. doi:10.1002/mds.26089
3. Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90. doi:10.1016/j.pediatrneurol.2012.05.002
4. Martino D, Johnson I, Leckman JF. What does immunology have to do with normal brain development and the pathophysiology underlying Tourette syndrome and related neuropsychiatric disorders. Front Neurol. 2020;11:567407. doi:10.3389/fneur.2020.567407
5. Conte G, Valente F, Fioriello F, Cardona F. Rage attacks in Tourette syndrome and chronic tic disorder: a systematic review. Neurosci Biobehav Rev. 2020;119:21–36. doi:10.1016/j.neubiorev.2020.09.019
6. Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry. 2015;2(1):68–87. doi:10.1016/S2215-0366(14)00132-1
7. Hirschtritt ME, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72(4):325–333. doi:10.1001/jamapsychiatry.2014.2650
8. Cavanna AE, Rickards H. The psychopathological spectrum of Gilles de la Tourette syndrome. Neurosci Biobehav Rev. 2013;37(6):1008–1015. doi:10.1016/j.neubiorev.2012.10.011
9. Virtanen S, Sidorchuk A, Fernández de la Cruz L, et al. Association of Tourette syndrome and chronic tic disorder with subsequent risk of alcohol- or drug-related disorders, criminal convictions, and death: a population-based family study. Biol Psychiatry. 2021;89(4):407–414. doi:10.1016/j.biopsych.2020.09.014
10. Roessner V, Eichele H, Stern JS, et al. European clinical guidelines for Tourette syndrome and other tic disorders-version 2.0. Part III: pharmacological treatment. Eur Child Adolesc Psychiatry. 2022;31(3):425–441. doi:10.1007/s00787-021-01899-z
11. Pringsheim T, Okun MS, Müller-Vahl K, et al. Practice guideline recommendations summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):896–906. doi:10.1212/WNL.0000000000007466
12. Liu ZS, Cui YH, Sun D, et al. Current status, diagnosis, and treatment recommendation for tic disorders in China. Front Psychiatry. 2020;11:774. doi:10.3389/fpsyt.2020.00774
13. Pringsheim T, Holler-Managan Y, Okun MS, et al. Comprehensive systematic review summary: treatment of tics in people with Tourette syndrome and chronic tic disorders. Neurology. 2019;92(19):907–915. doi:10.1212/WNL.0000000000007467
14. Huang J, Zhang C, Zhao X, et al. The jujube genome provides insights into genome evolution and the domestication of sweetness/acidity taste in fruit trees. PLoS Genet. 2016;12(12):e1006433. doi:10.1371/journal.pgen.1006433
15. Zhou QH, Zhou XL, Xu MB, et al. suanzaoren formulae for insomnia: updated clinical evidence and possible mechanisms. Front Pharmacol. 2018;9:76. doi:10.3389/fphar.2018.00076
16. Liu Z, Zhao X, Liu B, et al. Jujuboside A, a neuroprotective agent from semen Ziziphi spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ 1–42. Eur J Pharmacol. 2014;738:206–213. doi:10.1016/j.ejphar.2014.05.041
17. Fan F, Hao L, Zhang S, et al. Efficacy of the jingxin zhidong formula for tic disorders: a randomized, double blind, double dummy, parallel controlled trial. Neuropsychiatr Dis Treat. 2022;18:57–66. doi:10.2147/NDT.S347432
18. Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi:10.1186/1758-2946-6-13
19. Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13(6):6964–6982. doi:10.3390/ijms13066964
20. Tian S, Wang J, Li Y, Li D, Xu L, Hou T. The application of in silico drug-likeness predictions in pharmaceutical research. Adv Drug Deliv Rev. 2015;86:2–10. doi:10.1016/j.addr.2015.01.009
21. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi:10.1101/cshperspect.a020412
22. Wang S, Wang H, Lu Y. Tianfoshen oral liquid: a CFDA approved clinical traditional Chinese medicine, normalizes major cellular pathways disordered during colorectal carcinogenesis. Oncotarget. 2017;8(9):14549–14569. doi:10.18632/oncotarget.14675
23. The UniProt Consortium. UniProt: the universal protein knowledgebase [published correction appears in Nucleic Acids Res. Nucleic Acids Res. 2017;45(D1):D158–D169. doi:10.1093/nar/gkw1099
24. Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:
25. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43:D789–D798. doi:10.1093/nar/gku1205
26. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
27. Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25(1):25–29. doi:10.1038/75556
28. Du J, Yuan Z, Ma Z, Song J, Xie X, Chen Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol Biosyst. 2014;10(9):2441–2447. doi:10.1039/C4MB00287C
29. Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi:10.1038/s41467-019-09234-6
30. Adams PD, Afonine PV, Baskaran K, et al. Announcing mandatory submission of PDBx/mmCIF format files for crystallographic depositions to the Protein Data Bank (PDB). Acta Crystallogr D Struct Biol. 2019;75(Pt 4):451–454. doi:10.1107/S2059798319004522
31. Hu M, Yu Z, Wang J, et al. Traditional uses, origins, chemistry and pharmacology of Bombyx batryticatus: a review. Molecules. 2017;22(10):1779. doi:10.3390/molecules22101779
32. Yang P, Qin Y, Zhu Y, et al. Chaihu-Longgu-Muli decoction relieves epileptic symptoms by improving autophagy in hippocampal neurons. J Ethnopharmacol. 2020;259:112990. doi:10.1016/j.jep.2020.112990
33. Silva AG, Vila L, Marques P, et al. 1-(2’-Bromobenzyl)-6,7-dihydroxy-N-methyl-tetrahydroisoquinoline and 1,2-demethyl-nuciferine as agonists in human D2 dopamine receptors. J Nat Prod. 2020;83(1):127–133. doi:10.1021/acs.jnatprod.9b00921
34. Cao JX, Zhang QY, Cui SY, et al. Hypnotic effect of jujubosides from Semen Ziziphi spinosae. J Ethnopharmacol. 2010;130(1):163–166. doi:10.1016/j.jep.2010.03.023
35. Al-Reza SM, Yoon JI, Kim HJ, Kim JS, Kang SC. Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem Toxicol. 2010;48(2):639–643. doi:10.1016/j.fct.2009.11.045
36. Silman I, Sussman JL. Acetylcholinesterase: how is structure related to function? Chem Biol Interact. 2008;175(1–3):3–10. doi:10.1016/j.cbi.2008.05.035
37. Lane RM, Kivipelto M, Greig NH. Acetylcholinesterase and its inhibition in Alzheimer disease. Clin Neuropharmacol. 2004;27(3):141–149. doi:10.1097/00002826-200405000-00011
38. Silveyra MX, Cuadrado-Corrales N, Marcos A, et al. Altered glycosylation of acetylcholinesterase in Creutzfeldt-Jakob disease. J Neurochem. 2006;96(1):97–104. doi:10.1111/j.1471-4159.2005.03514.x
39. Quik M, Zhang D, Perez XA, Bordia T. Role for the nicotinic cholinergic system in movement disorders; therapeutic implications. Pharmacol Ther. 2014;144(1):50–59. doi:10.1016/j.pharmthera.2014.05.004
40. Hildonen M, Levy AM, Dahl C, et al. Elevated expression of SLC6A4 Encoding the Serotonin Transporter (SERT) in Gilles de la Tourette syndrome. Genes. 2021;12(1):86.
41. Gunther J, Tian Y, Stamova B, et al. Catecholamine-related gene expression in blood correlates with tic severity in Tourette syndrome. Psychiatry Res. 2012;200(2–3):593–601. doi:10.1016/j.psychres.2012.04.034
42. Jijun L, Zaiwang L, Anyuan L, et al. Abnormal expression of dopamine and serotonin transporters associated with the pathophysiologic mechanism of Tourette syndrome. Neurol India. 2010;58(4):523–529. doi:10.4103/0028-3886.68663
43. Wendland JR, Moya PR, Kruse MR, et al. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17(5):717–723. doi:10.1093/hmg/ddm343
44. Moya PR, Wendland JR, Rubenstein LM, et al. Common and rare alleles of the serotonin transporter gene, SLC6A4, associated with Tourette’s disorder. Mov Disord. 2013;28(9):1263–1270. doi:10.1002/mds.25460
45. Niesler B, Frank B, Hebebrand J, Rappold G. Serotonin receptor genes HTR3A and HTR3B are not involved in Gilles de la Tourette syndrome. Psychiatr Genet. 2005;15(4):303–304. doi:10.1097/00041444-200512000-00015
46. Li S, Zhang ZQ, Wu LJ, Zhang XG, Li YD, Wang YY. Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Syst Biol. 2007;1(1):51–60. doi:10.1049/iet-syb:20060032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.