Back to Journals » Drug Design, Development and Therapy » Volume 17
Mechanism of the Effect of Compound Anoectochilus roxburghii (Wall.) Lindl. Oral Liquid in Treating Alcoholic Rat Liver Injury by Metabolomics
Authors Huang T , Wu Y, Huang L , Lin R, Li Z , Wang X , Wu P , Huang L
Received 27 June 2023
Accepted for publication 7 November 2023
Published 15 November 2023 Volume 2023:17 Pages 3409—3428
DOI https://doi.org/10.2147/DDDT.S427837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Tingxuan Huang, Youjia Wu, Lingyi Huang, Renyi Lin, Zhenyue Li, Xiaoxiao Wang, Pingping Wu, Liying Huang
School of Pharmacy, Fujian Medical University, Fuzhou, Fujian, People’s Republic of China
Correspondence: Liying Huang; Pingping Wu, School of Pharmacy, Fujian Medical University, Xuefu North Road University Town, Fuzhou, Fujian, 350122, People’s Republic of China, Tel +8613860635139 ; +8615980271679, Fax +86059422862016, Email [email protected]; [email protected]
Purpose: Compound Anoectochilus roxburghii (Wall.) Lindl oral liquid (CAROL) is often as a hepatoprotective agent. The present study aimed to elucidate the protective mechanism of CAROL against alcoholic liver injury in rats by untargeted metabolomics combined with multivariate statistical analysis.
Methods: An alcoholic liver disease model was established in sprague-dawley (SD) rats by gavage of alcohol, and CAROL treatment was administered. The hepatoprotective effect of CAROL was evaluated by examining liver tissues changes and detecting biochemical index activities and cytokines in serum and liver homogenates. The metabolites in serum samples were examined using ultrahigh-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UHPLC–QTOF/MS) and multivariate statistical analysis to screen for differentially expressed metabolites and Kyoto Encyclopedia of Genes and Genomes (KEGG) to assess potential metabolic pathways.
Results: CAROL has the potential to downregulate inflammation levels and alleviate oxidative stress. The differential metabolites are mainly engaged in riboflavin metabolism, arginine and proline metabolism, phenylalanine, tyrosine and tryptophan biosynthesis metabolism, phenylalanine metabolism, pyrimidine metabolism, and vitamin B6 metabolism to achieve hepatoprotective effects.
Conclusion: CAROL may exhibit beneficial hepatoprotective effects by reducing inflammation, mitigating oxidative stress, and modulating metabolites and their metabolic pathways.This study has important implications for advancing the clinical application of CAROL.
Keywords: compound Anoectochilus roxburghii (Wall.) Lindl. oral liquid, alcoholic liver injury, untargeted metabolomics, UHPLC–QTOF/MS, metabolic pathway analysis
Graphical Abstract:
Introduction
Alcoholic liver disease (ALD) is the most common disease associated with long-term alcohol abuse. ALD is a spectrum of disease, from hepatic steatosis to alcoholic hepatitis, liver fibrosis to cirrhosis, and even to liver cancer, and it has become a public health problem by posing serious threat to people’s health.1,2 However, the precise pathogenesis of ALD remain unclear. Cell damage, mitochondrial dysfunction, and oxidative stress may affect the occurrence and development of ALD.3 No drugs have been approved for the treatment of ALD. The only effective treatment is supportive care and abstinence from alcohol.4 Therefore, developing or obtaining effective and safe drugs to treat ALD is needed. Traditional Chinese medicine (TCM) is now widely used for liver protection.5,6 Owing to their characteristics of multiple targets and small side effects, many TCM preparations have been used to treat ALD, including silymarin, glycyrrhizic acid preparations, and Yigan mingmu oral liquid.7,8 However, the efficacy of these drugs for ALD is not known. Therefore, obtaining new TCM preparations to treat ALD is necessary.
Compound Anoectochilus roxburghii (Wall.) Lindl oral liquid (CAROL) is a hospital preparation formulated by Mengchao Hepatobiliary Hospital of Fujian Medical University. It is commonly prescribed for the treatment of liver disorders and possesses several beneficial properties, including heat removal, detoxification, and blood cooling. It is also an excellent hepatoprotective drug. CAROL is a preparation made from two valuable herbs, Anoctochilus Roxborghii (Wall.) Lindl. (A. roxborghii) and Ganoderma lucidum (G. lucidum) extracted with water.9 A. roxborghii and G. lucidum are valuable traditional medicinal herbs containing various active ingredients with significant pharmacological activity in liver protection and the treatment of alcoholic liver injury.8,10 Published studies have reported that CAROL contains hepatoprotective active ingredients, such as nucleosides, flavonoids, and triterpenoids.9,11 More active substances in CAROL and the protective effect on alcoholic liver injury need to be further investigated.
Metabolomics is the qualitative and quantitative analysis of all endogenous low-molecular-weight metabolites (< 1.5 kDa) in the biological organism at a given time. By studying changes in metabolites in the body and their relationship with physiological and pathological changes, it is possible to identify biomarkers associated with diseases and explore the mechanisms from the metabolic pathway that are relevant to the pathogenesis of the organism or to the treatment of the drug. Metabolomics offers promising opportunities for the development of new diagnostic and prognostic biomarkers, as well as a deeper understanding of the underlying causes of various diseases and the processes involved in pharmacological treatment.12 Several research techniques are commonly employed in metabolomics, including nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC-MS), and liquid chromatography-mass spectrometry (LC-MS) et al.13 LC-MS is one of the most used technologies in metabolomics because of its high sensitivity, high selectivity, easy sample handling, high throughput analysis, and ease of operation. The metabolomic study of liver-protective effects of Chinese herbal medicines is one of the hot spots in clinical research.14,15 Liu Fang et al found that Panax ginseng was effective in restoring metabolic disorders caused by ethanol and identified 12 potential biomarkers for alcoholic fatty liver in a liver-protective metabolomics study.16 The metabolomic approach employed by Lian et al allowed for a comprehensive assessment of the metabolic changes induced by Zhi-Zi-Da-Huang decoction, shedding light on its potential therapeutic benefits for alcoholic liver damage.17
This study utilized ultra-high-performance liquid chromatography quadrupole time-of-flight/mass spectrometry (UHPLC-QTOF/MS) to perform qualitative analysis of the active ingredients in CAROL and evaluate its protective effect against alcohol-induced liver injury in rats by metabolomics. A rat model of alcohol-induced liver injury was established. Inflammatory cytokine levels were measured using enzyme-linked immunosorbent assay (ELISA) to assess the therapeutic impact of CAROL. Serum non-targeted metabolomics based on UHPLC-QTOF/MS and multivariate analysis were utilized to reveal the hepatoprotective mechanisms and metabolic pathways altered upon CAROL treatment. This study establishes a theoretical foundation for the clinical application of CAROL in the treatment of alcohol-induced liver injury and provides guidance for the clinical application of herbal formulations.
Materials and Methods
Materials and Reagents
AR224CN electronic balance (Orhaus Instrument Co., Ltd., China), R404A centrifuge (Eppendorf Company, Germany), KQ2200E type ultrasonic cleaner (Kunshan Ultrasonic Instruments Co., Ltd., China), net letter JXFSTPRP-24 homogenizer (Shanghai Industrial Development Co., Ltd., China), 1510 enzyme labeler (Thermo Company, USA), VG3S25-type vortex mixer (IKA Instrument Co., Ltd., Germany), DW-86L338J 86°C ultralow-temperature refrigerator and BCD-530WGPZV refrigerator (Haier Company, China), SZ-93 automatic double distill water (Shanghai Yarong Biochemical Instrument Co., Ltd., China), UltiMate 3000 UHPLC system (Thermo Co., Ltd., Germany), AB Sciex Triple TOF X500R high-resolution mass spectrometer (AB Sciex, USA), Agilent 1290 ultrahigh-performance liquid chromatograph (Agilent Co., Ltd., USA), and AB Sciex Triple TOF 6600 high-resolution mass spectrometer (AB Sciex, USA).
Sodium chloride (Xilong Scientific Co., Ltd., China), ethyl alcohol (Sinopharm Chemical Reagent Co., Ltd., China), 4% paraformaldehyde tissue fixative (Meilun Biotechnology Co., Ltd., China), methanol, acetonitrile, and ammonium acetate with tedia were purchased from CNW Technologies GmbH (Germany). Ammonium hydroxide was purchased from Fisher Chemical (USA). ALT kit, AST kit, SOD kit, GSH kit, MDA kit, and TG kit were purchased from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China). TNF-α kit, IL-6, and NF-κB were purchased from Fujian Misco Biotechnology Co., Ltd (Fujian, China).
CAROL (batch no. 20220104) was obtained from the Mengchao Hepatobiliary Hospital of Fujian Medical University. Silymarin (batch no. 210810) was purchased from Jiangsu Zhongxing Pharmaceutical Co., Ltd.
Identification of Active Substances in UHPLC–QTOF/MS of CAROL
Sample Pretreatment
A CAROL sample (500 µL) was placed in a centrifuge tube and mixed with three times the volume of methanol using a vortex mixer for 1 min. The mixture was then centrifuged at 17,000 rpm for 15 min at 4 °C. The supernatant was subjected to vacuum freeze-drying until nearly dry, followed by reconstitution in 50% methanolic water. The resulting solution was filtered through a 0.22 μm organic filter membrane to obtain the test solution.
UHPLC–QTOF/MS Conditions
The UltiMate 3000 UHPLC system coupled with a Q-TOF X500R was used to collect the active substances’ information of CAROL. Chromatographic separation was carried out on an ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 μm, Waters) equipped with a binary solvent system.
The mobile phases of the electrospray ionization source in positive ion mode (ESI+) were 0.1% formic acid water (A) and 0.1% formic acid acetonitrile (B). The mobile phases in negative ion mode (ESI–) were 2 mM ammonium acetate water (A) and acetonitrile (B). The elution-gradient program was as follows: 0–3.0 min, 5% B; 3.0–5.0 min, 5%–10% B; 5.0–40.0 min, 10%–40% B; 40.0–50.0 min, 95% B; 50.0–56.0 min, 95%–95% B; and 56.0–60.0 min, 95%–5%. The flow rate was 0.4 mL/min, the injection volume was 5 μL, and the column temperature was maintained at 40 °C.
The AB X500R Triple TOF mass spectrometer was used on the basis of information dependent acquisition (IDA) function for primary and secondary mass spectral data acquisition. In each data collection cycle, molecular ions with the strongest signal intensity and m/z greater than 100 were selected for the corresponding secondary mass spectral data acquisition by using the following parameters: first-level acquisition range of m/z 50–1200, bombardment energy of 30 eV, and 10 second-level spectrograms per 50 ms. The ESI ion-source parameters were set as follows: atomization pressure (GS1) of 60 psi, auxiliary pressure of 60 psi, curtain pressure of 35 psi, temperature of 650 °C, and spray voltage of 5000 V (ESI+) or –4000 V (ESI−).
Data Processing
First, the data were formatted. The MSDIAL version 4.6 software was used to process the data. The databases of Metlin, MassBank, MoNA, and HMDB that come with the system were applied to identify and analyze the components in CAROL on the basis of the information of primary mass spectra, secondary cleavage characteristic fragment ions, and isotopic distribution matched with the compounds in the spectral library. The setting identification parameters of MSDIAL were as follows: accurate mass tolerance (MS1) of 0.01 Da, MS2 of 0.05 Da, and identification score cutoff of 90 points.
Experimental Animals
SPF-grade 2-month-old SD male rats weighing 200 ± 20 g were purchased from Fujian Medical University Laboratory Animal Center (SCXK2017-0005, Fujian, China). The rats were housed in a room where the temperature and humidity were maintained at 25 °C ± 0.5 °C and 55% ± 5%, respectively, while alternating periods of light and darkness were maintained at 12/12 h. The animal experiment was approved by the Professional Committee of Animal Protection and Use of Fujian Medical University (no. FJMU IACUC 2021-NSFC-0010) in accordance with the Guidelines for Animal Experimentation.
Animal and Experimental Protocol
Fifty male rats were divided into five groups, including normal group, model group, positive control group, and high-dose and low-dose administration groups of CAROL (10 rats per group), and fed adaptively for 7 days before the experiment. The model group was gavaged with 5 g/kg (10 mL/kg) of alcohol solution once a day. The alcohol concentration was gradually increased from 40% to 45%, 50%, 55%, and 60%, of which 40%, 45%, 50%, and 55% were separately gavaged for 3 days, and finally maintained at 60% for 8 days. The positive control group was gavaged with alcohol in the same manner as the model group, and 100 mg/kg silymarin was gavaged once a day 30 min after gavage with alcohol. The method of alcohol gavage in the CAROL administration group was the same as that in the model group. After 30 min of alcohol gavage, 5.4 mL/kg (low-dose) and 10.8 mL/kg (high-dose) of CAROL were gavaged once a day. The normal group was given a similar dose of saline in the same manner.
Sample Collection and Processing
The rats were weighed after 12 h of fasting and deprived of water after the last dose. They were euthanized, and then liver and blood samples were taken. The blood samples were allowed to stand at room temperature for 1 h and then centrifuged at 3000 rpm for 10 min at 4 °C to isolate serum. The liver was weighed, and the liver lobules were taken at room temperature, fixed with 4% paraformaldehyde, dehydrated with ethanol, and embedded in paraffin. The liver tissue was cut into 5 μm slices, infiltrated with xylene, and stained with hematoxylin–eosin (H&E). The tissue sections were observed and evaluated under a light microscope. Another 0.2 g of liver was taken, and 10% liver homogenate was prepared by adding physiological saline. The rest of the liver tissues were frozen at –80 °C for storage.
Calculation of Liver Index (LI)
The weight of the rat’s liver was recorded. LI was calculated using the following formula to assess the extent of liver damage: LI = liver weight/body weight.
Detection of Biochemical Indicators and Cytokines
The alanine transaminase (ALT) and aspartate transaminase (AST) in serum and the superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA), and triglyceride (TG) in the homogenate of liver tissue were measured in accordance with the instructions provided by the kits’ supplier. The ELISA kit was used to detect cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and nuclear regulatory factor (NF)-κB, in the homogenate of liver tissue in accordance with the kit’s instructions.
Metabolomics Analysis
Serum Sample Preparation
The serum samples were prepared as follows: first, 100 μL of the sample was collected and mixed with four times the volume of extraction solvent (acetonitrile: methanol = 1:1, v/v) containing the isotopically labelled internal standard mixture was added. After vortex mixing, the samples were sonicated in an ice water bath for 15 min, incubated at −40 °C for 1 h, and then centrifuged at 4 °C and 12,000 rpm for 15 min. The supernatant (400 μL) was concentrated to dryness under vacuum at 37 °C. Subsequently, 200 μL of 50% acetonitrile was added, and the samples were ultrasonicated in ice water bath for 10 min. The samples were then centrifuged at 11,000 rpm for 20 min at 4 °C, and the obtained supernatant was utilized for UHPLC–QTOF/MS analysis. Quality control (QC) samples were obtained by mixing the same amount of supernatant from all serum samples to evaluate the reliability and stability of the analytical system. The QC samples were injected after each sample analysis.
UHPLC–QTOF/MS Conditions
The UHPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled with a 6600 Q-TOF was used to collect the metabolite profile information of serum samples. Chromatographic separation was carried out on a BEH C18 column (2.1 × 100 mm, 1.7 μm, Waters) equipped with a binary solvent system. The mobile phase was composed of an aqueous buffer (containing 25 mmol/L NH4Ac and 25 mmol/L ammonia, pH 9.75, A) and acetonitrile (B). The elution-gradient program was as follows: 0–0.5 min, 5% B; 0.5–7.0 min, 5%–35% B; 7.0–8.0 min, 35%–60% B; 8.0–9.0 min, 60% B; and 9.0–12.0 min, 60%–5% B. The flow rate was 0.5 mL/min, the injection volume was 2 μL, and the column temperature was maintained at 25 °C.
Data acquisition was performed in full scan/dd-MS2 mode at the scan range of m/z 50–1200, and the ESI source in positive and negative ion modes was used. The mass spectrometric parameters were as follows: atomizer gas and auxiliary gas of 60 psi; curtain gas of 35 psi; ion source temperature of 600 °C; declustering potential of 60 V; and ion-spray floating voltages of 5000 V (ESI +) and −4000 V (ESI−) in positive and negative modes, respectively.
Data Processing and Statistical Analysis
One-way analysis of variance (ANOVA) was used when the variation between groups followed a normal distribution; otherwise, the Games–Howell test was used. The results of body weight, LI, biochemical parameters, and cytokines were calculated and expressed as mean ± standard deviation. Statistical analysis was performed using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA), with P < 0.05 and P < 0.01 indicating significant differences and highly significant differences, respectively.
The MS data were processed through R packet. Multivariate statistical analysis containing principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) was performed afterwards. In accordance with the variable importance in projection (VIP), the criteria of VIP > 1 and P < 0.05 were selected as differential metabolites. Student’s t test was used for statistical analysis by using SPSS 22.0 software (SPSS Inc., Chicago, IL, USA).
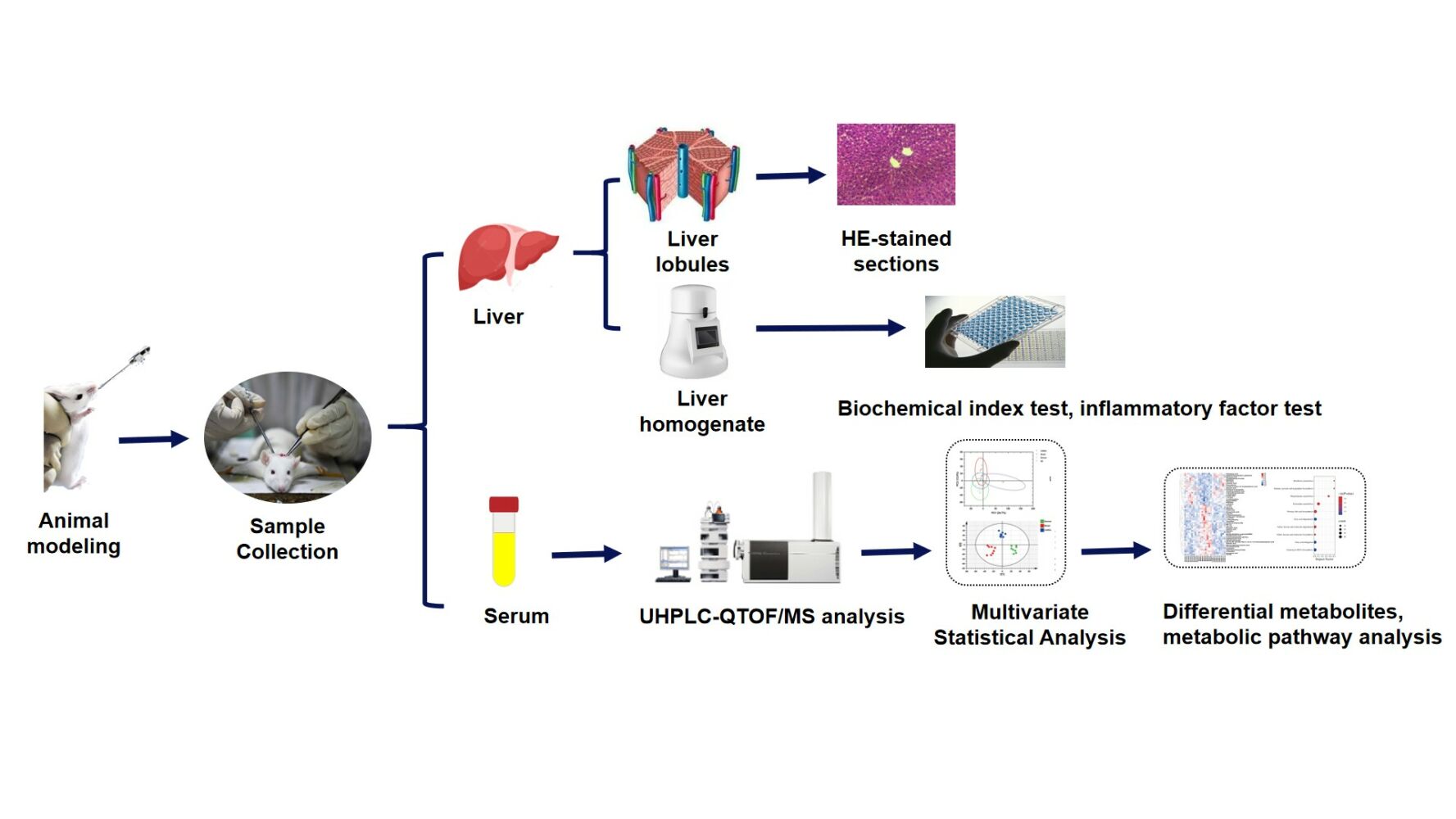
The metabolites were identified on the basis of public databases, including KEGG (http://www.genome.jp/kegg/) and HMDB (http://www.hmdb.ca/). Metabolic pathway analysis (MetPA) was used to explore the most relevant metabolic pathways. The mechanism of the protective effect of CAROL on alcoholic liver injury in rats was explored from the metabolite level. A flowchart of the whole experiment is shown in Supplementary Figure 1.
Results
CAROL as an Hepatoprotective Active Ingredient
A total of 22 kinds of active substances were identified from CAROL by UHPLC–QTOF/MS in accordance with the experimental method in “Identification of active substances in CAROL”. The results are shown in Table 1. These compounds included six flavonoids, five nucleosides, three organic acids, one coumarin, one vitamin, one alkaloid, one steroid, two terpenoids, and two others. Among them, 15 are kinds of liver-protective active substances, including vanillin, rutin, baicalein, hesperidin, uridine, guanosine, adenine, scopoletin, riboflavin, betaine, narcissoside, baicalin, ursolic acid, betulonic acid, and citric acid.
 |
Table 1 The 22 Active Ingredients in CAROL |
In-Vivo Hepatoprotective Effects of CAROL
Effects on Body Weight and LI
The body weight and LI of rats can reflect the health of the liver, as shown in Supplementary Figure 2. After alcohol ingestion, rats in the model group exhibited a depressed mood and significantly lower body weight compared to the normal group (P < 0.01) while their hepatic biochemical parameters were elevated (P < 0.05). Relative to the model group, the body weight of rats increased in all administration groups, but the highest dose of CAROL group showed the greatest increase (P < 0.05). However, no significant difference was found in the LI between the administration groups and the model group, which may be related to the relatively short duration of the experiment.
Effects on Biochemical Indicators
Serum levels of ALT and AST are commonly used indicators of liver injury. As shown in Figure 1, there was liver damage in the model group as indicated by the serum ALT and AST levels being significantly higher than in the normal group (P < 0.01). Both levels significantly decreased after CAROL administration (P < 0. 05). The serum AST levels of rats treated with high-dose CAROL (10.8 mL/kg) showed a more significant decrease than those administered with low-dose CAROL (5.4 mL/kg).
 |
Figure 1 Effect of CAROL on ALT and AST in alcoholic liver rats ( |
The biochemical parameters SOD, GSH, MDA, and TG in liver tissue homogenates are important indicators reflecting liver injury. As shown in Figure 2, SOD and GSH levels were significantly lower in the liver tissue of the model group compared to the normal group (P < 0.05), whereas the MDA content was significantly higher (P < 0.05). Moreover, the SOD content in the liver tissues of all administered groups was significantly higher than that in the model group (P < 0.01), whereas the MDA content was significantly lower (P < 0. 05). The GSH content in the high-dose CAROL groups significantly increased (P < 0.05). On the contrary, the GSH content in the low-dose group and the positive-control group increased, but there was no significant difference compared to the model group. As shown in Figure 3, the TG levels were significantly higher in the model group (P < 0.01) than those of the normal group, all TG levels decreased significantly (P < 0. 01) after administration of CAROL (P<0. 01).
 |
Figure 2 Effect of CAROL on GSH, SOD, MDA, and TG in alcoholic liver rats ( |
 |
Figure 3 Effect of CAROL on IL-6, TNF-α, and NF-κB in alcoholic liver rats ( |
Effects on Cytokines
The levels of IL-6, TNF-α, and NF-κB in the liver tissues of the model group significantly increased (P < 0.01), as depicted in Figure 3. After administration of CAROL, a significant reduction in TNF-α levels was observed (P < 0.01). Moreover, the high-dose CAROL groups showed a significant decrease in IL-6 and NF-κB levels (P < 0.01), whereas no such effect was observed in the low-dose CAROL groups.
Pathological Findings
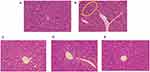
The H&E staining results of the liver tissues are presented in Figure 4. In the normal group (Figure 4A), the liver lobules exhibited a well-defined structural organisation, with hepatocytes appearing healthy and arranged radially around the central vein. The cytoplasm was uniformly stained and no signs of degenerative necrosis or inflammatory infiltrates were observed. Conversely, the model group displayed disorganised liver lobules accompanied by inflammatory infiltrates and slight fat vacuoles (highlighted in Figure 4B). Both the positive control group and CAROL groups (Figures 4C-E) showed clearer structures, more consistent staining, and rounder nuclei compared to the model group. Furthermore, the high-dose CAROL groups exhibited better delineation and alignment than the low-dose CAROL groups and the positive control group, suggesting a superior therapeutic effect.
Further, the high-dose CAROL groups were more clearly outlined and more regularly aligned than the low-dose CAROL groups and the positive-control group, indicating superior effect.
Analysis of the Metabonomic Profile
Identification of Serum Metabolites
The biochemical parameters and HE-stained sections of the liver demonstrated the superior therapeutic effects of high-dose CAROL over silymarin and low-dose CAROL. Therefore, samples of high-dose CAROL groups were selected for metabolomic analysis, hereafter collectively referred to as CAROL.
The serum samples were analysed separately by UHPLC–QTOF/MS in both positive and negative ion modes to achieve a broader coverage of metabolites and improved detection sensitivity. Preprocessing of the raw data involved procedures such as noise removal, outlier filtering, simulation and filling in of missing values, and normalization. A total of 3637 peaks were detected in both positive and negative ion mode.
Multivariate Statistical Analysis
PCA, as the most widely used unsupervised pattern recognition method, is used to reduce dimensions and observe the overall distribution between samples and stability of the entire analytical process. The difference of metabolites in the serum of the normal, model, and CAROL groups could be observed through a PCA diagram (Figure 5A). The horizontal and vertical coordinates indicate PC1 and PC2 and their contributions, respectively. The green, red, blue, and purple dots represent samples from the normal, model, CAROL, and QC groups, respectively. Each dot represents one sample. The PCA score plot of the QCS data showed clustering, and all samples fell inside the 95% confidence range, indicating that the data are reliable and stable This finding showed that the instrument system has good stability. The three sets of samples exhibited intra-group clustering and inter-group separation, indicating that the endogenous metabolites in the rat serum varied across the groups.
The data were further analysed using supervised PLS-DA, which is known for its superior ability to extract information about variation between groups compared to PCA.The results of PLS-DA analysis on serum metabolite data (Figure 5B) demonstrated significant separation among the three groups, substantial metabolic alterations following modeling. Moreover, differential metabolites were identified across the three groups. In serum samples, the R2Y and Q2 parameters for normal, model, and CAROL groups were determined as 0.991 and 0.85, respectively. All parameters exceeded 0.5, indicating a high interpretability rate and predictive capability of the established PLS-DA model. The dispersion of the groups (Figure 5B) obviously indicated that alcohol could induce hepatic metabolic disorder. Compared with the model group, the CAROL group showed a trend of returning to normal, suggesting potential reversal of alcohol-induced metabolic disorder by CAROL treatment. To ensure no overfitting in our models, we performed 200 permutation tests on the PLS-DA model as shown in Figure 5C, where Q2 < 0 indicated absence of overfitting.
ALD Rat Biomarker Analysis After CAROL Intervention
According to the methodology described in “Data processing and statistical analysis”, the statistical analysis method of Student’s T-test and the VIP of the multivariate data anlysis were used to identify metabolites that exhibited differential expression between the two comparison groups. A total of 52 differentially expressed metabolitesby removing duplicates were derived among the three groups, as shown in Table 2.
 |
Table 2 Differential Metabolites in Serum Samples |
Differential metabolites, including isocaproic acid, glycochenodeoxycholate, thymine, L-phenylalanine, pyridoxal (vitamin B6), thymidine, Val-Gln, 6-hydroxydopamine, Val-Asn, 5-hydroxytryptophol, Lys-Trp, NG-dimethy-L-arginine, cholic acid, Sn-glycerol-1-phosphate, glycitein, Met-Tyr, pregabalin, Lys-Pro, L-leucine, dihydroxyfumarat, L-carnitine, cholic acid, uracil, deoxycholic acid, (4Z,7Z,10Z,13Z,16Z,19Z)-4,7,10,13,16,19-docosahexaenoic acid dihomo-gamma-linolenic acid, thromboxane B2, palmitic acid, hippuric acid, helenalin, pentadecanoic acid, heptadecanoic acid, and ginkgolic acid were upregulated in the model group under the influence of alcohol. After the CAROL intervention was applied, 32 of these metabolites had a tendency to reverse modulation (downregulation), similar to the normal group. Meanwhile, the expression levels of riboflavin, alpha-N-phenylacetyl-L-glutamine, decanoyl-CoA, trimethylamine N-oxide, larixinic acid, pyrocatechol, stavudine, 12-oxo-2,3-dinor-10,15-phytodienoic acid, salicylic acid, deoxycytidine, raffinose, pyridoxal 5’-phosphate (PLP), 3-methylcytidine, pelargonic acid, 2-hydroxy-butanoic acid, L-asparagine, ferulic acid, L-proline, 3-guanidinopropanoate, and L-alanine were upregulated after CAROL invention compared to those in the model groups. These findings indicate that alcohol-induced alterations in metabolite profiles tend to regress with CAROL treatment.
In order to better illustrate the patterns in metabolite alterations and the counter-regulatory effects of CAROL, heatmaps were drawn based on the relative intensities of 52 metabolites in the normal, model, and CAROL groups (shown in Figure 6). Each row in Figure 6 represents the relative expression levels of a specific metabolite displayed on the right-hand side, while each column corresponds to the normal, model, and CAROL groups. The intensity level of the metabolite expression is shown by different colors, with red denoting high expression and blue denoting low expression.
 |
Figure 6 Heatmap of serum metabolites differently expressed. Rows correspond to samples, and columns correspond to metabolites. |
Analysis of Metabolic Pathways in CAROL Intervention of ALD Rats
By analyzing the multiple metabolic and regulatory pathways involved, the biological alterations in ALD rats under the effect of CAROL could be systematically understand, thereby elucidating the mechanism of CAROL’s hepatoprotective actions in ALD rats. The metabolic pathway was obtained by mapping differentially expressed metabolites into the KEGG pathway database. Enrichment and topological analyses were performed using the MetPA pathway database to screen the top 10 pathways most closely related to the effects of CAROL (Table 3). The degree of influence is proportional to the size of each bubble. Bubble color and ordinate (-ln P) represent the P value obtained from enrichment analysis, with deeper red hues indicating higher degrees of enrichment. Major pathways that affect metabolism were identified, including phenylalanine, tyrosine and tryptophan biosynthesis, riboflavin metabolism, phenylalanine metabolism, pyrimidine metabolism, vitamin B6 metabolism, and arginine and proline metabolism. To sum up, six main metabolic pathways were identified two related to vitamin B group metabolism, three related to amino acid metabolism, and one is nucleotide metabolism (Figure 7).
 |
Table 3 Metabolite Pathway Changes with MetPA |
 |
Figure 7 Path analysis bubble chart. |
Discussion
In this study, 22 active ingredients in CAROL were identified by UHPLC -QTOF-MS. It has been reported in the literature that these 22 active ingredients have 15 compounds were identified as having ameliorative effects on liver injury, including vanillin,18 rutin,19 baicalein,20 hesperidin,21 uridine,22 guanosine,23 adenine,24 scopoletin,25 riboflavin,26 betaine,27 narcissoside,28 baicalin,29 ursolic acid,30 betulonic acid,31 and citric acid.32 The hepatoprotective effects of CAROL are attributed to these 15 active ingredients.
The pathological mechanism of alcoholic liver injury is complex, the therapeutic effect of CAROL on alcoholic liver injury has not been reported in the literature. To investigate this, a sprague-dawley (SD) rats model of alcoholic liver injury was established and treated with CAROL. The hepatoprotective effect of CAROL and its mechanism were investigated by observing liver histological lesions, examining the changes of biochemical index activities and cytokines in serum and liver homogenates, as well as conducting metabolomic analysis. Indicators of liver weight and LI are macroscopic indicators of response to liver injury. Alcohol intake can cause macroscopic changes in the liver, while an increase in LI indicates successful modeling.33 The rats in the high dose administration group showed a significant increase in body weight. Although there was no significant change in LI between each dosing group and the model group, which may be related to the relatively short duration of the experiment. The ALT and AST in serum are commonly used indicators of liver injury, and they often manifest as elevated biochemical levels.34,35 Serum ALT and AST levels were significantly elevated in rats after gavage alcohol, and then decreased significantly after CAROL administration. It showed that the rat model of liver injury was successfully established and CAROL had a therapeutic effect on liver injury. And the effect of high-dose CAROL was better than that of low-dose CAROL group, indicating a certain dose-effect dependence. Reactive oxygen species (ROS) are highly produced when alcohol is consumed, and these ROS can lead to oxidative stress in the body. Oxidative stress can induce DNA damage and abnormal protein expression in the body, leading to irreversible cellular damage and ultimately resulting in the occurrence of diseases.36 MDA is the end product of lipid peroxidation and widely used to reflect the extent of cellular damage. GSH is a scavenger of toxic metabolites and free radicals, and oxidative stress leads to depletion of GSH. In addition, SOD is associated with inflammatory diseases and oxidative stress.37,38 The increase in SOD and GSH levels and the decrease in MDA content suggest that high doses of CAROL may protect the liver by inhibiting lipid peroxidation and enhancing the antioxidant defense system. In rats intervened with CAROL, the hepatoprotective effects of CAROL were, at least in part, caused by their radical scavenging and antioxidant activities. Fatty liver, which is caused by lipid accumulation, is the most common pathological change caused by alcohol and one of the first pathologies revealed in ALD.39 CAROL can regulate TG levels and thus alleviate liver injury.
In addition to oxidative stress, inflammatory factors may be involved in the development of ALD and predispose individuals to hepatitis. The elevated levels of IL-6, TNF-α and NF-κB in liver tissues of the model group may result from the activation of the NF-κB pathway, which subsequently accelerates the synthesis of inflammatory mediators, including IL-6 and TNF-α. The upregulation of the synthesis of these inflammatory factors leads to cytokine imbalance and immune dysfunction, ultimately resulting in liver hypofunction.38 However, high-dose CAROL intake can exert hepatoprotective effects by downregulating inflammatory factors and ultimately alleviate alcohol-induced hepatotoxicity.
Alcoholic liver injury is usually associated with metabolic disturbances caused by changes in the metabolite profile.40 Serum liver indices, as well as HE-stained sections, confirmed that CAROL can effectively reduce oxidative stress and hepatocyte injury with hepatoprotective effects. On this basis, UPLC-QTOF-MS non-targeted metabolomics technique was used to study the changes of metabolites in liver-injured rats after drug administration. It is hypothesised that CAROL may exert hepatoprotective effects by regulating metabolic pathways such as riboflavin metabolism; phenylalanine, tyrosine, and tryptophan biosynthesis metabolism; phenylalanine metabolism; pyrimidine metabolism; arginine and proline metabolism; and vitamin B6 metabolism.
Vitamin B metabolism is an important metabolic pathway affecting alcoholic liver injury, and riboflavin is a compound that regulates the metabolism of vitamin B2. In this experiment, the metabolism of riboflavin was downregulated in the model group compared to that in the normal group, which may indicate that alcohol interferes with the digestion and absorption of riboflavin from food in rats.41 Riboflavin is a necessary for GSH production, which is crucial for antioxidant defense, especially when active oxygen is produced due to alcohol induction.42 Therefore, a deficiency in riboflavin could lead to the destruction of mitochondrial respiratory chain, thus overwhelming the cellular antioxidant, and oxidative stress is conducive to the occurrence of liver disease.43 Compared to the alcohol group, CAROL upregulated this metabolism. This finding is consistent with the results of the biochemical index tests in section “Effects on biochemical indicators”. Research showed that vitamin B6 could significantly improve inflammatory cell infiltration and liver cell edema and degeneration.44,45 PLP is a compound that regulates the metabolism of vitamin B6. The absorption of ethanol could change the liver metabolism of PLP in an acute and chronic manner. Acetaldehyde produced by ethanol has a harmful effect on the metabolism of PLP, and it could enhance the enzymatic hydrolysis of PLP by phosphatase.46 Compared to the alcohol group, the CAROL group affected the metabolism of Vitamin B6 by upregulating PLP. In summary, CAROL could improve alcoholic liver injury by regulating the vitamin B metabolic pathway.
The disorder of amino acid metabolism often occurs in patients with liver disease or mild liver injury, indicating that amino acid metabolism could be significantly interfered by liver injury. Some studies have shown that liver injury has a specific and repeatable plasma amino acid pattern, such as an increase in the concentration of aromatic amino acids (AAAs) and a reduction in the concentration of branched chain amino acids (BCAAs). This phenomenon is known as Fisher’s ratio (BCAAs/AAAs), which has been reported as an important indicator for determining the liver’s metabolism, reserve of liver function, and degree of liver disease.47,The biosynthesis and biological metabolism of tyrosine, tryptophan, and phenylalanine are all influenced by oxidative stress, inflammation, and lipid metabolism.48 The increased content of phenylalanine, tyrosine, and tryptophan could interfere with the mitochondrial TCA cycle and thus affect liver injury.49 In the present study, the metabolism of phenylalanine, tyrosine, and tryptophan was one of the metabolic pathways of ALD regulation by CAROL. Phenylalanine was significantly upregulated in rat serum after alcohol intake, in agreement with the literature.50 Compared to the alcohol group, the concentration of phenylalanine in the serum of ALD rats after CAROL intervention was reduced. CAROL may improve in-vivo lipid metabolism, inflammation, and oxidative stress in ALD rats by regulating the biosynthesis of phenylalanine, tyrosine, and tryptophan.
Proline is closely related to arginine and proline metabolism, playing a significant role in protecting the liver from damage. It possesses antioxidant and ROS scavenging properties, helping to maintain stable levels of glutathione. Furthermore, proline metabolism in the mitochondria can prevent liver damage associated with cholestasis, contributing significantly to the liver’s defense. In addition, proline has an inhibitory effect on liver inflammation.51 In this study, the model group exhibited downregulated level of proline compared to the normal group. Alcohol enhanced oxidative stress by downregulating proline levels, leading to liver injury. Therefore, CAROL may ameliorate liver injury by regulating arginine and proline metabolism and upregulating proline. This is consistent with literature reporting that proline is upregulated to protect against liver injury.51
Pyrimidine nucleotides are essential for base pairing in DNA synthesis. The derivatives of pyrimidine nucleotides may regulate cell signaling and energy metabolism to maintain intracellular homeostasis.52 Different metabolites of thymidine, thymine, uracil, and deoxycytidine are closely associated with pyrimidine metabolism. Alcohol upregulated uracil, thymidine, and thymine and downregulated deoxycytidine compared to the normal group; however, CAROL intervention reversed this trend. A report has shown that liver damage could be caused by disrupted metabolism of pyrimidines.53 The interference of pyrimidine metabolism may occur simultaneously with major liver cell dysfunction, such as oxidative stress, inhibition of bile acid production, and GSH biosynthesis pathway.54 CAROL could alleviate ALD by regulating pyrimidine metabolism. The literature also reports that ethanol extract of Baizhi Gentiana can treat alcoholic liver injury by regulating the pyrimidine metabolic pathway.55
In conclusion, riboflavin metabolism; biosynthetic metabolism of phenylalanine, tyrosine, and tryptophan; phenylalanine metabolism; pyrimidine metabolism; arginine and proline metabolism; and vitamin B6 metabolism may be the important metabolic pathways for the protective effect of CAROL on alcoholic liver injury in rats. The protection from CAROL against alcoholic liver injury in rats may act by affecting multiple important metabolic pathways as shown in Figure 8. This work did not quantify the changes in serum levels of several core metabolites in rats after CAROL intervention, and the levels of several core metabolites in serum will be further verified quantitatively by LC-QqQ/MS technique in the future.
Conclusion
In this study, 22 active substances in CAROL were identified, 15 of which have hepatoprotective activity. The outcomes of the trial demonstrated that CAROL protects rats’ livers from damage brought on by alcohol. By using multivariate statistical analysis and UPLC-QTOF/MS, the potential differential metabolites were identified. A total of 52 differential metabolites that could be reversed by CAROL were identified in positive and negative ion modes, respectively. The analysis of differential metabolites revealed that riboflavin metabolism; phenylalanine, tyrosine, and tryptophan biosynthesis metabolism; phenylalanine metabolism; pyrimidine metabolism; arginine and proline metabolism; and vitamin B6 metabolism are important metabolic pathways related with CAROL therapy in rats with alcoholic liver injury. Comprehensive analysis of the mechanism inferred that CAROL could inhibit inflammatory factors, enhance the activity of antioxidant enzymes, regulate the TCA cycle, and reduce lipid peroxidation to alter related metabolites and their metabolic pathways, thus achieving hepatoprotective effects. This study revealed the hepatoprotective mechanism of CAROL based on metabolomics research findings, thus providing a theoretical basis for studying its clinical application as well as a strategy for exploring the mechanism behind the hepatoprotective effect of Chinese compound medicinal preparations. The target proteins in the key pathways will be further validated by Western blotting (WB) to explore the mechanism of CAROL attenuating liver injury in a more comprehensive and in-depth manner.
Abbreviations
CAROL, Compound Anoectochilus roxburghii (Wall.) Lindl oral liquid; ALD, Alcoholic liver injury; UHPLC-QTOF/MS Ultra-high performance liquid chromatography quadrupole time-of-flight mass spectrometry; SD, Sprague-Dawley; KEGG, Kyoto Encyclopedia of Genes and Genomes; TCM, Traditional Chinese medicine; A. roxborghii, Anoctochilus Roxborghii (Wall.) Lindl; G. lucidum, Ganoderma lucidum; NMR, Nuclear magnetic resonance; GC/MS, Gas chromatography/mass spectrometry; LC/MS, Liquid chromatography/mass spectrometry; ELISA, Enzyme-linked immunosorbent assay; ESI, Electrospray ionization source in positive mode; IDA, Information dependent acquisition; LI, Liver index; ALT, Alanine transaminase; AST, Aspartate aminotransferase; SOD Superoxide dismutase; GSH, Glutathione; MDA, Malondialdehyde; TG, Triglyceride; TNF-α, Tumor necrosis factor-α; IL-6, Interleukin-6; NF-κB, Nuclear regulatory factor-κB; QC, Quality control; ANOVA, One-way analysis of variance; PCA, Principal component analysis; PLS-DA, Partial least squares discriminant analysis; ROS, Reactive oxygen species; VIP, Variable importance in the projection; MetPA, Metabolic pathway analysis; PLP, Pyridoxal 5 ‘- phosphate; AAAs, Aromatic amino acids; BCAAs, Branched chain amino acids; TCA, Tricarboxylic acid cycle; WB, Western blotting.
Ethics Approval and Consent to Participate
The ethical approval of this study was provided by the Institutional Ethical Committee of Fujian Medical University, Fujian, China (No. FJMU IACUC 2021-NSFC-0010).
Acknowledgments
The authors wish to express their gratitude to Xue Mi (Public Technology Center of Fujian Medical University), Public Technology Center of Fujian Medical University for the support on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Natural Fund Project of Fujian Province, China (No. 2020J01623 and 2023J01311), the Joint Funds at the Innovation of Science and Technology, Fujian Province, China (No. 2021Y9008), the Young Teachers Education Foundation of Fujian Province, China (No. JAT220080), the High-Level Talent Foundation of Fujian Medical University (No. XRCZX2021005).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Park SH, Lee YS, Sim J, et al. Alcoholic liver disease: a new insight into the pathogenesis of liver disease. Arch Pharm Res. 2022;45(7):447–459. doi:10.1007/s12272-022-01392-4
2. Zhang H, Zuo Y, Zhao H, et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis. Front Nutr. 2022;9:989311. doi:10.3389/fnut.2022.989311
3. Liu J, Kong D, Ai D, et al. Insulin resistance enhances binge ethanol-induced liver injury through promoting oxidative stress and up-regulation CYP2E1. Life Sci. 2022;303:120681. doi:10.1016/j.lfs.2022.120681
4. Tang L, Yu X, Zou L, et al. Qinggan Huoxue recipe protects against experimental alcoholic Liver fibrosis through CXCL16 inhibition. Evid Based Complement Alternat Med. 2023;2023:5642713. doi:10.1155/2023/5642713
5. Liu Z, Xu B, Ding Y, et al. Guizhi Fuling pill attenuates liver fibrosis in vitro and in vivo via inhibiting TGF-β1/Smad2/3 and activating IFN-γ/Smad7 signaling pathways. Bioengineered. 2022;13(4):9357–9368. doi:10.1080/21655979.2022.2054224
6. Delgado-Montemayor C, Cordero-Pérez P, Torres-González L, et al. Development of a hepatoprotective herbal drug from turnera diffusa. Evid Based Complemen Alternative Med. 2022;2022:1–10. doi:10.1155/2022/5114948
7. Mo Q, Zhou G, Xie B, et al. Evaluation of the hepatoprotective effect of Yigan mingmu oral liquid against acute alcohol-induced liver injury in rats. BMC Complement Med Ther. 2020;20(1):32–42. doi:10.1186/s12906-020-2817-9
8. Gao L, Chen X, Fu Z, et al. Kinsenoside Alleviates alcoholic liver injury by reducing oxidative stress, inhibiting endoplasmic reticulum stress, and regulating AMPK-Dependent Autophagy. Front Pharmacol. 2021;12:747325. doi:10.3389/fphar.2021.747325
9. Zhang Q, Huang L, Wu Y, et al. Study on quality control of Compound Anoectochilus roxburghii (Wall.) Lindl. by liquid chromatography-tandem mass spectrometry. Molecules. 2022;27(13):4130. doi:10.3390/molecules27134130
10. Lv XC, Wu Q, Cao YJ, et al. Ganoderic acid A from Ganoderma lucidum protects against alcoholic liver injury through ameliorating the lipid metabolism and modulating the intestinal microbial composition. Food Funct. 2022;13(10):5820–5837. doi:10.1039/D1FO03219D
11. Xu X, Huang L, Wu Y, et al. Synergic cloud-point extraction using [C4mim][PF6] and Triton X-114 as extractant combined with HPLC for the determination of rutin and narcissoside in Anoectochilus roxburghii (Wall.) Lindl. and its compound oral liquid. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1168:122589. doi:10.1016/j.jchromb.2021.122589
12. Fang ZZ, Gonzalez FJ. LC-MS-based metabolomics: an update. Arch Toxicol. 2014;88(8):1491–1502. doi:10.1007/s00204-014-1234-6
13. Fu T, Qin S, He HJ, et al. Mechanisms of Ardisia japonica in the treatment of hepatic injury in rats based on LC-MS metabolomics. Metabolites. 2022;12(10):981. doi:10.3390/metabo12100981
14. Dong Y, Qiu P, Zhao L, et al. Metabolomics study of the hepatoprotective effect of Phellinus igniarius in chronic ethanol-induced liver injury mice using UPLC-Q/TOF-MS combined with ingenuity pathway analysis. Phytomedicine. 2020;74:152697. doi:10.1016/j.phymed.2018.09.232
15. Li S, Wang Y, Li C, et al. Study on hepatotoxicity of Rhubarb based on metabolomics and network pharmacology. Drug Des Devel Ther. 2021;15:1883–1902. doi:10.2147/DDDT.S301417
16. Liu F, Wang M, Wang Y, et al. Metabonomics Study on the Hepatoprotective Effect of Panax notoginseng Leaf Saponins Using UPLC/Q-TOF-MS Analysis. Am J Chin Med. 2019;47(03):559–575. doi:10.1142/S0192415X19500290
17. An L, Lang Q, Shen W, et al. Dynamic metabolic profiling of urine biomarkers in rats with alcohol‑induced liver damage following treatment with Zhi‑Zi‑Da‑Huang decoction. Mol Med Rep. 2016;14(3):2093–2100. doi:10.3892/mmr.2016.5494
18. Zheng WV, Li Y, Cheng X, et al. Uridine alleviates carbon tetrachloride‐induced liver fibrosis by regulating the activity of liver‐related cells. J Cell Mol Med. 2021;26(3):840–854. doi:10.1111/jcmm.17131
19. Yu Z, Li Q, Wang Y, et al. A potent protective effect of baicalein on liver injury by regulating mitochondria-related apoptosis. Apoptosis. 2020;25(5–6):412–425. doi:10.1007/s10495-020-01608-2
20. Yang J-Y, Li M, Zhang C-L, et al. Pharmacological properties of baicalin on liver diseases: a narrative review. Pharmacol Rep. 2021;73:1230–1239. doi:10.1007/s43440-021-00227-1
21. Tang N, Hong F, Hao W, et al. Riboflavin ameliorates mitochondrial dysfunction via the AMPK/PGC1α/HO‑1 signaling pathway and attenuates carbon tetrachloride‑induced liver fibrosis in rats. Exp Ther Med. 2022;24(4):608. doi:10.3892/etm.2022.11545
22. Nasehi Z, Kheiripour N, Taheri MA, et al. Efficiency of hesperidin against liver fibrosis induced by bile duct ligation in rats. Biomed Res Int. 2023;2023:1–12. doi:10.1155/2023/5444301
23. Li Y, Guo S, Ren Q, et al. Pharmacokinetic comparisons of multiple triterpenic acids from jujubae fructus extract following oral delivery in normal and acute liver injury rats. Int J Mol Sci. 2018;19(7):2047. doi:10.3390/ijms19072047
24. Hiroki Nishi D, Masuda M, YukiGoda KI. Alteration of serum amino acidprofles by dietary adeninesupplementation inhibits fatty liverdevelopment in rats. Sci Rep. 2020;10(1):22110. doi:10.1038/s41598-020-79234-w
25. Ham JR, Lee H-I, Choi R-Y, et al. Anti-obesity and anti-hepatosteatosis effects of dietary scopoletin in high-fat diet fed mice. J Funct Foods. 2016;25:433–446. doi:10.1016/j.jff.2016.06.026
26. Gan D, Zhang W, Huang C, et al. Ursolic acid ameliorates CCl4‐induced liver fibrosis through the NOXs/ROS pathway. J Cell Physiol. 2018;233(10):6799–6813. doi:10.1002/jcp.26541
27. Santos Szewczyk K D, Pietrzak W, Klimek K, et al. LC-ESI-MS/MS identification of biologically active phenolics in different extracts of alchemilla acutiloba opiz. Molecules. 2022;27(3):621–633. doi:10.3390/molecules27030621
28. Bellaver B, Souza DG, Bobermin LD, et al. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinergic Signal. 2015;11(4):571–580. doi:10.1007/s11302-015-9475-2
29. Arumugam MK, Chava S, Perumal SK, et al. Acute ethanol-induced liver injury is prevented by betaine administration. Front Physiol. 2022;13:940148. doi:10.3389/fphys.2022.940148
30. Amal MH, Ghanim NSY, Heba A. Metwaly iecturer vanillin augments liver regeneration effectively in hioacetamide induced liver fibrosis rat model. Life Sci. 2021;286:120036. doi:10.1016/j.lfs.2021.120036
31. Ahmed OM, Elkomy MH, Fahim HI, et al. Rutin and Quercetin counter doxorubicin-induced liver toxicity in Wistar rats via their modulatory effects on inflammation, oxidative stress, apoptosis, and Nrf2. Oxid Med Cell Longev. 2022;2022:1–19.
32. Abdel-Salam OME, Youness ER, Mohammed NA, et al. Novel neuroprotective and hepatoprotective effects of citric acid in acute malathion intoxication. Asian Pac J Trop Med. 2016;9(12):1181–1194. doi:10.1016/j.apjtm.2016.11.005
33. Zhang Z, Zhou H, Bai L, et al. Protective effects of probiotics on acute alcohol-induced liver injury in mice through alcohol metabolizing enzymes activation and hepatic TNF-α response reduction. J Funct Foods. 2019;59:234–241. doi:10.1016/j.jff.2019.05.018
34. Wang C, Zhao S, Xu Y, et al. Integrated microbiome and metabolome analysis reveals correlations between gut microbiota components and metabolic profiles in mice with methotrexate-induced hepatoxicity. Drug Des Devel Ther. 2022;16:3877–3891. doi:10.2147/DDDT.S381667
35. Chinnappan R, Mir TA, Alsalameh S, et al. Aptasensors are conjectured as promising ALT and AST diagnostic tools for the early diagnosis of acute liver injury. Life. 2023;13(6):1273. doi:10.3390/life13061273
36. Yang CL, Lin YS, Liu KF, et al. Hepatoprotective Mechanisms of taxifolin on carbon Tetrachloride-Induced acute liver injury in mice. Nutrients. 2019;11(11):2655. doi:10.3390/nu11112655
37. Pereira CMC, Junior GJD, Lima J, et al. Phosphatidylinositol 3-kinase gamma participates in nimesulide-induced hepatic damage. J Pharm Pharmacol. 2021;73(4):496–504. doi:10.1093/jpp/rgaa049
38. Zhou J, Zhang J, Wang C, et al. Acai (Euterpe oleracea Mart.) attenuates alcohol-induced liver injury in rats by alleviating oxidative stress and inflammatory response. Exp Ther Med. 2018;15(1):166–172. doi:10.3892/etm.2017.5427
39. Xue Y, Li X, Tian Y, et al. Salmon sperm DNA prevents acute liver injury by regulating alcohol-induced steatosis and restores chronic hepatosis via alleviating inflammation and apoptosis. J Food Biochem. 2022;46(10):e14346. doi:10.1111/jfbc.14346
40. Fan X, Wang X, Lian J, et al. Flos Carthami exerts hepatoprotective action in a rat model of alcoholic liver injury via modulating the metabolomics profile. Evid Based Complement Alternat Med. 2022;2022:8158699. doi:10.1155/2022/8158699
41. Pinto J, Huang YP. Mechanisms underlying the differential effects of ethanol on the bioavailability of riboflavin and flavin adenine dinucleotide. J Clin Invest. 1987;79(5):1343–1348. doi:10.1172/JCI112960
42. Dutta PSJ, Halpin D, Pinto J, Rivlin R. Acute ethanol exposure alters hepatic glutathione metabolism in riboflavin deficiency. Alcohol. 1995;12:43–47. doi:10.1016/0741-8329(94)00068-O
43. Zhu YY, Thakur K, Feng JY, et al. Riboflavin bioenriched soymilk alleviates oxidative stress mediated liver injury, intestinal inflammation, and gut microbiota modification in B (2) depletion-repletion Mice. J Agric Food Chem. 2022;70:3818–3831. doi:10.1021/acs.jafc.2c00117
44. Mei M, Liu D, Tang X, et al. Vitamin B6 metabolic pathway is involved in the pathogenesis of liver diseases via multi-omics analysis. J Hepatocell Carcinoma. 2022;9:729–750. doi:10.2147/JHC.S370255
45. Ma T, Li Y, Zhu Y, et al. Differential Metabolic Pathways and metabolites in a C57BL/6J mouse model of alcoholic liver disease. Med Sci Monit. 2020;26:e924602. doi:10.12659/MSM.924602
46. Veuch RL, Lumeng L, Li T-K. Vitamin B6 metabolism in chronic alcohol abuse. J Clin Invest. 1975;55:1026–1032. doi:10.1172/JCI108003
47. Choe H, Yun I, Kim Y, et al. Effect of herbal extracts and supplement mixture on alcohol metabolism in Sprague Dawley-rats. J Food Sci Technol. 2022;59(12):4915–4923. doi:10.1007/s13197-022-05580-4
48. Liu Y, Luo Y, Wang X, et al. Gut microbiome and metabolome response of Pu-erh Tea on metabolism disorder induced by chronic alcohol consumption. J Agric Food Chem. 2020;68(24):6615–6627. doi:10.1021/acs.jafc.0c01947
49. Baltazar-Diaz TA, Gonzalez-Hernandez LA, Aldana-Ledesma JM, et al. Escherichia/Shigella, SCFAs, and metabolic pathways-The triad that Orchestrates Intestinal Dysbiosis in patients with decompensated alcoholic cirrhosis from western Mexico. Microorganisms. 2022;10:1231. doi:10.3390/microorganisms10061231
50. Liu C, Hua H, Guo Y, et al. Study on the hepatoprotective effect of Sporidiobolus pararoseus polysaccharides under the “gut microbiome-amino acids metabolism” network. Food Biosci. 2022;49:101928. doi:10.1016/j.fbio.2022.101928
51. Heidari R, Mohammadi H, Ghanbarinejad V, et al. Proline supplementation mitigates the early stage of liver injury in bile duct ligated rats. J Basic Clin Physiol Pharmacol. 2018;30(1):91–101. doi:10.1515/jbcpp-2017-0221
52. Le TT, Ziemba A, Urasaki Y, et al. Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J Lipid Res. 2013;54:1044–1057. doi:10.1194/jlr.M034249
53. Xu L, Wang Y, Ma Z, et al. Urine Metabolomics Study on potential hepatoxic biomarkers identification in rats induced by Aurantio-Obtusin. Front Pharmacol. 2020;11:1237. doi:10.3389/fphar.2020.01237
54. Ruan LY, Li MH, Xing YX, et al. Hepatotoxicity and hepatoprotection of Polygonum multiflorum Thund. as two sides of the same biological coin. J Ethnopharmacol. 2019;230:81–94. doi:10.1016/j.jep.2018.10.032
55. Cao H, Guo Y, Jin L. A study on gentiana dahurica fisch ethanol extract alleviating alcoholic liver disease in Mice: a metabolomic analysis of the liver. Evid Based Complement Alternat Med. 2021;2021:5569538. doi:10.1155/2021/5569538
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.