Back to Journals » Clinical Epidemiology » Volume 13
Measuring Medication Adherence in a Population-Based Asthma Administrative Pharmacy Database: A Systematic Review and Meta-Analysis
Authors Asamoah-Boaheng M , Osei Bonsu K, Farrell J , Oyet A, Midodzi WK
Received 15 August 2021
Accepted for publication 30 September 2021
Published 22 October 2021 Volume 2021:13 Pages 981—1010
DOI https://doi.org/10.2147/CLEP.S333534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Vera Ehrenstein
Michael Asamoah-Boaheng,1 Kwadwo Osei Bonsu,2 Jamie Farrell,1 Alwell Oyet,3 William K Midodzi1
1Faculty of Medicine, Memorial University of Newfoundland, St John’s, NL, Canada; 2School of Pharmacy, Memorial University of Newfoundland, St John’s, NL, Canada; 3Department of Mathematics and Statistics, Memorial University of Newfoundland, St John’s, NL, Canada
Correspondence: Michael Asamoah-Boaheng
Faculty of Medicine, Memorial University of Newfoundland, St John’s, NL, Canada
Tel +1 7093513861
Email [email protected]
Background: Limited studies have systematically reviewed the literature to identify and compare the various database methods and optimal thresholds for measuring medication adherence specific to adolescents and adults with asthma. In the present study, we aim to identify the methods and optimal thresholds for measuring medication adherence in population-based pharmacy databases.
Methods: We searched PubMed, Embase, International Pharmaceutical Abstracts (IPA), Web of Science, Google Scholar, and grey literature from January 1, 1998, to March 16, 2021. Two independent reviewers screened the studies, extracted the data, and assessed the quality of the studies. A quantitative knowledge synthesis was employed.
Results: Thirty-eight (38) retrospective cohort studies were eligible. This review identified 20 methods for measuring medication adherence in adolescent and adult asthma administrative health records. Two measures namely the medication possession ratio (MPR) and proportion of days covered (PDC) were commonly reported in 87% of the literature included in this study. From the meta-analysis, asthma patients who achieved adherence threshold of “ 0.75– 1.00” [OR: 0.56, 95% CI: 0.41 to 0.77] and “> 0.5” [OR: 0.71, 95% CI: 0.54 to 0.94] were less likely to experience asthma exacerbation.
Conclusion: Despite their limitations, the PDC and the MPR still remain the most common measures for assessing adherence in asthma pharmacy claim databases. The evidence synthesis showed that an adherence threshold of at least 0.75 is optimal for classifying adherent and non-adherent asthma patients.
Keywords: medication adherence, adherence measures, asthma, adherence thresholds, meta-analysis, administrative health databases, review
Introduction
Achieving targeted clinical outcomes–asthma control, reduced asthma exacerbation and improved lung function – in asthma patients require a certain degree of medication use. Medication adherence evaluates the degree to which patients use their medications as prescribed by their healthcare providers.1,2 While adherence to treatment is essential to optimize the benefits of therapy, nonadherence has been associated with poor clinical outcomes, increased healthcare cost and low quality of life.3–5 Medication adherence in adult asthma patients ranges from 30% to 70%6–8 and these estimates differ by country, age, sex, and ethnicity.9
Several methods have been developed to measure medication adherence and the use of records on prescribed medications to indirectly estimate adherence has gained prominence due to increasing availability of electronic health records and administrative data.10,11 The accurate evaluation of medication adherence in large populations using administrative data is important for assessing medication effectiveness, identifying risk factors associated with suboptimal adherence as well as introducing effective interventions for improving adherence.12,13 However, the use of administrative and pharmacy claim databases–have several shortcomings including incomplete, or missing data and inability to confirm if patients actually ingested their acquired medication.14,15 Nonetheless, these adherence measures could reflect real-life setting15 and improve clinical outcomes if the database captures complete coverage of prescribed medications and refill medication history.12
Using administrative data, researchers and clinicians are often faced with a dilemma of choosing an appropriate adherence measure from a wide range of measures and approaches in the literature.16 In particular, the availability of different adherence measures and their variations commonly used in estimating adherence to asthma medications often worsen the plight of researchers in this area. While some investigators have consistently reported common methods for measuring adherence, a wide variety of threshold classification exist.11,17,18 Two of the most widely used adherence measures that could be estimated from administrative data are the medication possession ratio (MPR) and proportion of days covered (PDC), which estimates the proportion of the time a patient has medication available.11 The PDC and MPR adherence rate data can be reported as continuous or converted to a dichotomous measure when a patient attains a certain degree of compliance. To identify patients who are adherent to their medication using these measures, a threshold of ‘≥0.80ʹ is conventionally used regardless of the clinical contexts; nonetheless, the threshold may differ across medication therapeutic classes or disease condition.11,19 There is no ideal threshold for measuring adherence to prescribed medications and the selection of arbitrary cut-off value/threshold is of great concern to researchers since there is lack of pharmacological basis underlying decision to choose cut-off values.11,20 In addition, several studies have proposed and used disease-specific measures to assess adherence to medications among patients with various conditions including asthma.19,21 Thus, it remains unclear which adherence measure would be appropriate to assess adherence to asthma medications in patient population already known to have high non-adherence rates.
To our knowledge, limited studies have systematically summed up the evidence around adherence measures to identify an appropriate measure for patients with adolescent and adult asthma. In addition, there is dearth of studies that have identified an optimal adherence threshold for the appropriate adherence measure and their association with clinical outcomes in adolescents and adults with asthma. In view of this, we aim to systematically review evidence in extant literature to identify and compare various methods for measuring medication adherence; optimal thresholds for assessing adherence to medications and their association to targeted clinical outcomes in adolescents and adults with asthma.
Materials and Methods
We followed the recommended checklist, the Preferred Reporting Item of Systematic Reviews and Meta-Analyses (PRISMA)22 to conduct and report the comprehensive systematic review of the selected studies. The protocol of this review was registered in PROSPERO with registration number CRD42020168922.
Literature Search and Search Strategy
The search strategy was developed by the author (MA-B) in consultation with a Health Science Librarian at the Faculty of Medicine, Memorial University of Newfoundland. We performed a comprehensive search in PubMed, Embase, International Pharmaceutical Abstracts (IPA) and hand search in Google Scholar, Web of Science, grey literature (thesis, unpublished papers), ResearchGate and other research platforms. The authors started the exhaustive search on February 1, 2020, and ended on February 5, 2020, and was subsequently updated up to March 16, 2021. The search included articles published from January 1, 1998, to March 16, 2021. The search criteria comprised “MeSH” terms in PubMed, “Emtree” in Embase, keywords and a combination of “MeSH” terms and Keywords and finally “Emtree” and Keywords. MeSH terms used for the search were (“medication adherence”[Mesh]), and (“Asthma”[Mesh]). Keywords used included (prescription[tiab] OR medication[tiab] OR puffer[tiab] OR “inhaled corticosteroid”[tiab]) AND (adherence[tiab] OR compliance[tiab] OR filling[tiab] OR dispensing[tiab] OR dispensed[tiab] OR filled[tiab]) AND (“Asthma”[Mesh] OR asthma[tiab]). Our search focused on human studies and was limited to studies involving asthma patients aged 12 years and older. Additionally, studies published in English language were included in this review. We manually screened the reference lists of the relevant studies to identify additional articles. Also, content experts were contacted to inquire about other related studies. The final search strategy in all the research databases is summarized in the Supplementary Material in Table S1.
Study Eligibility and Selection
Two reviewers (MA-B, KOB) independently screened the titles and abstracts yielded by the three bibliographic databases for eligibility at the initial stage. The Rayyan software (a free web and mobile app reference manager)23 was used to expedite the initial screening of the abstracts and titles. Further, Rayyan was used to remove duplicates and sort inclusions and exclusions of the retrieved abstracts. Any disagreement in the selection of the studies was resolved by consensus or arbitration by an independent researcher. After their relevance was assessed, selected articles were further screened. Studies were eligible for inclusion if they met the following criteria: a) included individuals 12 years and older with physician diagnosis of asthma; Physician diagnosis of asthma was defined as any diagnosis based on ICD codes for asthma in claim/administrative databases as well as prescribed asthma-related medications; b) using population-based administrative claim databases; c) studies reporting claim databases medication adherence measures for asthma; d) studies published from January 1, 1998, to March 2021; e) articles written in English and f) studies published only on humans.
Quality Assessment and Risk of Bias
The reviewers independently assessed the risk of bias and quality assessment of the included studies. We adopted the Joanna Briggs Institute checklist24 to evaluate the risk of bias of the cohort studies. Using the checklist, we assessed the quality of the individual studies based on 10 domains (see Table S2 in the Supplementary Material). Any disagreement that arose from the assessment of the risk of bias of the studies was resolved by an arbitrator (third reviewer). Further, we determined the confidence in the evidence of studies included in the meta-analysis using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE).25
Data Extraction
The reviewers used a standardized spreadsheet based on some generic items and relevant information to independently extract the following data: (a) Study ID or author’s name, (b) study population, (c) study design, (d) name of the administrative database, (e) location, (f) outcome assessed/study objectives, (g) medication adherence measures/related measures, (h) definition of the measure, (i) Strength and weaknesses/limitations of the measures, and (j) estimated rate of adherence measured/study results. The data extraction process was simultaneously performed by the reviewers (MA-B and KOB). We resolved the disagreements in the data extraction by mutual agreement.
Evidence Synthesis
A priori, we anticipated significant variations particularly in study design and objectives of studies included in this review. This could introduce heterogeneity and impact on conclusions drawn from our evidence synthesis. To mitigate the impact of heterogeneity on evidence synthesized, two separate approaches – quantitative and narrative – were used to synthesize evidence from retrieved studies. Specifically, we presented outcome data which were practicable to quantitatively combine in a meta-analysis. We used narrative/qualitative synthesis for data with significant heterogeneity and impracticable to combine in a quantitative synthesis. This was done to ensure that, sound and solid conclusions could be made from the evidence gleaned from the various studies included in our systematic review.
Qualitative/Narrative Data Synthesis
We conducted a narrative synthesis of studies meeting the inclusion criteria. Narrative synthesis is an approach to the systematic review and synthesis of findings from multiple sources which primarily uses text to summarize and explain the findings of the synthesis.26 It is used when statistical meta-analysis is not feasible particularly due to substantial methodological and clinical heterogeneity between studies identified.26 This study sought to find appropriate adherence measures and further determine the optimal adherence threshold for adults with asthma using administrative data. Thus, this narrative synthesis focused on adherence measures reported in the various claims/administrative databases and study findings were grouped by type, definition/equation, cut-off values or thresholds determination of medication adherence measures.
Quantitative Data Synthesis
The main summary measure for the quantitative synthesis was the odds ratio (OR). Review Manager, version 5.4, and Comprehensive Meta-analysis (CMA) software’s were used to analyze data for the quantitative synthesis. We employed the random effects model to synthesize the available evidence due to the suspected between study heterogeneity. The effects estimates were synthesized using the generic inverse variance method to estimate the contribution of each study (expressed in weights) to the pooled effect. Meta-regression was conducted to investigate the source of the between-study heterogeneity. The authors performed a publication bias check by using the ‘Orwin’s fail-safe Ns’, Egger’s regression test and Funnel plot.
Results
Identification of Studies
The database search generated a total of 7268 citations (PubMed = 2456, Embase = 4479, IPA = 321, and additional searches from other sources = 12) [see Figure 1]. Duplicate studies were removed using the Rayyan web app reference manager leaving 2756 records. The titles and abstracts of the 2756 records were screened for relevance. After the screening, we retrieved and downloaded 70 articles for full text review and finally excluded 32 studies based on the study’s inclusion and exclusion criteria. The remaining 38 retrospective/prospective cohort studies met the inclusion criteria for this review. The flow diagram in Figure 1 summarizes studies identified and excluded at each stage of the review.
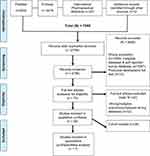 |
Figure 1 Flow diagram depicting article inclusion and exclusion along with reasons. |
Study Characteristics
The general characteristics of the 38 included articles are presented in Table 1. Most of the studies (n = 33) were retrospective cohort studies with pharmacy claims data.14,15,18,21,27–55 Three studies employed a retrospective design with prospective assessment45,53,56 and two other studies conducted by Bidwal et al19 and Vaidya et al57 were retrospective in design with cross-sectional assessment of medication adherence without follow-up. All 38 articles were published between 2010 and 2020. More than half of the studies were conducted in North America (USA = 23, Canada = 5)14,15,19,21,26–30,32–34,38,41,43,45–48,50–52,55–60.The remaining articles were mostly performed in Europe (Netherlands = 1, Denmark = 1, Spain = 2, United Kingdom (UK) = 8, Germany = 1 and France = 1) and one study each were conducted in South Korea (n = 1) and Australia (n = 1).18,27,33,38,39,41,44,46,51,55,56,63–65 The study population consisted of 1,001,662 adolescents and adults with physician diagnosis of asthma in any population-based administrative database. More than one-third (n = 13) of the studies observed adherence and clinical outcomes (ie, asthma exacerbation, emergency room visits) simultaneously14,15,17,18,28,30,31,34,37,40,45,48,56 while three studies assessed the association between medication adherence and cost of asthma.43,49,57 The occurrence of the targeted clinical outcome was assessed from 12 months to 10 years.
 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
Table 1 Summary of Study Findings |
In view of this, the various asthma databases employed were of great interest in this review. As reported in Table 1, majority of the administrative databases used were pharmacy claim databases capturing patients’ medical records, prescription refills and records of drugs dispensed. Notable among them were the pharmacy claim databases from the IQVIATM Health Plan Claims Data, Danish Registry of Medicinal Product Statistics, HMO-claim records/database, Quebec Provincial Health Insurance administrative databases, Maintenance et Exploitation des Donnees pour l’Etude de la Clientele Hospitaliere (MED-ECHO), Québec prescription claims databases, Optimum Patient Care Research Database (OPCRD), Administrative insurance claims database, Medstat MarketScan database, and Clinical Practice Research Datalink (CPRD).
Measures of Medication Adherence
The assessment of medication adherence varied across studies. This review identified 20 different metrics used in measuring medication adherence in asthma patients. Some of the reported measures were medication possession ratio (MPR), proportion of days covered (PDC); Continuous Measure of Medication Acquisition (CMAq), proportion of prescribed days covered (PPDC); Persistence with inhaled therapies; Continuous Medication Availability (CMA), Refill Rate, Annual Prescription Possession Ratio (PPR); Group-Based Trajectory Modelling (GBTM) and others (see Tables 1 and 2). The MPR and PDC were commonly reported as the primary measures of medication adherence. Thus, approximately 87% of the included studies reported the use of both PDC and MPR as the main/primary metrics for asthma patients’ medication adherence in the long term. Specifically, 20 studies (53%) employed MPR and 13 (34%) used PDC as a measure of medication adherence. The majority of studies chose a fixed time-frame for the refill interval than using the last refill as the endpoint for the refill interval and did not exclude the last refill in the estimation of MPR. Additionally, some studies14,21,29,47,52,55 adopted multiple asthma adherence metrics (specifically: Med-Total and MPR; MPR and persistence; PDC and MPR; Prescription fills and PDC; Refilling and PDC; and MPR and persistence metric). Modifications of the two commonly reported measures (MPR and PDC) were also reported. Blais et al54 developed the annual proportion of prescribed days covered (PPDC) method as a modification of the PDC measure. The PPDC has the ability to account for prescribing patterns used in the administrative databases. A number of studies reported the continuous measure of availability as adherence metric which is an MPR calculated across multiple refills.37,61 Hardstock et al33 and Visaria et al62 compared the weighted average MPR and adjusted MPR to other measures in identifying non-adherent asthma patients.
 |
Table 2 Distribution of the Adherence Metric Reported by the Included Studies |
Definition/Equation of the Adherence Measures
There was variation in the definition and calculation of the two commonly reported adherence measures – MPR and PDC. With regards to the MPR-related measures, the denominator of the MPR formula varied from study to study. For instance, the majority of the studies estimated MPR as the sum of the days’ supply for medication fills divided by the time from the first supply fill until the end of the measurement period.19,27,31,40 Similarly, MPR was calculated in other studies as the sum of days of medication supply divided by the total time treated or evaluated.55,57 Other adherence calculations of MPR adopted a fixed denominator within the year representing the days between the first and the last refill. In a study by Martin et al,60 MPR was computed as the sum of the number of days’ supply of inhaled corticosteroids (ICS) divided by 365 days and multiplying the overall expression by 100% to provide an adherence percentage value. Measures such as the Med-Total, Medication Refill Adherence (MRA), and Continuous (Multiple Interval Measures of) Medication Availability (CMA 4 and 7) used formulae similar to MPR definitions.
The MPR fixed interval is generally applied for assessing seasonal use of medication as well as for assessing medication use in patients with allergies.66 The MPR takes a range of positive values from 0 inclusive through to “at least 1”. A zero MPR denotes no adherence, while an MPR value of 1 measure optimal adherence. In some extreme cases, an MPR above one shows that the patient took more than prescribed medication, while MPR value below 1 indicates less than prescribed medication within a specified period.67
Similar to MPR, there were variations in calculation of PDC-related measures in majority of studies estimating the PDC as a quotient value of the days covered divided by the days in the measurement period. It was also estimated as the percentage of days a patient had access to medication depending on the amount of medication obtained. A fixed interval PDC was calculated as the ratio of the number of days a patient had medication on hand to the total number of post-index days (ie, 365 days).15,21,29,30,33,35,36,42,47,50,51,53,59
Three studies assessed medication adherence using the CMA measure with slight variations in formulae.17,39,45 The CMA was calculated as the cumulative days’ supply obtained over a series of intervals divided by the total days from the beginning to the end of the time period in the study. The overall average of all participants’ CMA provided the adherence value of the entire time period of the study and evaluates the relationship of adherence and drug effect. It has been suggested that the CMA and MPR as well as Medication Refill Adherence (MRA) provide identical adherence measuring power.17,39,45
The AMR was calculated as the ratio of units of controller medication to the sum of units of controller medication and rescue medication. Two studies–Bidwal et al19 and Stanford et al21 –assessed medication adherence with the AMR metric and further evaluated the impact of treatment groups on adherence among adults with persistent asthma.
Six studies assessed persistence as another measure of medication adherence which was estimated as the total time between index treatment/date and time to discontinuation of the therapy.21,42,47,55 Several variations in calculation of persistence were reported among included studies.14,21,39,42,47,55 While drug persistence was calculated based on prescriptions filled within 30 days and between 31 and 180 days after provision of prescription in some studies,39,42,55 others estimated persistence based on the absence of treatment gap ‘≥30ʹ days.14 Table 1 gives a detailed description of the formulae and equations for the remaining adherence methods.
The Continuous, Multiple Interval Measure of Medication Gaps (CMG) measures were used in only one study45 to assess level of adherence and the impact of treatment on adherence. According to Williams et al, the CMG was obtained by dividing the total number of days in treatment gaps by the duration of the time period of interest in order to recognize any time intervals without drug exposure. Any negative value was set to 0. The CMG essentially calculates nonadherence values for cumulative periods without considering the possibility of early refill or overfill.17
Adherence Measures and Cut-Off Value (Threshold)
In this review, studies used various cut-off values or thresholds to estimate level of medication adherence among adolescents and adults. For MPR, the cut-off values or thresholds for good/high medication adherence ranged from ‘>0.75 to >1.00ʹ (See Table S8 in the Supplementary Material). Adherence metrics identified in this review were commonly categorized into two or more levels during assessment and for testing associations with study outcomes. The cut-offs or thresholds distinguish adherence and nonadherence or adherence from partial adherence. Categorizing adherence metrics into two distinct levels (adherence vs non-adherence) was common observation among most of the studies. Among studies which dichotomized adherence score, ten (10) assessed adherence using MPR, seven (7) with PDC, two (2) assessed adherence with AMR and one (1) employed the CMA measure. An arbitrary cut-off value or threshold of ‘≥0.80ʹ was commonly employed in most of the studies for both MPR and PDC.14,19,37,38,45,49 The adherence cut-off value for AMR measure reported in this review was >0.50.19,21 Four studies categorized adherence metrics into three or more categories. They were either categorized based on arbitrary cut-offs/thresholds or around suitable quintiles of the adherence scores. A study by Bidwal et al19 set the cut-off point for good adherence at MPR ≥ 0.80, medium at MPR ≥ (0.5–0.80) and low at MPR < 0.5: compared to D’Ancona et al56 study with adherence levels; good adherence (MPR > 0.75), intermediate (MPR: 0.74–0.51) and poor (MPR ≤ 0.5). Good adherence cut-off value for the PDC ranged from at least 0.50 to 0.80 and considered any value <0.5 as non-adherent. Three studies estimated adherence thresholds by computing median and 75th percentile of the adherence scores40,43,57 and the values above the medians denoted good or high adherence cut-off value. In the same vein, adherence scores ≥1 denoted optimal and excess adherence [see Tables S3 and S8]. Only two studies assessed adherence and the impact of treatment groups or covariates on it as a continuous variable.30,45 Thus, researchers did not set adherence cut-off.
Adherence Threshold Determination
Several methods were used to model or link clinical outcomes and adherence rates or determine adherence rates and their determinants in the retrieved articles. Seven studies used descriptive and unadjusted analytical methods to link the various clinical outcomes and adherence rates.29,32,51,53,54,56,58 The remaining studies employed a wide range of statistical methods to determine the adherence cut-offs as well as link the adherence rates to targeted clinical outcomes. The statistical methods ranged from simple to more advanced adjusted regressions. Logistic regression analyses (binary and multivariate) were used to assess the association between adherence and a range of clinical outcomes including asthma hospitalizations, emergency department (ED) visits and asthma exacerbation in some of the studies.19,31,36,37,40,55
Studies using logistic regression compared the odds ratio of different adherence rate groups for asthma related ED visit or for asthma-related hospitalization14,31 or intubation or all-cause hospitalization14 or short acting-beta 2 agonist (SABA) use14,37 or asthma exacerbation.40 A combination of advanced statistical approaches such as propensity score with various survival analysis, and multivariate generalized linear models were used in examining association between adherence thresholds and various targeted outcomes (see Table S9).
For propensity score with survival analysis, log rank statistics generated two adherence groups that separated most significantly either by shifting the threshold and comparing the resulting dichotomized adherence groups or risk of discontinuation.21,50 Adjusted Poisson regressions were employed to determine adherence thresholds or cut-offs and their associations with targeted clinical outcomes in two studies.45,49
Meta-Analysis for Threshold Determination
In addition to the narrative/qualitative synthesis, we performed meta-analyses to quantitatively summarize the effect estimates [odds ratios (OR)] for asthma exacerbation associated with specific adherence thresholds. The meta-analysis (Figure 2) focused on the MPR adherence thresholds and asthma exacerbation. The forest plot was sub-grouped into 3 MPR adherence thresholds (“0.75–1.00”, “0.5”, and “mean/median/75th percentile of MPR value”). Using inverse variance random effects model, we found a significant association between achieving a ‘0.75–1.00ʹ range of MPR adherence thresholds and reduction in asthma exacerbations with pooled effects estimate [odds ratio (OR): 0.56; 95% confidence interval (CI): 0.41–0.77]. The pooled effect size was heterogenous across the included studies with I2 = 74%. Similarly, achieving an MPR adherence threshold of “0.50 or more” was associated with lower risk of asthma exacerbations [OR = 0.71, 95% CI = (0.54–0.94)] with I2 = 65%. In summary, patients who achieved an adherence threshold between ‘0.75 and 1.00ʹ reduced their risk of exacerbation by 44% compared to those with a cut-off value less than 0.75.
 |
Figure 2 Forest plot of the association between achieving specific MPR adherence thresholds and risk of asthma exacerbations. |
Further, we employed meta-regression to identify the source of the between study heterogeneity. We identified “differences in adherence thresholds”, “different study locations”, and “varied study durations” as the main sources of the study heterogeneity in the meta-summary analysis (See Tables S4 and S5 in the Supplementary Material).
Publication Bias
The Eggers test recorded a t-statistics of 0.0096, Egger’s regression intercept of 0.051 with 95% confidence limits of (−12.04 to 12.15) indicating no substantial publication bias in this review (See Table S6). The study was limited to smaller number of studies included in the meta-analysis for estimating the pooled effect of asthma exacerbation among adherent and non-adherent asthma patients. Thus, resulting in a wider confidence interval with unprecise estimate for the Eggers test intercept. Also, the Fail-safe N test and the funnel plot showed no substantial publication bias. (See Table S7 and Figure S1 in the Supplementary Material).
Outcomes and Adherence Cut-Off/Threshold
The prevalence of nonadherence to asthma medications varied with adherence cut-offs/thresholds set by retrieved studies. Seven (7) studies reported data on adherence prevalence or reported some data which enabled estimation of medication adherence prevalence. With AMR cut-off of >0.50, non-adherence prevalence was the least and ranged from 10.7% to 34.6% (See Table S9 for detailed estimates of adherence prevalence on all 7 studies in the Supplementary Material). While some lower thresholds were associated with improved targeted outcomes of asthma18,29,37,41,55,61 it appears there is a trend which shows that adherence thresholds of at least 0.50 for MPR, PDC, CMA and AMR could result in significant reduction in asthma-related ED visits, asthma-related hospitalization, and SABA use.
Quality Assessment
Using the Joanna Briggs checklist for cohort studies, we performed quality assessment on all 38 studies included in the review. Overall, studies were evaluated to have a low to medium risk of bias with good methodological quality (see Table S2 in the Supplementary Material). Overall, confidence in the evidence from the reviews of the quantitative research ratings of the included studies was moderate (see Table S2a in the Supplementary Material).
Discussion
This review provides evidence of medication adherence measures in adolescent and adult asthma using administrative health databases. A total of 38 retrospective cohort studies were eligible for inclusion in this review using a stringent criterion. We observed low to medium overall risk of bias across the included studies with a substantial good methodological quality. Overall, a total of 1,001,662 adolescents and adults with physician diagnosis of asthma were included in this review. The authors identified 20 medication adherence measures from the various asthma databases. The measures were calculated using pharmacy claims databases comprising dispensed medications and refill records.
Data on prescription refills offer information about possession of medication and does not necessarily provide details of the actual use of the drug. Hence, information on the prescription refills provide a rough estimate of the adherence and probable overestimation of patients’ adherence.10 The use of administrative data for assessment of medication adherence has limitations which include inability of researchers to confirm whether or not the patients have actually ingested their prescribed medications. Also, the administrative health databases does not always capture detailed patient data including their physical examinations, clinical outcomes and laboratory tests.14 In spite of these limitations, adherence measured from administrative data has widely been demonstrated to correlate well with objective adherence measures and with clinical outcomes in various disease conditions. There is also documented evidence demonstrating concordance between healthcare database adherence rates and rates estimated from objective measures of adherence such as pill counting, and electronic monitoring.68,69 In particular, adherence measured from administrative data has been shown to improve clinical outcomes such as asthma exacerbation and highly sensitive in predicting improved asthma outcomes and reflects real-life situation of medication use.11 Additionally, the International Society for Pharmacoeconomics and Outcome Research (ISPOR) working group has proposed both the MPR and the PDC for measuring medication compliance in claim databases while the Pharmacy Quality Alliance (PQA) has recommended the PDC as the preferred method for assessing adherence for use in their Medicare Star Ratings.70 Moreover, the administrative electronic health databases are easy to use, linkable to other health databases, and inexpensive in assessing adherence to prescribed medications in patients with asthma.71
The MPR and PDC were the two commonly reported methods (representing 87% of the included studies). We found some evidence of subtle distinction in the operationalization of the MPR and PDC measures. For instance, the PDC numerator measured the sum of days of medication covered and MPR numerator measured the sum of days of medication supplied. A cut-off value is advised for the adherence measures in classifying patients’ as being adherent or non-adherent.72,73 Majority of the studies reported threshold for good adherence for the MPR-related measures as ‘≥0.8ʹ and the PDC-related measures ranged from at least “0.5 to (≥0.80)” [See Table S3]. To identify the optimal threshold capable of reducing important clinical events in asthma patients, we linked the varying thresholds (“0.5”, “0.75–0.80” and “median/75 percentile MPR thresholds”) to a clinical outcome of interest (asthma exacerbation). In particular, we found significant association between achieving MPR threshold of “0.5–1.00” and reduced risk of asthma exacerbation. The use or choice of thresholds between “0.75–1.00” and “≥0.50” were capable of ensuring good asthma control with a reduced asthma exacerbation (OR: 0.56, 95% CI: 0.41–0.77) and (OR: 0.71; 95CI: 0.54–0.94), respectively. The choice of the optimal adherence threshold was based on the cut-off value that reduced asthma exacerbation to a larger extent. Here, patients who achieved a threshold from “0.75–1.00 were 44% less likely to experienced asthma exacerbation compared to adherence rates less than 0.50. Also, individuals who attained an adherence value of at least 0.5 reduced subsequent exacerbations by 29% compared to less than 0.5. Thus, achieving an adherence threshold within “0.75–1.00” is optimal in reducing important clinical events in asthma patients.
The PDC is known to provide a more conservative estimate of medication adherence compared to other measures in cases of concomitant multiple medication usage.30 It is recommended for assessing medication adherence of patients on multiple therapies as compared to the MPR measure. This measure is also capable of avoiding double counting of days of medication coverage when two refills overlap. Additionally, the PDC provides a more accurate representation of medication adherence because it eliminates the possibility of being unreasonably elevated as it does not include possibility of overlapping days such as refilling a medication early. Major groups and institutions including the Pharmacy Quality Alliance recommend the use of the PDC measure for assessing medication adherence of patients on multiple therapies at the same time.
On the other hand, the MPR is unable to cover multiple therapies and it is mainly used for measuring single medication use. One of the strengths of the MPR measure is ease of accessibility and low-cost.74 Even though, the MPR is widely used in assessing adherence in most chronic disease medication intake, there exist some limitations associated with it. The MPR is estimated as a function of prescription issued and does not directly measure patients’ usage of the prescribed drug or medications. The MPR measures the total days of supply of medications from all medication records over a period for adherence calculations. As a result, it leads to double counting the days patients refill their medications before the previous prescription runs out. This drawback is likely to overestimate the usage of some maintenance medications such ICS. Also, the MPR is likely to cause a downward or upward bias,75 if patients were instructed to use their medications at a different dosage than implied by the days and quantity of supplied information on each claim.32
A common limitation using administrative filled claim databases for adherence calculation was the inability to determine whether the medication was ingested by the patient. Also, the definitions of the common methods (namely MPR and PDC) reported by some studies differed slightly from each other. Notwithstanding, most of the studies reportedly used almost the same definition for the calculation of MPR and PDC measures.
Also, adherence measures do not include medication usage during inpatient visits and hospitalizations due to limitations such as incomplete coverage of some databases. When patients pay out-of-pocket to obtain refills from multiple pharmacies and do not submit an insurance claim, administrative claim databases could be incomplete and limited.71 We believe that if the patient records in an administrative database are complete (by accounting for patients’ likelihood of obtaining medications from pharmacies not captured in the database), the derived methods can be considered to have a high sensitivity.
In choosing an adherence measure using asthma databases, some general issues should be considered and addressed. The measurement of adherence over a short period of time is likely to be imprecise due to unplanned circumstances–hospitalizations–which may be unrelated to adherence. Andrade et al12 recommended adjusting for the measure of adherence for the hospitalized patients after determining the number of days the patient was hospitalized.
Conclusion
This review identified two commonly reported measures – MPR and PDC – for measuring medication adherence in adolescents and adults with diagnosis of asthma. Other measures identified for measuring the various divisions of adherence included: persistence, Multiple Interval Measure of Medication Gaps (CMG), medication implementation/adherence and prescription fills. Using meta-analysis, we identified an adherence threshold of at least 0.75 as optimal for achieving targeted clinical outcomes such as reduced risk of asthma exacerbation. These measures were found to be consistently used in assessing adherence among asthma patients in administrative claim databases. While we admit that adherence measures assess medication acquisition rather than ingestion, the identified measures were highly sensitive with a complete coverage of patients’ medication records in the database. Despite their limitations, the two database adherence measures are objective and reflect medication use in real-world setting. Future studies should investigate in detail medication adherence thresholds (considering varying thresholds) in relation to asthma clinical outcomes using administrative health databases.
Acknowledgments
We acknowledge the effort of Alison Farrell, a Health Science Librarian at the Memorial University of Newfoundland, for her effort and assistance in performing an exhaustive literature search. M.A.-B. was supported by the Research and Graduate studies (RGS) scholarship at Memorial University of Newfoundland and TPMI/NL SUPPORT Educational scholarship.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang S, Huang Z, Traubenberg S. Measuring Medication Adherence with Simple Drug Use and Medication Switching. SAS Glob Forum; 2013:1–9.
2. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705. doi:10.1111/j.1365-2125.2012.04167.x
3. Mäkelä M, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005
4. Bender B, Milgrom H, Apter A. Adherence intervention research: what have we learned and what do we do next? J Allergy Clin Immunol. 2003;112(3):489–494. doi:10.1016/S0091-6749(03)01718-4
5. Lindsay J, Heaney L. Nonadherence in difficult asthma - facts, myths, and a time to act. Patient Prefer Adherence. 2013;7:329–336.
6. Bateman E, Hurd S, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi:10.1183/09031936.00138707
7. Bender B, Bender S. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25:107–130. doi:10.1016/j.iac.2004.09.005
8. Rand C, Wise R. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149:S69–S76. doi:10.1164/ajrccm/149.2_Pt_2.S69
9. Williams L, Joseph C, Peterson EL, et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol. 2007;120:1153–1159. doi:10.1016/j.jaci.2007.08.020
10. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122.
11. Engelkes M, Janssens HM, De Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi:10.1183/09031936.00075614
12. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi:10.1002/pds.1230
13. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:1–12.
14. Ismaila A, Corriveau D, Vaillancourt J, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30(7):1417–1425. doi:10.1185/03007995.2014.908827
15. Friedman HS, Navaratnam P, McLaughlin J. Treatment and outcomes - adherence and asthma control with mometasone furoate versus fluticasone propionate in adolescents and young adults with mild asthma. J Asthma. 2010;47(9):994–1000. doi:10.1080/02770903.2010.513076
16. Karve S, Cleves M, Helm M, Hudson T, West D, Martin B. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009;12(6):989–995. doi:10.1111/j.1524-4733.2009.00543.x
17. Williams LK, Pladevall M, Xi H. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114:1288–1293. doi:10.1016/j.jaci.2004.09.028
18. Kang HR, Song HJ, Nam JH, et al. Risk factors of asthma exacerbation based on asthma severity: a nationwide population-based observational study in South Korea. BMJ Open. 2018;8(3):1–10. doi:10.1136/bmjopen-2017-020825
19. Bidwal M, Lor K, Yu J, Ip E. Evaluation of asthma medication adherence rates and strategies to improve adherence in the underserved population at a Federally Qualified Health Center. Res Soc Adm Pharm. 2017;13(4):759–766. doi:10.1016/j.sapharm.2016.07.007
20. Farmer K. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. ClinicalTherapeutics. 1999;21(6):1074–1090.
21. Stanford RH, Averell C, Parker ED, Blauer-Peterson C, Reinsch TK, Buikema AR. Assessment of adherence and asthma medication ratio for a once-daily and twice-daily inhaled corticosteroid/long-acting β-agonist for asthma. J Allergy Clin Immunol Pract. 2019;7(5):1488–1496.e7. doi:10.1016/j.jaip.2018.12.021
22. Moher D, Liberati A, Tetzlaff J, Ltman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi:10.1136/bmj.b2535
23. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. doi:10.1186/s13643-016-0384-4
24. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: Aromataris EM, editor. Z: Joanna Briggs Institute Reviewer’s Manual. Adelaide, South Australia: The Joanna Briggs Institute; 2017.
25. Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook: handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach, Cochrane collaboration, London, United Kingdom; 2013. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
26. Popay J, Roberts H, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. A product from the ESRC methods programme version. Bailrigg Lancaster Univ. 2006;1:1–92.
27. Darbà J, Ramírez G, Sicras A, García-Bujalance L, Torvinen S, Sánchez-de La Rosa R. Identification of factors involved in medication compliance: incorrect inhaler technique of asthma treatment leads to poor compliance. Patient Prefer Adherence. 2016;10:135–145. doi:10.2147/PPA.S95303
28. Delea TE, Stanford RH, Hagiwara M, Stempel DA. Association between adherence with fixed dose combination fluticasone propionate/salmeterol on asthma outcomes and costs. Curr Med Res Opin. 2008;24(12):3435–3442. doi:10.1185/03007990802557344
29. Feehan M, Ranker L, Durante R, et al. Adherence to controller asthma medications: 6-month prevalence across a US community pharmacy chain. J Clin Pharm Ther. 2015;40(5):590–593. doi:10.1111/jcpt.12316
30. Gelzer AD, Gao W, Keleti D, et al. Multifaceted interventions improve medication adherence and reduce acute hospitalization rates in Medicaid patients prescribed asthma controllers. J Asthma. 2019;56(2):190–199. doi:10.1080/02770903.2018.1439954
31. Guo JJ, Kelton CM, Tsai K, Cai B, Bian B, Wigle PR. PRS30 inhaled corticosteroid and long-acting beta-agonist medication compliance in patients with moderate and severe asthma. Value Health. 2012;15(4):A57. doi:10.1016/j.jval.2012.03.314
32. Hagiwara M, Delea TE, Stanford RH. Risk of asthma exacerbation, asthma-related health care utilization and costs, and adherence to controller therapy in patients with asthma receiving fluticasone propionate/salmeterol inhalation powder 100 μg/50 μg versus mometasone furoate inhalation powd. J Asthma. 2013;50(3):287–295. doi:10.3109/02770903.2012.754028
33. Hardtstock F, Maywald U, Timmermann H, Unmuessig V, Mueller S, Wilke T. Prs70 applying different measures to assess patients’ non-adherence: results of a linked data study of patients with asthma in Germany. Value Health. 2019;22:S885. doi:10.1016/j.jval.2019.09.2559
34. Kelloway JS, Wyatt R, DeMarco J, Adlis S. Effect of salmeterol on patients’ adherence to their prescribed refills for inhaled corticosteroids. Ann Allergy Asthma Immunol. 2000;84(3):324–328. doi:10.1016/S1081-1206(10)62781-0
35. Navaratnam P, Friedman HS, Urdaneta E. Treatment with inhaled mometasone furoate reduces short-acting β2 agonist claims and increases adherence compared to fluticasone propionate in asthma patients. Value Health. 2011;14(2):339–346. doi:10.1016/j.jval.2011.01.001
36. Makhinova T, Barner JC, Richards KM, Rascati KL. Asthma controller medication adherence, risk of exacerbation, and use of rescue agents among Texas Medicaid patients with persistent asthma. J Manag Care Pharm. 2015;21(12):1124–1132.
37. Papi A, Ryan D, Soriano JB, et al. Relationship of inhaled corticosteroid adherence to asthma exacerbations in patients with moderate-to-severe asthma. J Allergy Clin Immunol Pract. 2018;6(6):1989–1998.e3. doi:10.1016/j.jaip.2018.03.008
38. Sicras-Mainar A, Huerta A, Sánchez D, Navarro-Artieda R. Use of resources and costs associated with non-adherence to inhaled corticosteroid treatment in asthma. Semergen. 2018;44(1):13–22. doi:10.1016/j.semerg.2017.03.005
39. Souverein PC, Koster ES, Colice G, et al. Inhaled corticosteroid adherence patterns in a longitudinal asthma cohort. J Allergy Clin Immunol Pract. 2017;5(2):448–456.e2. doi:10.1016/j.jaip.2016.09.022
40. Stern L, Berman J, Lumry W, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97(3):402–408. doi:10.1016/S1081-1206(10)60808-3
41. Taylor A, Chen LC, Smith MD. Adherence to inhaled corticosteroids by asthmatic patients: measurement and modelling. Int J Clin Pharm. 2014;36(1):112–119. doi:10.1007/s11096-013-9862-0
42. Svedsater H, Parimi M, Ann Q, Gray C, Nixon M, Boxall N. A retrospective database study of persistence abd adherence in patients with asthma in the UK (UK-THIN): Fluticasone furoate/Vilanterol (FF/ VI) versus Beclometasone Dipropionate/Formoterol (BDP/FM). In: Thorax-2019-Btsabstracts2019372. BMJ Publishing Group Ltd; 2019:A1–A262.
43. Vaidya V, Tak S, Hong SH. Impact of patient cost sharing on medication adherence among asthmatic patients on dual-controller therapy. J Pharm Health Serv Res. 2013;4(4):227–233. doi:10.1111/jphs.12035
44. van Boven JFM, Koponen M, Lalic S, et al. Trajectory analyses of adherence patterns in a real-life moderate to severe asthma population. J Allergy Clin Immunol Pract. 2020;8:1961–1969.e6. doi:10.1016/j.jaip.2019.12.002
45. Williams S, Trudo F, Suchower L, et al. Is history of patient adherence to asthma controller medication associated with initial choice of prescription for inhaled corticosteroid and long-acting β2-adrenergic agonist combination therapy? J Manag Care Pharm. 2012;18(7):551.
46. Vervloet M, van Dijk L, Spreeuwenberg P, et al. The relationship between real-world inhaled corticosteroid adherence and asthma outcomes: a multilevel approach. J Allergy Clin Immunol Pract. 2020;8(2):626–634. doi:10.1016/j.jaip.2019.09.003
47. Wu AC, Butler MG, Li L, et al. Primary adherence to controller medications for asthma is poor. Ann Am Thorac Soc. 2015;12(2):161–166. doi:10.1513/AnnalsATS.201410-459OC
48. Woodcroft K, Yu Y. Inhaled corticosteroid and inhaled corticosteroid/long- acting beta agonist adherence and exacerbations among patients with persistent asthma in an integrated healthcare system. J Manag Care Spec Pharm. 2016;22:S65–6.
49. Zhang S, Dang-Tan T, Ismaila A, et al. The impact of adherence and exacerbation frequency on health care utilization and associated direct costs in severe asthma. Chest. 2016;150(4):827A. doi:10.1016/j.chest.2016.08.927
50. Averell CM, Stanford RH, Laliberté F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2019;1–10. doi:10.1080/02770903.2019.1663429
51. Backer V, Stensen L, Sverrild A, Wedge E, Porsbjerg C. Objective confirmation of asthma diagnosis improves medication adherence. J Asthma. 2018;55(11):1262–1268. doi:10.1080/02770903.2017.1410830
52. Balkrishnan R, Christensen DB. A comparison of medication adherence indices to assess long-term inhaled corticosteroid medication use. J Asthma. 2001;38(1):91–98. doi:10.1081/JAS-100000026
53. Blais L, Kettani FZ, Forget A, Beauchesne MF, Lemière C, Ducharme FM. Assessing adherence to inhaled corticosteroids in asthma patients using an integrated measure based on primary and secondary adherence. Eur J Clin Pharmacol. 2017;73(1):91–97. doi:10.1007/s00228-016-2139-5
54. Blais L, Kettani FZ, Beauchesne MF, Lemière C, Perreault S, Forget A. New measure of adherence adjusted for prescription patterns: the case of adults with asthma treated with inhaled corticosteroid monotherapy. Ann Pharmacother. 2011;45(3):335–341. doi:10.1345/aph.1P719
55. Covvey JR, Mullen AB, Ryan M, et al. A comparison of medication adherence/persistence for asthma and chronic obstructive pulmonary disease in the United Kingdom. Int J Clin Pract. 2014;68(10):1200–1208. doi:10.1111/ijcp.12451
56. D’Ancona G, Kavanagh J, Roxas C, et al. Adherence to inhaled corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55:1–7. doi:10.1183/13993003.02259-2019
57. Vaidya V, Gabriel MH, Patel P, Gupte R, James C. The impact of racial and ethnic disparities in inhaled corticosteroid adherence on healthcare expenditures in adults with asthma. Curr Med Res Opin. 2019;35(8):1379–1385. doi:10.1080/03007995.2019.1586221
58. Blais L, Vilain A, Kettani FZ, et al. Accuracy of the days’ supply and the number of refills allowed recorded in Quebec prescription claims databases for inhaled corticosteroids. BMJ Open. 2014;4(11):1–8. doi:10.1136/bmjopen-2014-005903
59. Korgaonkar S, Banahan B, Pittman E, Noble S. Effect of depression on adherence to controller medications and healthcare resource utilization in asthma patients. Value Health. 2018;21:S231. doi:10.1016/j.jval.2018.04.1567
60. Martin R, Price D, Krishnan J, et al. Excess inhaled corticosteroid adherence may be a marker of uncontrolled asthma. Eur Respir Soc Ann Congress. 2013;2013:10–11.
61. Williams KL, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid non-adherence. J Allergy Clin Immunol. 2011;128(6):1185–1191. doi:10.1016/j.jaci.2011.09.011
62. Visaria J, Frazee S, Henderson R. Is it appropriate to measure asthma controller adherence using pharmacy claims data? Value Health. 2012;15(4):A61–2. doi:10.1016/j.jval.2012.03.339
63. Belhassen M, Nolin M, Ginoux M, Van GE. Adherence to inhaled corticosteroids before and after an asthma-related hospitalisation: distinct trajectories. Eur Respir J. 2018;52:PA4478.
64. Dima A, Souverein P, Koster E, Chisholm A, Price D, Gene C. REG study: real-life, longitudinal ICS adherence patterns in a UK asthma population. Eur Respir J. 2015;2015:PA1238.
65. Souverein P, Koster E, Dima A, Colice G. Longitudinal inhaled corticosteroid adherence using multiple methods to calculate medication possession ratios. Pharmacoepidemiol Drug Saf. 2015;24:1–587. doi:10.1002/pds.3838
66. Barner J. Medication Adherence: Focus on Secondary Database Analysis. ISPOR Student Forum; 2010.
67. Sperber CM, Samarasinghe SR, Lomax GP. An upper and lower bound of the medication possession ratio. Patient Prefer Adherence. 2017;11:1469–1478. doi:10.2147/PPA.S136890
68. Hudson M, Rahme E, Richard H, Pilote L. Comparison of measures of medication persistency using a prescription drug database. Am Heart J. 2007;153(1):59–65. doi:10.1016/j.ahj.2006.10.018
69. Gue ́nette L, Moisan J, Pre ́ville M, Boyer R. Measures of adherence based on self-report exhibited poor agreement with those based on pharmacy records. J Clin Epidemiol. 2005;58(9):924–933. doi:10.1016/j.jclinepi.2005.02.002
70. Patel N. The difference between primary measures of medication adherence: PDC and MPR; 2018. Available from: https://www.usciences.edu/blog/noteworthy/posts/the-difference-between-primary-measures-of-medication-adherence-pdc-and-mpr.html.
71. Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. doi:10.1345/aph.1H018
72. Bonafede M, Johnson B, Tang D, Shah N, Harrison D, Collier D. Etanercept-methotrexate combination therapy initiators have greater adherence and persistence than triple therapy initiators with rheumatoid arthritis. Arthritis Care Res. 2015;67:1656–1663. doi:10.1002/acr.22638
73. Chu L, Kawatkar A, Gabriel S. Medication adherence and attrition to biologic treatment in rheumatoid arthritis patients. Clin Ther. 2015;37:660–666. doi:10.1016/j.clinthera.2014.10.022
74. Boulet L, Vervloet D, Magar Y, et al. Adherence: the goal to control asthma. Clin Chest Med. 2012;33:405–417. doi:10.1016/j.ccm.2012.06.002
75. World Health Organization (WHO). Adherence to long-term therapies: evidence for action. World Health Organization; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_report/en/.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
