Back to Journals » OncoTargets and Therapy » Volume 12
Matrix metalloproteinase 2 contributes to aggressive phenotype, epithelial-mesenchymal transition and poor outcome in nasopharyngeal carcinoma
Received 20 January 2019
Accepted for publication 30 April 2019
Published 17 July 2019 Volume 2019:12 Pages 5701—5711
DOI https://doi.org/10.2147/OTT.S202280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Siyi Li,*,1 Weiren Luo*,1,2
1Department of Pathology, The Second Affiliated Hospital of Southern University of Science and Technology, Shenzhen Third People’s Hospital, National Clinical Research Center for Infectious Diseases, Shenzhen, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research, School of Medicine of Southern University of Science and Technology, Shenzhen, People’s Republic of China
*Both authors contributed equally to this work
Background: Though matrix metalloproteinase 2 (MMP-2) involvement in tumor aggressiveness and invasion is well-known, its prognostic impacts still remain largely controversial. Furthermore, the correlations between MMP-2 and epithelial-mesenchymal transition (EMT) have not been directly established in nasopharyngeal carcinoma (NPC).
Materials and methods: The purpose of this study was to investigate MMP-2 expression in NPC. Tissue microarrays from 144 patients with NPC and 45 non-cancerous pharynx tissues were analyzed for MMP-2 expression by immunohistochemistry. MMP-2 expression in relation to clinicopathological characteristics and EMT were assessed in NPC. Tumor-invasive potential affected by exogenous expression of MMP-2 in NPC cells was also detected in vitro.
Results: Compared to normal nasopharyngeal epithelium, high expression of tumoral MMP-2 was detected in 47.9% of NPC samples. Significant association was found between MMP-2 expression and various aggressive features including T classification, M classification and tumor stage (P<0.05). Of note, high expression of MMP-2 was prominently observed at tumor invasive front, neoplastic spindle cells migrating into the stroma and vessel invasion. Importantly, high MMP-2 expression predicted worse survival in patients with stage III–IV (P=0.039). Overexpression of MMP-2 could decrease cell-cell adhesion, promote tumor invasion and EMT including downregulation of E-cadherin and upregulation of N-cadherin, Fibronectin and Slug of NPC cells.
Conclusion: Our findings demonstrate that MMP-2 expression contributes to tumor aggressiveness and poor prognosis, and induces the occurrence of EMT in NPC.
Keywords: MMP-2, epithelial-mesenchymal transition, nasopharyngeal carcinoma, prognosis, immunohistochemistry
Introduction
Nasopharyngeal carcinoma (NPC) is the most frequently diagnosed malignancy in Southern China (especially in people of Cantonese ancestry region), with a high incidence rate of 20–50 cases per 100,000 people each year.1 Different from other head and neck cancers, most types of NPCs are undifferentiated squamous cell carcinomas, which are more aggressive and tend to have distant organ metastases.2 Unfortunately, the precise molecules responsible for the progression and prognosis of NPC still remain incompletely understood.
Degradation of extracellular matrix (ECM) and penetration of basement membranes by matrix metalloproteinases (MMPs) are of eminent importance in invasion and metastasis.3 Matrix metalloproteinase 2 (MMP-2), an important member of the MMPs family, has been shown to facilitate tumor invasion and metastasis and regulated by a variety of pathway.4–7 For example, Kenny HA and colleagues reported that MMP-2 regulated varian cancer (OvCa) invasion and metastasis through cleavage of ECM proteins Fibronectin (FN) into small fragments and promoted binding of OvCa cells to these FN fragments.7 Our report recently has also demonstrated that MMP-2 could regulate non-small cell lung cancer invasion and modulated by LATS2.8 Moreover, several MMP inhibitors have been considered extremely potential to attenuate tumor invasion and progression.9–12 Importantly, an increased expression of MMP-2 has been reported in a number of tumors including renal cell carcinoma, prostate cancer and ovarian cancer, and contributes to unfavorable outcome of patients.13–15 These advances indicate that MMP-2 might be crucial for the development and progression of tumors. However, the prognostic impacts of tumoral MMP-2 expression on patients remain largely controversial.16–18 For example, Pellikainen JM shows that high MMP-2 expression in carcinoma cells possessed no prognostic value for breast cancer.16 Even more, Wong JC and colleagues had the opposite conclusion. They found that absence of tumoral MMP2 expression correlated with poor clinical outcomes in rectal cancer.18
In consequence, the purpose of this study was to investigate and clarify the prognostic significance of neoplastic expression of MMP-2 in patients with NPC. Furthermore, the direct and functional impacts of MMP-2 overexpression on the invasive potential of NPC in vitro were also assessed.
Materials and methods
Patients and samples
One hundred and forty-four cancer tissues with NPC (median age, 49.4 y; range, 19–75 y; 107 male, 37 female) and 45 non-cancerous pharynx tissues were collected from Affiliated Hospital of Guangdong Medical College and the People’s Hospital of Gaozhou City, China. Prior to unitizing these tumor samples, approval from the Institutional Research Ethics Committee of Guangdong Medical College was obtained. Informed consent was obtained from all patients and the study was conducted in accordance with the principles of the Declaration of Helsinki. No radiation/chemotherapy treatment was applied to any of the patients included in this study. According to the WHO histological classification (2005), all of 144 NPC samples were classified as non-keratinizing carcinoma.19 All of the tumors were classified based on the UICC (2002) TNM classification and the clinicopathological features were described in detail as listed in Table S1. The survival time was counted from the date of diagnosis to the follow-up deadline or date of death. The follow-up deadline was August 2011, and it was ranged from 10 to 106 months (median follow-up time, 65.7 months).
Immunohistochemical staining
Immunohistochemistry (IHC) analysis was performed as previously reported.20 In brief, paraffin-embedded sections were baked at 60 °C for 2 h followed by being deparaffinized in xylenes for 20 min and rehydrated in an ethanol gradient. The sections were submerged into EDTA buffer and boiled for 2 mins with high-pressure for antigenic retrieval. After natural cooling, the slides were treated with 3% H2O2 to quench endogenous peroxidase activity, followed by incubation with 1% bovine serum albumin (BSA) to block non-specific binding. The slides were incubated with the MMP-2 rabbit polyclonal antibody (catalog ab110186, dilution 1:500; AbCam) overnight at 4 °C. PBS buffer was used as negative controls, and colon cancer tissue was used as positive control. After PBS washing, the sections were reacted with the biotinylated secondary antibody (Zymed, San Francisco, CA). Sections were then visualized with 3,3′-diaminobenzidine (DAB) for 2 min, counterstained with hematoxylin and were mounted under a light microscopy. Pan-cytokeratin was used to distinguish neoplastic spindle cells from fibroblast cells or other stromal cells in NPC tissues.
Evaluation of immunohistochemical data
The degree of immunostaining were examined and scored independently by two pathologists based on the proportion of positively stained tumor cells and intensity of staining. The proportion of positive tumor cells was evaluated as follows: 0 (no positive tumor cells), 1 (<10% positive tumor cells), 2 (10–50% positive tumor cells), and 3 (>50% positive tumor cells). Staining intensity was graded according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (modest staining, yellow brown), and 3 (strong staining, brown). We evaluated the expression of MMP-2 in NPC specimens (including tumor cells and tumor stroma) and non-cancerous nasopharyngeal tissues by determining the staining index (0, 1, 2, 3, 4, 6, or 9), which was calculated by multiplication of proportion and intensity. The cutoff value for low and high expression level of MMP-2 was ≤4 (including 0, 1, 3, 4) and ≥6 (including 6 and 9), respectively.21
Plasmids, lentivirus production, and transduction
To produce virus particles expressing vector or MMP-2, LV-pGV208 (used as vector control) or LV-pGV208-MMP-2 along with packaging plasmids pHelper 1.0 and pHelper 2.0 were transfected into HEK293T cells (maintained in 10% FBS) using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturers’ instruction. After 48 h transfection, virus supernatant was harvested from these cells, and then used to infect CNE-1 and SUNE-1 cells. To get 100% percentage of infected cells, lentiviral infected cells were sorted by Flow Cytometry based on EGFP assay. The successful overexpression of MMP-2 was verified by Western blot and qRT-PCR.
Cell culture, immunofluorescence analysis
Human NPC cell lines CNE-1 (epithelial-like) and SUNE-1 were provided by Cancer Research Institute of Southern Medical University, Institutional Review Boards of Southern Medical University approved the use of these cell lines. Two cell lines were grown in RPMI 1640 culture medium (100 units/ml of penicillin and 100 μg/ml streptomycin) in a humidified 5% CO2 atmosphere at 37 °C. For immunofluorescence analysis, cells were grown on sterile 12-mm glass coverslips for 24 h at 37 °C. After washing twice with PBS, cell lines were plated on culture slides (Costar, MA) and after 24 hrs were rinsed with PBS and fixed in 4% paraformaldehyde for 15 min, then permeabilized with 0.05% triton X-100. The cells were then blocked for 30 min in 10% BSA (Sigma, MO) in PBS and then incubated with the appropriate primary antibodies including E-cadherin (1:500), N-cadherin (1:100), Fibronectin (1:200), Slug (1:300). Finally, cell nuclei were stained with DAPI (Boisynthesis biotechnology, Beijing, China) and representative images were examined using a confocal microscope (FV300 Olympus, Tokyo, Japan).
RNA isolation, reverse transcription and qRT-PCR
For mRNA analyses, total RNA from NPC cells was extracted with Trizol Reagent (TaKaRa) according to the protocol provided by the manufacturer. Total RNA was reversely transcribed with the PrimeScript RT reagent Kit (TaKaRa). Expression of mRNA analysis was performed using SYBR Green Master Mix (TaKaRa) as described using GAPDH for normalization on a Stratagene Mx3000P qRT-PCR System. The primers used for the amplification of the indicated genes were as previously described.21,22 All samples were normalized to internal controls and fold changes were calculated through relative quantification (2−ΔΔCt).
Cell-cell homotypic adhesion experiment
CNE-1 and SUNE-1 cells were digested and diluted to 5×104 cells/mL. Cells were seeded into a 24-well plate (1 mL/well); each kind of cell was placed in three wells. Cells were then cultured at 37 °C in 5% CO2 until cells fused in a single layer without any gaps. Meanwhile, CNE-1 and SUNE-1 cells were digested to prepare unicell suspension (1×105 cells/mL). After removal of culture solutions in the 24-well plate, the prepared unicell suspensions (1 mL/well) were added into corresponding wells containing the same type of cells. The 24-well plate was oscillated at 37 °C on a shaker slowly to make the cells fully adhere. Non-adhered tumor cells were carefully collected at 15 min, 45 min and 75 min after oscillation, respectively, and counted under a light microscope. Cell-cell adhesion rate (%) = ([total cell count] –[non-adhered cell count]/[total cell count])×100%。All values are presented as mean ± SD of three experiments.
Tumor invasion assays
Before invasion assay, matrigel (BD Biosciences) was added in the upper chamber overnight for dry. For transwell migration assay, 1×105 cells were seeded into the upper chamber (with 8.0 μm pores, BD Biosciences) in serum-free RPMI 1640. RPMI 1640 with 10% FBS was loaded in the lower compartment as chemo-attractant. After 20 hrs, the migrated or invaded cells were fixed with 100% methanol, stained with hematoxylin solution (Sigma), and counted in three randomly selected optical fields.
Statistical analysis
All statistical analyses were carried out using the SPSS13.0 (SPSS Inc., Chicago, IL). The correlation between the clinicopathologic features and MMP-2 expression was analyzed by the χ2 test. Survival curves were estimated using the Kaplan-Meier method and the log-rank test. Correlation coefficients between MMP-2 expression and variable factors were examined by the Spearman correlation method. Data were presented as mean ± SEM unless otherwise indicated of at least 3 independent experiments. Two-tailed Student’s t-test was used for comparisons of 2 independent groups. Statistical significance was assessed by the independent-samples t-test (*P<0.05; **P<0.01).
Results
Cytoplasmic MMP-2 is highly expressed in tumor cells of NPC samples
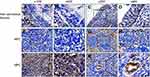
The immunohistochemical staining for MMP-2 expression in 144 paraffin-embedded tissues of NPC were presented in Table S1. Among 45 noncancerous tissues, negative or weak signals of MMP-2 were observed in non-cancerous nasopharyngeal epithelium (Figure 1A and B), only 7 samples (15.6%) were highly expressed for MMP-2 (Figure 1C and D). MMP-2 was localized in the cytoplasm of cancer cells. Of the 144 tumors, 75 (52.1%) cases exhibited negative (Figure 1E and F) or weak (Figure 1G and H) cytoplasmic immunoreactivity for MMP-2, and 69 (47.9%) cases displayed high cytoplasmic staining (Figure 1I and J). There was a significant increase in MMP-2 expression in NPC samples compared with noncancerous tissues. In addition, MMP-2 was high expressed in endothelial cells in tumor specimens and was used as internal positive controls (Figure 1K and L).
Association of MMP-2 expression with clinicopathological variables and prognosis in NPC
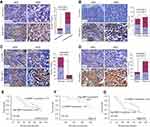
The relationship between MMP-2 expression and clinicopathological variables of NPC was further analyzed. As summarized in Table 1, there were no statistically significant differences between the expression levels of MMP-2 and sex (P=0.155), age (P=0 0.889), N classification (P=0.076). On the other side, MMP-2 protein expression was significantly associated with tumor histology (Figure 2A; P=0.022), T classification (Figure 2B; P=0.004), M classification (Figure 2C; P=0.023) and tumor stage (Figure 2D; P=0.000). Similarly, spearman correlation analysis revealed the positive correlation between MMP-2 and tumor histology, T classification, M classification and tumor stage, with a spearman correlation coefficient of 0.190, 0.239, 0.189 and 0.310, respectively. As listed in Table 2, univariate analysis shows that M classification was the most significant factor for the overall survival of NPC patients (P=0.000), followed by tumor stage (P=0.001), T classification (P=0.004) and N classification (P=0.013). However, neoplastic MMP-2 expression showed no prognostic value for overall survival time of patients (P=0.363; Table 2). As showed in Figure 2E, there was no significant difference in the postoperative survival according to MMP-2 expression in tumor cells. The average overall survival time for patients with high MMP-2 expression was 70.2 months (95% confidence interval, 61.7–78.7), when it was 78.3 months (95% confidence interval, 70.9–85.7) for patients with low MMP-2 positivity (P=0.359).
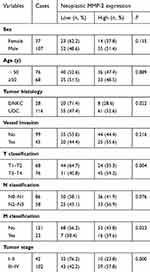 |
Table 1 Association of MMP-2 expression with clinical characteristics in 144 patients with nasopharyngeal carcinoma (NPC) |
 |
Table 2 Univariate and multivariate analysis on overall survival of nasopharyngeal carcinoma (NPC) patients |
Furthermore, we have investigated the prognostic value of MMP-2 on different subgroups of clinical stage. As shown in Figure 2G, patients with high MMP-2 expression in advanced clinical stage (III–IV) had shorter survival time than those with low expression of MMP-2 (P=0.039). The median survival time of patients with high MMP-2 expression was 43.00 months (95% confidence interval, 38.30–47.70), whereas the median survival time of patients with low MMP-2 expression was 90.00 months (95% confidence interval, 80.76–99.24). On the other hand, no significant difference was found in the subgroup of patients withⅠ-Ⅱ tumor stage (P=0.143). (Figure 2F)
Overexpression of MMP-2 contributes to tumor invasion and EMT in NPC
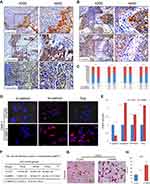
In cancer tissues, high expression of MMP-2 was prominently observed at tumor invasive front, cancer cells migrating into the stroma and vessel invasion (Figure 3A), indicating this protein might be correlated with the invasive and metastatic properties of NPC. It is noted that these malignant cells often displayed spindle-shaped cells morphology. As shown in Figure 3C, MMP-2 expression in tumor cells was significantly associated with E-cadherin (r =−0.227, P=0.006), N-cadherin (r =0.234, P=0.005), Fibronectin (r =0.176, P=0.035) and Slug (r =0.233, P=0.001) in NPC samples, whereas not with Vimentin (r =0.114, P=0.172) and Snail expression (r =0.083, P=0.324). Representative images shows that high MMP-2 expression in tumor cells correlated closely with increased expression of N-cadherin expression (Figure 3B).
To understand whether MMP-2 overexpression directly induces EMT and invasion and motility of NPC cells, we detected the EMT-related markers and phenotypic changes of NPC cells including CNE-1 cells and SUNE1 cells with ectopic expression of MMP-2. The immunofluorescence and qRT-PCR results demonstrated ectopic expression of MMP-2 of CNE-1 cells could significantly reduced the expression of epithelial marker E-cadherin and increased the expression of mesenchymal markers including N-cadherin, FN and Slug (Figure 3D and E). Reduced expression of E-cadherin and enhanced expression of N-cadherin, FN and Slug were also found in the overexpressing-MMP-2 group compared with control group in SUNE1 cell lines. The cell-cell adhesion assay showed that cell-cell adhesive abilities of CNE-1 cells were decreased significantly (Figure 3F; P<0.001). Similar results were found in the SUNE1 cells groups. Compared with the controls, the rates of cell-cell adhesion of overexpressing-MMP-2 group was 18.35±1.26 vs 12.82±0.72, 42.60±1.39 vs 29.63±1.08, 68.37±2.28 vs 0.46.55±1.90 in 15 min, 45 min and 75 min, respectively. Transwell invasion assay implied that the invasive activities of CNE-1 cells were up-regulated (Figure 3G and H; P<0.001) in MMP-2 overexpressing groups. Similarlly, the invasive abilities of SUNE1 cells were increased in MMP-2 overexpressing groups in comparision with the control groups (82.45±6.20 vs 32.65±4.60, P<0.001).
Discussion
MMP-2 belongs to a Zn2+ dependent endopeptidase, which is known to be crucial for tumor invasion and metastasis due to their ability to degrade type IV collagen.3 Moreover, increased expression of MMP-2 has been detected in a variety of human malignancies, and correlated closely with aggressive behaviors.4–7 In NPC, several indirect observations have indicated that the induction of MMP-2 by various pathways was involved in tumor cell invasion in vitro.23,24 Based on these observations, the determination of MMP-2 expression in NPC tissues seems to be important for better understanding of tumor biology and for the development of new therapy approaches. However, to our knowledge, prognostic significance of tumoral MMP-2 expression and its correlation with EMT have not been fully established.
In the current study, we observed that the expression levels of neoplastic MMP-2 expression were highly increased in NPC tissues compared with non-tumoral epithelium, suggesting that this molecule might be involved in the pathogenesis of NPC. Furthermore, high expression of MMP-2 linked strongly with various clinicopathologic features including tumor histology, T classification (depth of tumor infiltration), M classification and tumor stage. Our results suggest that MMP-2 is involved in the development and progression of NPC. On the other hand, Cui D et al reported that the expression levels of MMP-2 were positively correlated with lymph node metastasis in NPC.25 In contrast, we failed to demonstrate a significant relationship between them, though patients with high MMP-2 expression had a tendency to lymph node metastasis. The inconsistency would be likely due to the different numbers of specimens used. Studies have demonstrated that growth factors and other genes, such as TGF-b, bone sialoprotein and prostaglandin E2, can regulate the expression levels of MMP-2 in various tumors.26–29 In NPC HONE-1 cells, Chen MK et al recently showed that at treatment with the bcl-2 inhibitor HA14-1 had an inhibitory effect on tumor migration and MMP-2 expression.30 Our recent report indicates that FoxM1 increases expression of MMPs including MMP1 and MMP9, whereas FoxM1 knockdown has the opposite effect.31 As we know, EBV plays an important role in NPC pathogenesis through the regulation of MMPs. For example, Lu J et al showed that the EBV-related protein LMP1 could regulate the expression and activity of MMP-1 and confered the invasive properties of NPC.32 Further studies are needed to investigate the correlation between EBV-related factors and MMP-2 induction in NPC.
Epithelial-mesenchymal transition (EMT) is crucial for the initiation of invasion and metastasis for tumor progression.33 Of note, as other groups reported previously, EMT may occur more frequently in the invasive front of tumors, including colorectal cancer, oesophageal squamous cell carcinoma, craniopharyngioma and papillary thyroid carcinoma.34–37 Recently, we also found that nuclear Vimentin and other EMT-related markers were aberrantly expressed in tumor invasive front of NPC.38 Interestingly, MMP-2 protein was also found to be overexpressed at the tumor invasive front, such as head and neck cancer, hepatocellular carcinoma and cervical cancer.39–41 These findings suggest a possible relationship between MMP-2 and EMT in cancer progression. Actually, experimental data has shown that cancer cells that undergo EMT can enhance MMP-2 expression to facilitate cell invasion and metastasis.42–44 In the current study, we observed that MMP-2 was markedly distributed at the invasive front of cancers. In some cases, the expression of MMP-2 was increased in tumor cells migrating the stroma with mesenchymal phenotypes, and these cells have been shown to be conferred with EMT properties in our recent study.45 Furthermore, we found that neoplastic MMP-2 expression correlated closely with EMT-related markers including E-cadherin, N-cadherin, Fibronectin and Slug in NPC samples. Based on these observations, it seems likely that cancer cells expressing MMP-2 in NPC may have an advantage to invade and metastasize as compared to MMP-2-negative cancer cells. At last, a direct linking between EMT and MMP-2 in NPC in vitro was further examined. As expected, we found that upregulation of MMP-2 triggered EMT-like cellular marker changes and spindle-shaped morphology (a more aggressive phenotype), and enhanced the motility and invasion of NPC cells. These findings were strongly supported by MMP-2-mediated EMT in NPC specimens as described above. For the first time, we here clarify the correlation between EMT and MMP-2 in NPC.
In the past years, MMP-2 overexpression in tumor cells has been shown to contribute to unfavorable prognostic outcomes in various types of tumors.13–15 For example, Passlick B et al found a significant correlation of MMP-2 overexpression with shortened cancer-related survival in 193 patients with non-small cell lung cancer (NSCLC).46 However, controversy still exists about the prognostic impacts of tumoral expression of MMP-2. Conflicted with Passlick B et al’s results in NSCLC, for instance, Ishikawa S and colleagues suggested that 5-year survival rate of patients with strong MMP-2 expression was not significantly worse than those with weak MMP-2 in breast cancer.17 Such a conclusion was also obtained by Pellikainen JM groups reported in breast cancer.16 Similar to these findings, our findings also showed no prognostic significance of MMP-2 overexpression for overall time with NPC patients. However, we found that high MMP-2 expression could stratify survival time of patients in advanced clinical stage (III–IV). As we know, patients diagnosed at stage III–IV tend to have local recurrence and distant metastases after treatment. In consequence, we suggest that tumoral MMP-2 expression might be used as a potential biomarker for patients with late-stage.
Conclusion
We here show that high expression of MMP-2 was associated strongly with tumor aggressive features/phenotypes and poor survival in patients with stage III–IV. Finally, overexpression of MMP-2 enhanced the invasive abilities and induced EMT in NPC. Our results provide a deep insight into tumor aggressiveness and clinical outcomes of MMP-2 in tumor cells in NPC.
Abbreviation list
Nasopharyngeal carcinoma, NPC; matrix metalloproteinase 2, MMP-2; epithelial-mesenchymal transition, EMT; tissue microarray, TMA; immunohistochemistry, IHC; immunofluorescence labelling of formalin-fixed, FFPE; differentiated nonkeratinizing carcinoma, DNKC; undifferentiatied carcinoma, UDC; tumor size, T; hazard ratio, HR; confidence interval, CI.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81202125, 81872202); the Natural Science Foundation of Guangdong Province (Grant Nos. 2015A030313263, 2018A030313778); the Natural Science Novel Project of Department of Education of Guangdong Province (Grant No. 2013KJCX0093); and Guangdong Provincial Key Laboratory of Cell Microenvironment and Disease Research (Grant No. 2017B030301018).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Perri F, Della Vittoria Scarpati G, Caponigro F, et al. Management of recurrent nasopharyngeal carcinoma: current perspectives. Onco Targets Ther. 2019;12:1583–1591. doi:10.2147/OTT.S188148
3. Hua H, Li M, Luo T, Yin Y, Jiang Y. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68(23):3853–3868. doi:10.1007/s00018-010-0573-6
4. Siqueira AS, Carvalho MR, Monteiro AC, Freitas VM, Jaeger RG, Pinheiro JJ. Matrix metalloproteinases, TIMPs and growth factors regulating ameloblastoma behaviour. Histopathology. 2010;57(1):128–137. doi:10.1111/j.1365-2559.2010.03596.x
5. Tester AM, Waltham M, Oh SJ, et al. Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice. Cancer Res. 2004;64(2):652–658. doi:10.1158/0008-5472.CAN-0384-2
6. Xu X, Wang Y, Chen Z, Sternlicht MD, Hidalgo M, Steffensen B. Matrix metalloproteinase-2 contributes to cancer cell migration on collagen. Cancer Res. 2005;65(1):130–136. doi:10.1158/0008-5472.CAN-04-4557
7. Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–1379. doi:10.1172/JCI33775
8. Wu A, Li J, Wu K, et al. LATS2 as a poor prognostic marker regulates non-small cell lung cancer invasion by modulating MMPs expression. Biomed Pharmacother. 2016;82:290–297. doi:10.1016/j.biopha.2016.04.008
9. Koppikar SJ, Choudhari AS, Suryavanshi SA, Kumari S, Chattopadhyay S, Kaul-Ghanekar R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer. 2010;10:210. doi:10.1186/1471-2407-10-663
10. O’Grady A, Dunne C, O’Kelly P, Murphy GM, Leader M, Kay E. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007;51(6):793–804. doi:10.1111/his.2007.51.issue-6
11. Banerji A, Chakrabarti J, Mitra A, Chatterjee A. Effect of curcumin on gelatinase A (MMP-2) activity in B16F10 melanoma cells. Cancer Lett. 2004;211(2):235–242. doi:10.1016/j.canlet.2004.02.007
12. Rah B, Amin H, Yousuf K, et al. A novel MMP-2 inhibitor 3-azidowithaferin A (3-azidoWA) abrogates cancer cell invasion and angiogenesis by modulating extracellular Par-4. PLoS One. 2012;7(9):e44039. doi:10.1371/journal.pone.0044039
13. Kallakury BV, Karikehalli S, Haholu A, Sheehan CE, Azumi N, Ross JS. Increased expression of matrix metalloproteinases 2 and 9 and tissue inhibitors of metalloproteinases 1 and 2 correlate with poor prognostic variables in renal cell carcinoma. Clin Cancer Res. 2001;7(10):3113–3119.
14. Trudel D, Fradet Y, Meyer F, Harel F, Têtu B. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 2003;63(23):8511–8515.
15. Kamat AA, Fletcher M, Gruman LM, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–1714. doi:10.1158/1078-0432.CCR-05-2338
16. Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10(22):7621–7628. doi:10.1158/1078-0432.CCR-04-1061
17. Ishikawa S, Takenaka K, Yanagihara K, et al. Matrix metalloproteinase-2 status in stromal fibroblasts, not in tumor cells, is a significant prognostic factor in non-small-cell lung cancer. Clin Cancer Res. 2004;10(19):6579–6585. doi:10.1158/1078-0432.CCR-04-0272
18. Wong JC, Chan SK, Schaeffer DF, et al. Absence of MMP2 expression correlates with poor clinical outcomes in rectal cancer, and is distinct from MMP1-related outcomes in colon cancer. Clin Cancer Res. 2011;17(12):4167–4176. doi:10.1158/1078-0432.CCR-10-1224
19. Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85(2):74. doi:10.1177/014556130608500201
20. Wang SC, Lin XL, Wang HY, et al. Hes1 triggers epithelial-mesenchymal transition (EMT)-like cellular marker alterations and promotes invasion and metastasis of nasopharyngeal carcinoma by activating the PTEN/AKT pathway. Oncotarget. 2015;6(34):36713–36730. doi:10.18632/oncotarget.5457
21. Cai LM, Lyu XM, Luo WR, et al. EBV-miR-BART7-3p promotes the EMT and metastasis of nasopharyngeal carcinoma cells by suppressing the tumor suppressor PTEN. Oncogene. 2015;34(17):2156–2166. doi:10.1038/onc.2014.341
22. Wang Y, Kong W, Yue J, et al. Vascular endothelial growth factor165-regulated nasopharyngeal carcinoma cell lines invasion and migration involve expression and activation of matrix metalloproteinase-2. J Huazhong Univ Sci Technolog Med Sci. 2006;26(5):621–624. doi:10.1007/s11596-006-0538-z
23. Lin ML, Lu YC, Chung JG, et al. Down-regulation of MMP-2 through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol Carcinog. 2010;49(9):783–797.
24. Li XY, Lin YC, Huang WL, et al. Zoledronic acid inhibits proliferation and impairs migration and invasion through downregulating VEGF and MMPs expression in human nasopharyngeal carcinoma cells. Med Oncol. 2012;29(2):714–720. doi:10.1007/s12032-011-9904-1
25. Cui D, Zhang X, Fu Y. Expressions of COX-2 and MMP-2 in nasopharyngeal carcinoma and the their relationship with lymph node metastasis. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2008;22(15):692–694.
26. Ellenrieder V, Hendler SF, Ruhland C, Boeck W, Adler G, Gress TM. TGF-beta-induced invasiveness of pancreatic cancer cells is mediated by matrix metalloproteinase-2 and the urokinase plasminogen activator system. Int J Cancer. 2001;93(2):204–211. doi:10.1002/ijc.1330
27. Karadag A, Ogbureke KU, Fedarko NS, Fisher LW. Bone sialoprotein, matrix metalloproteinase 2, and alpha(v)beta3 integrin in osteotropic cancer cell invasion. J Natl Cancer Inst. 2004;96(12):956–965. doi:10.1093/jnci/djh169
28. Ito H, Duxbury M, Benoit E, et al. Prostaglandin E2 enhances pancreatic cancer invasiveness through an Ets-1-dependent induction of matrix metalloproteinase-2. Cancer Res. 2004;64(20):7439–7446. doi:10.1158/0008-5472.CAN-04-1177
29. Kumar B, Koul S, Petersen J, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res. 2010;70(2):832–841. doi:10.1158/0008-5472.CAN-09-2918
30. Chen MK, Yang SF, Lai JC, et al. Expression of bcl-2 correlates with poor prognosis and modulates migration of nasopharyngeal carcinoma cells. Clin Chim Acta. 2010;411(5–6):400–405. doi:10.1016/j.cca.2009.12.010
31. Luo W, Gao F, Li S, Liu L. FoxM1 promotes cell proliferation, invasion, and stem cell properties in nasopharyngeal carcinoma. Front Oncol. 2018;8:483. doi:10.3389/fonc.2018.00483
32. Lu J, Chua HH, Chen SY, Chen JY, Tsai CH. Regulation of matrix metalloproteinase-1 by Epstein-Barr virus proteins. Cancer Res. 2003;63(1):256–262.
33. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi:10.1038/nrc3447
34. Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98(18):10356–10361. doi:10.1073/pnas.171610498
35. Usami Y, Satake S, Nakayama F, et al. Snail-associated epithelial-mesenchymal transition promotes oesophageal squamous cell carcinoma motility and progression. J Pathol. 2008;215(3):330–339. doi:10.1002/path.2351
36. Qi ST, Zhou J, Pan J, Zhang C, Silky C, Yan XR. Epithelial-mesenchymal transition and clinicopathological correlation in craniopharyngioma. Histopathology. 2012;61(4):711–725. doi:10.1111/j.1365-2559.2012.04254.x
37. Liu Z, Kakudo K, Bai Y, et al. Loss of cellular polarity/cohesiveness in the invasive front of papillary thyroid carcinoma, a novel predictor for lymph node metastasis; possible morphological indicator of epithelial mesenchymal transition. J Clin Pathol. 2011;64(4):325–329. doi:10.1136/jcp.2010.083956
38. Luo W, Fang W, Li S, Yao K. Aberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinoma. Int J Cancer. 2012;131(8):1863–1873. doi:10.1002/ijc.27467
39. Ondruschka C, Buhtz P, Motsch C, et al. Prognostic value of MMP-2, −9 and TIMP-1,-2 immunoreactive protein at the invasive front in advanced head and neck squamous cell carcinomas. Pathol Res Pract. 2002;198(8):509–515. doi:10.1078/S0344-0338(04)70292-7
40. Giannelli G, Bergamini C, Marinosci F, et al. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int J Cancer. 2002;97(4):425–431. doi:10.1002/ijc.1635
41. Davidson B, Goldberg I, Kopolovic J, et al. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma–a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Onco. 1999;73(3):372–382. doi:10.1006/gyno.1999.5381
42. Pulyaeva H, Bueno J, Polette M, et al. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin Exp Metastasis. 1997;15(2):111–120. doi:10.1023/A:1018444609098
43. Yokoyama K, Kamata N, Fujimoto R, et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol. 2003;22(4):891–898.
44. Gordon KJ, Kirkbride KC, How T, Blobe GC. Bone morphogenetic proteins induce pancreatic cancer cell invasiveness through a Smad1-dependent mechanism that involves matrix metalloproteinase-2. Carcinogenesis. 2009;30(2):238–248. doi:10.1093/carcin/bgn274
45. Luo WR, Chen XY, Li SY, Wu AB, Yao KT. Neoplastic spindle cells in nasopharyngeal carcinoma show features of epithelial-mesenchymal transition. Histopathology. 2012;61(1):113–122. doi:10.1111/j.1365-2559.2012.04254.x
46. Passlick B, Sienel W, Seen-Hibler R, et al. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6(10):3944–3948.
Supplementary material
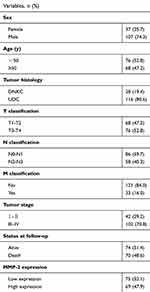 |
Table S1 Overview of clinicopathologic characteristics of 144 nasopharyngeal carcinoma (NPC) patients and MMP-2 expression |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.