Back to Journals » Drug Design, Development and Therapy » Volume 16
Matrine Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review
Authors Lin Y, He F, Wu L, Xu Y, Du Q
Received 23 November 2021
Accepted for publication 3 February 2022
Published 1 March 2022 Volume 2022:16 Pages 533—569
DOI https://doi.org/10.2147/DDDT.S349678
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Yingda Lin,1,2,* Fuming He,2 Ling Wu,1 Yuan Xu,1 Qiu Du3,4,*
1Department of Pharmacy, the Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, 225012, People’s Republic of China; 2Medical College, Yangzhou University, Yangzhou, 225001, People’s Republic of China; 3Department of Neurosurgery, the Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, 225012, People’s Republic of China; 4Department of Central Laboratory, the Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, 225012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiu Du, Department of Neurosurgery, the Affiliated Hospital of Yangzhou University, Yangzhou University, 368 Hanjiang Middle Road, Yangzhou, 225012, People’s Republic of China, Email [email protected] Yuan Xu, Department of pharmacy, the Affiliated Hospital of Yangzhou University, Yangzhou University, 368 Hanjiang Middle Road, Yangzhou, 225012, People’s Republic of China, Email [email protected]
Abstract: As The main effective monomer of the traditional Chinese medicine Sophora flavescens Ait, matrine has a broad scope of pharmacological activities such as anti-tumor, anti-inflammatory, analgesic, anti-fibrotic, anti-viral, anti-arrhythmia, and improving immune function. These actions explain its therapeutic effects in various types of tumors, cardiopathy, encephalomyelitis, allergic asthma, rheumatoid arthritis (RA), osteoporosis, and central nervous system (CNS) inflammation. Evidence has shown that the mechanism responsible for the pharmacological actions of matrine may be via the activation or inhibition of certain key molecules in several cellular signaling pathways including the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR), transforming growth factor-β/mothers against decapentaplegic homolog (TGF-β/Smad), nuclear factor kappa B (NF-κB), Wnt (wingless/ integration 1)/β-catenin, mitogen-activated protein kinases (MAPKs), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathways. This review comprehensively summarizes recent studies on the pharmacological mechanisms of matrine to provide a theoretical basis for molecular targeted therapies and further development and utilization of matrine.
Keywords: matrine, pharmacological effects, signaling pathways, review
Introduction
Sophora flavescens Ait is a well-known traditional Chinese herbal medicine and has been widely utilized in the clinical practice for its effects on various damp-heat syndromes, heat-toxin syndromes like carbuncle and sore, and skin itching.1 Modern pharmacological studies have shown that matrine (molecular formula: C15H24N2O, molecular weight: 248.36 g/mol) is a naturally occurring quinolizidine alkaloids rich in Sophora flavescens Ait as well as Sophora alopecuroides Linn, Sophora tonkinensis, which mediates a variety of biological effects such as anti-tumor, anti-inflammatory, analgesic, anti-fibrotic, anti-viral, anti-arrhythmia, antimicrobial, and insecticidal, illustrating that it has immense potential for clinical application with various pharmacological activities.1,2 At present, studies have reported that the pharmacological effects of matrine involve the PI3K/AKT/mTOR, TGF-β/Smad, NF-κB, Wnt/β-catenin, MAPKs, JAK/STAT, and other significant signaling pathways, and closely related to multiple biological processes such as cell proliferation, differentiation, apoptosis, and immune regulation.1–3
However, few studies have classified and summarized the underlying molecular mechanisms by which matrine exerts its pharmacological effects through different signaling pathways. In this review, the pharmacological activities of matrine and the molecular mechanisms of its pharmacological effects through the targeted regulation of different signaling pathways will be comprehensively expounded, so as to provide theoretical support for further expanding the potential clinical application of matrine.
Matrine Exerts Pharmacological Effects Through the PI3K/AKT/mTOR Signaling Pathway
PI3K is a heterodimeric lipid kinase composed of a catalytic subunit (p110α, p110β, or p110δ; encoded by the PIK3CA, PIK3CB, and PIK3CD genes, respectively) and a regulatory subunit (p85).4 PI3K is mainly activated by receptor tyrosine kinase (RTK) signaling, via phosphorylation of the inositol lipid phosphatidylinositol 4, 5-bisphosphate (PI (4, 5) P2). It then forms the second messenger molecule phosphatidylinositol (3, 4, 5)-trisphosphate (PI (3, 4, 5) P3) that recruits and activates pleckstrin homology (PH) domain-containing proteins such as AKT and phosphoinositide-dependent kinases (PDK) 1, which leads to downstream signaling events crucial for proliferation, survival, and migration.5,6 The serine/threonine-protein kinase AKT is a critical mediator of signaling downstream of PI3K, which is rapidly activated by PI3K or PDK as well as growth factors, inflammation, and DNA damage.7 AKT is recruited to the plasma membrane through the interaction of its PH domain with PI (3, 4, 5) P3, resulting in a conformational change and activation by phosphorylation, and plays a central role in multiple cellular processes essential for cell growth, metabolism, and survival.8 mTOR is a key kinase downstream of PI3K/AKT. It is an evolutionarily conserved atypical serine/threonine-protein kinase that belongs to the PI3K-related kinase (PIKK) protein family. mTOR participates in two distinct complexes, mTOR complex 1 (mTORC1) and mTORC2. Both complexes consist of a structure of many large proteins that aid in the stimulation and inhibition of key pathways and cascades in the cell. mTOR is the molecule that activates, regulates, and inhibits the functions of the two large complexes and has important functions in regulating cell growth, proliferation, motility, survival, protein synthesis, and transcription.9
The PI3K/AKT/mTOR pathway is crucial to many aspects of cell growth and survival in physiological as well as pathological conditions. Phosphorylation of PI3K leads to activation of AKT, which regulates several downstream molecules, including mTOR. These pathways are interconnected and therefore, in a certain sense, could be regarded as a single, unique pathway.10 The PI3K/AKT/mTOR pathway serves important roles in cellular processes such as survival, proliferation, growth, metabolism, angiogenesis, and metastasis. It is frequently hyper-activated or altered in many types of human cancer, and may serve as a valuable target for disease therapy.11,12
Chinese medicine plays a unique role in anti-tumor research. Studies have shown that matrine may be widely utilized in the inhibition of malignant tumors and adjuvant chemoradiotherapy through modulation of the PI3K/AKT/mTOR pathway. Matrine is a promising treatment for lung cancer, either alone or in combination with chemotherapeutic agents. Niu et al13 found that matrine inhibited cell proliferation and induced apoptosis in A549 and 95D lung cancer cells in a dose- and time-dependent manner and exerted its cancer-killing effect by promoting apoptosis in lung cancer cells via the PI3K/AKT/mTOR signaling pathway and downregulation of the expression of the inhibitor of apoptosis protein (IAP) family proteins. Wan et al14 evaluated the anti-lung adenocarcinoma effect of matrine and found that matrine suppressed the proliferation of A549 lung adenocarcinoma cells by inducing apoptosis and autophagy, accompanied by the upregulation of Bcl-2 associated X (Bax), microtubule associated protein 1 light chain 3-II (LC3-II), and Beclin-1, as well as the suppression of B cell leukemia/lymphoma 2 (Bcl-2) and sequestosome 1 (p62). This study demonstrated that mechanistically, the growth inhibitory effects of matrine were dependent on the suppression of PI3K/AKT and the upregulation of caveolae associated protein 3 (Cavin3). Xie et al15 showed that matrine significantly suppressed proliferation and colony formation, induced cellular apoptosis, and inhibited the migration of lung cancer cells, and further found that matrine markedly inhibited the phosphorylation of AKT and glycogen synthase kinase-3β (GSK-3β), indicating that the inhibitory effects of matrine in lung cancer are possibly through suppression of the AKT/GSK-3β signaling pathway. Liao et al16 found that matrine treatment significantly inhibited the proliferation, migration, and invasion of human NCI-H1299 lung cancer cells in a concentration-dependent manner, and the mechanism may be related to the upregulation of microRNA (miR)-133a expression and inhibition of the epidermal growth factor receptor (EGFR)/AKT/matrix metallopeptidase (MMP) -9 pathway.
For breast cancer, matrine has shown therapeutic and/or adjuvant therapeutic effects, but its anti-breast cancer mechanism remains unclear. Du et al17 showed that matrine significantly inhibited MCF-7 cells growth by inducing apoptosis and autophagy, and suppressed the phosphorylation of AKT and mTOR, suggesting that matrine may achieve its anti-breast cancer effect by inhibiting AKT/mTOR pathway. Yu et al18 demonstrated that matrine markedly suppressed the proliferation of highly metastatic breast cancer cells mDA-MB-231, and displayed synergistic effects with existing anticancer drugs such as celecoxib, trocomycin A, and rosiglitazone. They also found that matrine induced apoptosis and cell cycle arrest, and significantly reduced the invasion of MDA-MB-231 cells, mechanistically, the anti-breast cancer effects of matrine may via the EGF/vascular endothelial growth factor (VEGF)/VEGFR1/AKT/NF-κB signaling pathway. Li et al19 verified that matrine inhibited MCF-7 cell growth in a concentration-and time-dependent manner, and upregulated phosphatase and tensin homolog (PTEN) by downregulating miR-21 which in turn dephosphorylated AKT, resulting in accumulation of Bcl-2 associated agonist of cell death (Bad), Cyclin dependent kinase inhibitor 1A (p21, Cip1) and Cyclin dependent kinase inhibitor 1B (p27, Kip1), revealing that miR-21/PTEN/AKT pathway is a signaling mechanism for the anti-breast cancer action of matrine.
Studies have evaluated the therapeutic effects of matrine on hepatocellular carcinoma (HCC). Wang et al20 investigated the effects and related mechanisms of matrine in hepatoma HepG2 and Bel7402 cells and showed that both autophagy and apoptosis were induced by treatment with matrine. They also found that autophagy was induced via inhibition of the PI3K/AKT/mTOR pathway and upregulation of Beclin-1. In addition, apoptosis induced by matrine was enhanced in a caspase-dependent manner via treatment with the autophagy inhibitor chloroquine (CHQ) and Beclin-1 small-interfering RNA. These results indicated that inhibition of autophagy may promote matrine-induced apoptosis in HCC, and the combination of matrine and an autophagy inhibitor may be a promising treatment. Wang et al21 confirmed the inhibitory function of matrine on the invasive and metastatic capabilities of HCC. Additionally, increased epithelial (E)-cadherin expression and decreased Vimentin, MMP-2, MMP-9, snail family transcriptional repressor (Snai)-1, and Snai-2 expression induced by matrine are attributed to enhanced PTEN activity and inhibited AKT signaling. This may contribute to the inhibitory effects of matrine on invasion and metastasis in HCC, revealing the potential therapeutic application of matrine as an anti-metastatic treatment for HCC.
Chi et al22 explored the anti-tumor effects of matrine in glioma cells and confirmed that matrine suppressed cell viability and induced apoptosis and autophagy, decreased the expression of circular RNA (circRNA)-104075 and Bcl-9, while overexpression of circRNA-104075 or Bcl-9 counteracted the effects of matrine and activated the PI3K/AKT and Wnt/β-catenin signaling pathways inhibited by matrine. This study indicated that matrine down-regulated the expression of circ-104075 and Bcl-9 via inhibition of the PI3K/AKT and Wnt/β-catenin pathways to induce apoptosis and autophagy in glioma cells, providing a potential candidate drug for the treatment of glioma. Wang et al23 treated human glioma U251 and U87 cell lines with matrine, and found that matrine significantly inhibited the proliferation, migration and invasion of glioma cells, and decreased the phosphorylation of mitogen-activated protein kinase 14 (p38 MAPK) and AKT, as well as the expression of N-cadherin, increased the expression of E-cadherin, suggesting that matrine play an anti-tumor metastasis role by inhibiting the p38-MAPK and AKT signaling. Zhou et al24 showed that matrine treatment effectively suppressed the proliferation of glioblastoma cells by inducing cellular senescence, inhibited the growth of orthotopic xenografts and prolonged the overall survival of mice, and decreased the expression of insulin like growth factor 1 (IGF1), PI3K, and phosphorylated (p-)AKT. This study suggested that matrine may inhibit cell growth by inhibiting the IGF1/PI3K/AKT signaling pathway, providing a potential treatment for glioblastoma. Shen et al25 found that matrine obviously inhibited neuroblastoma cells proliferation, induced G2/M cell cycle arrest, and suppressed cell migration by upregulating the expression of tribbles pseudokinase 3 (TRB3) and thereby blocking the activation of the PI3K/AKT pathway, indicating the potential therapeutic effects of matrine on neuroblastoma.
Several studies have explored the therapeutic effects of matrine on leukemia. Wu et al26 demonstrated that matrine inhibited the growth acute myelocytic leukemia (AML) cells by inducing apoptosis and autophagy both in vitro and in vivo. They also found that matrine inhibited the phosphorylation of AKT, mTOR and downstream substrates ribosomal protein S6 kinase B1 (p70S6K) and eukaryotic translation initiation factor 4E binding protein 1 (4EBP1), suggesting that matrine exerts anti-tumor effects through the AKT/mTOR pathway. Hao et al27 revealed that matrine treatment inhibited the proliferation of AML THP-1 cells and induced apoptosis, and decreased the expression of p-PI3K, p-AKT, and p-mTOR in a concentration-dependent manner, indicating that the inhibition of PI3K/AKT/mTOR signaling pathway may be related to the anti-tumor effects of matrine. Zhang et al28 proved that matrine exerted excellent anti-leukemic effects, they found that matrine could inhibit cell viability and induce apoptosis of AML cells through mitochondria-mediated pathway and inhibition of the AKT and extracellular regulated protein kinases (ERK)1/2 pathways. Given these findings, matrine may serve as a promising candidate chemotherapeutic agent against AML.
Yang et al29 treated human T24 bladder cancer (BC) cells with matrine and found that matrine inhibited cell proliferation and invasion, decreased the p-PI3K and p-AKT expression in a dose-dependent manner, and subsequently increased the expression of cell cycle-inhibiting molecules cyclin dependent kinase inhibitor 2A (p16), p21 and p27, but decreased the expression of invasion genes MMP-2 and MMP-9, indicating that matrine could play an anti-bladder cancer role through the PI3K/AKT signaling pathway. Li et al30 verified that matrine restrained tumor growth in vivo and inhibited BC cell proliferation, migration, invasion but promoted apoptosis via upregulation of long intergenic non-coding RNA (LINC) 00472/programmed cell death protein 4 (PDCD4) by blocking PTEN/PI3K/AKT pathways. Liao et al31 confirmed that matrine combined with cisplatin has better anti-tumor effect by downregulating the VEGF/PI3K/AKT signaling pathway. They found that matrine and cisplatin combined treatment of urothelial bladder cancer (UBC) EJ and T24 cells, synergistically and significantly inhibited cell proliferation, migration and invasion, induced G1/S cell cycle arrest and apoptosis, and increased reactive oxygen species (ROS) levels, suggesting that matrine may be a potential option for combination therapy of UBC.
Peng et al32 examined the anti-tumor effect of matrine on gastric cancer (GC) cells and found that matrine significantly inhibited the proliferation and migration of SGC 7901 cells, downregulated the protein level of U-plasminogen activator (uPA) and the phosphorylation of ERK and AKT. Further studies showed that blocking the PI3K/AKT pathway with PI3K inhibitor LY294002 suppressed cell growth and uPA expression. This study indicates that matrine may inhibit GC cells by inactivating the PI3K/AKT/uPA signaling pathway, suggesting that matrine may be a potential drug for GC treatment. Li et al33 found that autophagy increased in a dose- and time-dependent manner in GC cells exposed to matrine, and inhibition of autophagy increased matrine-induced apoptosis; in addition, matrine treatment did not inhibit the phosphorylation of AKT and its downstream effectors mTOR and p70S6K, whereas the total levels of AKT and mTOR decreased. These results suggest that the AKT/mTOR/p70S6K signaling pathway may not be involved in matrine-induced autophagy in SGC-7901 cells, but inhibition of autophagy may be a promising strategy to enhance the anti-GC potential of matrine.
Bai et al34 demonstrated that matrine effectively inhibited the proliferation of prostate cancer PC-3 and RWPE1 cells, activated forkhead box O3 (FoxO3a), and then upregulated the expression of downstream target proteins Bcl-2 like 11 (Bim) and p27, leading to apoptosis and cell cycle arrest. Additionally, the expression of p-AKT and AKT were decreased after matrine treatment, resulting in the retention of FoxO3a, indicating that AKT and FoxO3a signaling pathways may be involved in matrine-mediated suppression of prostate cancer. Through high-throughput sequencing analysis, Li et al35 found that matrine exhibited extensive regulatory effects on the mRNA expression profile of PC-3 and DU145 cells, and differentially expressed genes played important roles in cell metabolism, growth, anatomical structure formation, cell component organization and biological regulation; besides, the forkhead box sub-group O (FOXO) and PI3K/AKT signaling pathways showed significant differences. They further found that matrine treatment obviously inhibited the proliferation, migration and invasion but promoted apoptosis of PC-3 and DU145 cells through FOXO and PI3K/AKT signaling pathways, suggesting that matrine may be used as a complementary agent for prostate cancer chemotherapy.
The antitumor activity of matrine in colon cancer has been testified, but the latent mechanism is still indistinct. Zhang et al36 treated colon cancer LoVo cells with matrine and found that matrine inhibited cell proliferation in a dose- and time-dependent manner, induced cell cycle arrest in G1 phase and apoptosis, and significantly reduced AKT phosphorylation; further studies showed that specific inhibition of p-AKT induced cell apoptosis and synergistically inhibited LoVo cell proliferation with matrine, while activation of AKT counteracted the inhibitory effect of matrine on cell proliferation. This study suggests that matrine exerts an anti-colon cancer effect by inhibiting the AKT signaling pathway, and may serve as a potential treatment for colon cancer.
Among gynecological cancers, ovarian cancer displays the highest mortality rate and a poor response to anticancer therapy. Therefore, Zhang et al37 used epithelial ovarian cancer cells A2780, SKOV3, and cisplatin-resistant cells A2780/DDP to test the therapeutic effect of matrine, and found that matrine retarded cancer-related signaling transduction by reducing the phosphorylation levels of ERK1/2, mitogen-activated extracellular signal-regulated kinase (MEK)1/2, PI3K, AKT, mTOR, Focal adhesion kinase (FAK), Ras homolog family member A (RhoA), VEGFR2, and TEK receptor tyrosine kinase (Tie2) in vitro and in vivo, and then modulated cell proliferation, apoptosis, and autophagy to inhibit the occurrence and progression of ovarian cancer, indicating that matrine, as a natural agent, is expected to be a targeted drug against ovarian cancer with the potential to overcome chemotherapy resistance and reduce toxic side effects.
Jin et al38 evaluated the anti-tumor potential of matrine in V600EBRAF harboring melanoma M21 cells and found that matrine effectively inhibited cell proliferation, induced cell cycle arrest at G0/G1 phase and apoptosis in a dose-dependently manner; mechanistically, matrine inhibited the PI3K/AKT pathway by activating PTEN, ultimately leading to upregulation of P21 and Bax in M21 cells. These findings suggest that activation of PTEN holds promise as a practicable strategy for melanoma treatment, and matrine may serve as a potent drug candidate.
Xu et al39 found that matrine inhibited the proliferation, induced apoptosis and G0/G1 phase cell cycle arrest of human osteosarcoma (OS) MG63, U2OS, and SAOS2 cell lines in a dose-dependent manner in vitro, and was associated with increased expression of p27/Kip1 and the pro-apoptotic factor Bax and decreased expression of AKT, glycogen synthase kinase 3 (GSK3)-β (Ser9), and cyclin D1, indicating that matrine may be an effective anti-OS drug due to cell growth inhibitory effect possibly through modulation of the AKT signaling. Yu et al40 investigated the effects and molecular mechanisms of matrine, arsenic trioxide, and their combination on myeloma RPMI8226 and U266 cell lines, and demonstrated that matrine and arsenic trioxide significantly inhibited proliferation and promoted apoptosis of myeloma cells, and synergistic effects produced when the two were combined; mechanistically, by activating caspase-3 and poly ADP-ribose polymerase (PARP), up-regulating the expression of Bim, down-regulating the expression of Bcl-2 and survivin, and inhibiting p-AKT, matrine-mediated apoptosis was promoted.
Zhao et al41 and Li et al42 found that treatment of different subtypes of thyroid cancer cells with matrine inhibited cell proliferation, induced apoptosis and G1 phase cell cycle arrest, and downregulated miR-21 and p-AKT expression, but up-regulate PTEN expression, indicating that the miR-21/PTEN/AKT pathway may be one of the mechanisms of matrine-induced growth inhibition of thyroid cancer cells, and miR-21 is expected to be a potential target of matrine for the treatment of follicular thyroid carcinoma.
Matrine also has potential therapeutic effects on benign tumors. Li et al43 demonstrated that matrine inhibited the cell viability of pituitary tumor RC-4B/C cells and GH3 cells, decreased the levels of AKT and Foxo3a, increased the nuclear localization of Foxo3a and the expression of apoptosis-related gene Bcl-2, and inhibited the level of cytoplasmic Foxo3a, indicating that matrine inhibits the proliferation of pituitary tumor cells may be related to the AKT/Foxo3a signaling pathway.
In addition to anti-tumor activity, matrine exhibits protective effects in vascular injury, cardiopathy, immune system diseases, and the inflammatory reaction. Xu et al44 showed that matrine enhanced viability, inhibited apoptosis, and alleviated oxidative stress in H2O2-induced H9c2 cardiomyocytes. They also confirmed that matrine modulated H2O2-induced myocardial oxidative stress repair through the HOX transcript antisense RNA (HOTAIR)/miR-106b-5p axis via the AKT and STAT3 signaling pathways, providing a potential therapeutic target for oxidative stress-associated heart diseases. Zhang et al45 demonstrated that matrine effectively improved lipid metabolism, inflammation, and vascular wall thickness in mice fed a high-fat diet, reduced the phosphorylation of nitric oxide synthase 3 (ENOS)-Thr497, and increased the phosphorylation of ENOS-S1177, thereby resulted in an increase in NO production, which is mediated by PI3K/AKT and protein kinase C Alpha (PKCα), revealing the molecular mechanism underlying the protective effect of matrine in high-fat diet-induced vascular disease. Ma et al46 pre-treated human coronary smooth muscle cells (HCSMCs) with matrine and then exposed them to advanced glycation end products (AGEs). They found that matrine pre-treatment significantly reduced collagen content, increased smooth muscle myosin heavy chain 11 (MYH11) and DNA polymerase delta interacting protein 2 (Poldip2) expression, decreased the expressions of collagen I, β1-integrin, PI3K and nuclear p-p70S6k, and reduced the phosphorylation of AKT and mTOR in HCSMCs exposed to AGEs. These results indicate that matrine inhibits AGEs-induced contraction synthesis phenotypic transformation and the fibrosis response by regulating the Poldip2/mTOR signaling pathway. Liu et al47 showed that matrine pre-treatment partially alleviated subarachnoid hemorrhage (SAH)-induced early brain injury (EBI) in rats, inhibited apoptosis and reduced increased levels of the inflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β. This study also found partial suppression of SAH-induced AKT and NF-κB inhibitor alpha (IκB-α) phosphorylation and degradation, decreased NF-κB p65 protein levels, and upregulated expression of Kelch like ECH associated protein 1 (Keap1), nuclear factor erythroid 2-related factor 2 (Nrf2), and Heme oxygenase 1 (HO-1). Mechanistically, matrine may alleviate EBI after experimental SAH in rats possibly via PI3K/AKT-mediated NF-κB inhibition and Keap1/Nrf2-dependent HO-1 induction.
Matrine has been approved for the prevention and therapy of cancer cachexia, such as skeletal muscle atrophy, which is associated with poor prognosis and suboptimal responses. Chen et al48 evaluated the mechanism of matrine on skeletal muscle atrophy and found that matrine increased muscle fiber size and muscle mass in a CT26 colon adenocarcinoma cachexia mouse model, and significantly improved the differentiation of C2C12 myoblasts, reduced C2C12 myotube atrophy and apoptosis, down-regulated the levels of specifically expressed E3 ubiquitin ligases muscle-specific ring finger protein 1 (MuRF1) and muscle atrophy F-box protein (MAFbx) in skeletal muscle, and increased the phosphorylation of AKT, mTOR and FoxO3α in atrophic C2C12 myotube, indicating that matrine may alleviate muscle atrophy and improve myoblast differentiation by inhibiting E3 ubiquitin ligases and activating the AKT/mTOR/FoxO3α signaling pathway.
Matrine has been reported to be beneficial in experimental autoimmune encephalomyelitis (EAE) mainly through its anti-inflammatory effect, but the underlying mechanism is unclear. Liu et al49 induced EAE in C57BL/6 mice and found that matrine treatment effectively inhibited the severity of EAE, increased the expression of protein lipoprotein, a marker protein of CNS myelin, significantly increased the number of mature oligodendrocytes, and activated the PI3K/AKT/mTOR signaling pathway to promote oligodendrocyte differentiation and myelination, thereby protecting myelin from CNS inflammation-induced damage. Chen et al50 showed that matrine significantly prevented ovariectomy-induced bone loss and inhibited osteoclastogenesis in vivo with decreased serum levels of tartrate-resistant acid phosphatase 5 (TRAcp5b), TNF-α, and IL-6, and significantly inhibited osteoclast differentiation in bone marrow monocytes and RAW264.7 cells. Mechanistically, matrine blocked receptor activator for NF-κB ligand (RANKL)-induced activation of NF-κB, AKT, and MAPK pathways, and inhibited the expression of osteoclastogenesis-related markers, including MMP-9, nuclear factor of activated T cells 1 (NFATc1), tartrate-resistant acid phosphatase (TRAP), proto-oncogene tyrosine-protein kinase Src (c-Src), and cathepsin K, suggesting that matrine inhibits osteoclastogenesis by regulating multiple signaling pathways and may serve as a promising agent for the treatment of osteoclast-related diseases such as osteoporosis.
In addition to the biological activities mentioned above, recent studies have also shown that matrine exhibits antidepressant effects. Wu et al51 found that matrine exhibited significant antidepressant effects in a mouse model of depression induced by forced swim test and tail suspension test, completely reversed the mild depression-like symptoms caused by chronic unpredictable stress. Further, they demonstrated that matrine treatment restored the attenuating effects of these stresses on PI3K/AKT/mTOR signaling pathway in the hippocampus, thereby exerting antidepressant-like effects. This study sheds light on the development of novel antidepressants with better efficacy and fewer side effects and further extends the understanding of the pharmacological effects of matrine.
Although natural matrine alkaloids have significant pharmacological activities, their shortcomings such as low bioavailability, poor activity, large dosage, and strong side effects restrict their wide application. Therefore, the structural modification of matrine alkaloids to develop derivative compounds with higher activity is a valuable research direction. For instance, Feng et al52 reported a more effective thio-derivative of matrine (MD-1) and showed that it more effectively inhibited the proliferation and migration of hepatic stellate cells (HSCs) -T6 cells and induced cell cycle G0/G1 phase arrest and apoptosis to a greater extent than matrine. Further, they found that MD-1 bound to the EGFR on the surface of HSC-T6 cells, further inhibiting the phosphorylation of EGFR and its downstream target AKT, resulting in decreased expression of cyclin D1 and eventual inhibition of the activation of HSC-T6 cells, thus exhibiting an anti-hepatic fibrosis role. Moreover, in rats with dimethylnitrosamine (DMN)-induced hepatic fibrosis, MD-1 delayed the development and progression of hepatic fibrosis, protected hepatic parenchymal cells, and improved hepatic functions, suggesting that it may serve as a potential anti-hepatic fibrosis drug. Sun et al53 obtained the novel derivative WM622 (C26H35ON3S2) after structural modification of matrine, which had better biological activity and a greater inhibitory effect on transplanted tumors in vivo than matrine. WM622 also significantly inhibited the proliferation and promoted apoptosis of HCC cells, induce G0/G1 cell cycle arrest, and dose-dependently decreased the phosphorylation levels of EGFR, AKT, PI3K, and GSK3β. These results indicate that WM622 can inhibit the proliferation of the HCC both in vivo and in vitro by inducing apoptosis, blocking cell cycle in the G0/G1 phase, and inhibiting the PI3K/AKT signal pathway.
Qian et al54 modified the structure of matrine and obtained a novel derivative WM130 (C30N4H40SO5F) with pharmacological activity superior to matrine. They found that WM130 significantly inhibited the proliferation, invasion and migration, induced apoptosis of HCC cells, and inhibited Huh-7 xenograft tumors growth in a dose-dependent manner. Furthermore, the expressions of p-EGFR, p-ERK, p-AKT, MMP-2, Ki67, and Bcl-2/Bax were down-regulated, while the expression of PTEN was up-regulated in HCC after WM130 treatment. It is concluded that WM130 inhibits HCC by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Zou et al55 evaluated the anticancer properties of MASM ([(6aS, 10S, 11aR, 11bR, 11cS) 210-Methylamino-dodecahydro-3a, 7a-diaza-benzo (de) anthracene-8-thione]), a potent matrine derivative, on A549 (non-small-cell lung cancer cell line), MCF-7 and MDA-MB-231 (breast cancer cell lines), and Hela (cervical cancer cell line), and found that MASM induced apoptosis and autophagy in all cancer lines and possessed higher pharmaceutical potency than matrine, but removal of ROS with N-acetylcysteine rescued tumor cells from apoptosis and autophagy. In addition, the inhibition of autophagy can further enhance MASM-induced apoptosis through ROS-mediated PI3K/AKT/mTOR, ERK and p38 signaling pathways, suggesting that modulating autophagy during MASM administration may further enhance its therapeutic effects. Wu et al56 found that matrine derivative 5I bearing benzo-α-pyranone structure effectively inhibited the proliferation of lung cancer cells in vivo and in vitro without obvious side effects. In addition, compound 5I induced G1 cell cycle arrest and autophagy in lung cancer cells through up-regulating P27, down-regulating cyclin dependent kinase 4 (CDK4) and Cyclin D1 (CCND1) and attenuating PI3K/Akt/mTOR pathway, and suppression of autophagy significantly attenuated the inhibitory effect of 5I on cell proliferation; thus providing a potential paradigm for exploiting the anticancer properties of 5I.
Sophora flavescens alkaloid (SFA) gels, a compound Traditional Chinese Medicine, its effective ingredients mainly include matrine and oxymatrine. Zhou et al57 proved that SFA gels can inhibit the proliferation and metastasis of cervical cancer cells, induce cell cycle arrest in G2/M phase and apoptosis; further they showed that SFA gels may exert anti-cervical cancer effects by suppressing the AKT/mTOR signaling pathway. The results suggest that SFA gels may be a potential anti-tumor therapeutic agent for the treatment of cervical cancer.
Xie et al58 treated nasopharyngeal carcinoma cell lines (CNE1, CNE2, and HONE1) with 14-thienyl methylene matrine (YYJ18) and demonstrated that YYJ18 significantly inhibited proliferation and induced apoptosis of these three cell lines in a dose-dependent manner; further, YYJ18 treatment significantly suppressed the phosphorylation of p38, but upregulated the phosphorylation of ERK1/2 and AKT, activated the downstream signaling Bax, caspase-3, and inhibited the activity of Bcl-2, confirming that the inhibitory effect of YYJ18 on nasopharyngeal carcinoma cells may be related to the modulation of MAPK and PI3K/AKT signaling pathways.
Postmenopausal osteoporosis (POMP) is a metabolic bone disorder characterized by decreased bone density, micro-architectural deterioration of bone tissue, and over-activated osteoclastogenesis. Chen et al59 developed a novel matrine derivative MASM (M19) based on sophocarpine, which significantly inhibited RANKL/macrophage colony stimulating factor (M-CSF) induced osteoclastogenesis through suppressing NF-κB, MAPKs, and PI3K/AKT pathways in vitro and ameliorated bone loss in ovariectomized mice in vivo, and confirmed that M19 targeted and stabilized ribosomal protein S5 (RPS5), while overexpressing of RPS5 synergized with M19 significantly inhibited osteoclastogenesis. These results indicated that RPS5 may be a potential candidate target for inhibiting osteoclastogenesis and treating osteoporosis, and M19 is a promising drug for the treatment of POMP. Xin et al60 found that M54, a bioactive derivative of matrine, can also target RPS5 and inhibit osteoclastogenesis through a mechanism similar to that of M19, which may also serve as a potential clinical drug for treating POMP. The above results suggest that matrine derivatives indeed have more efficient and less toxic biological activities, and are more likely to be valuable drug candidates for the therapy of various diseases.
We have summarized the relevant studies on the pharmacological effects of matrine through the PI3K/AKT/mTOR signaling pathway in Table 1.
 |  |  |  |
Table 1 The Relevant Studies on the Pharmacological Effects of Matrine Through the PI3K/AKT/mTOR Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the TGF-β Signaling Pathway
TGF-β belongs to a group of newly discovered superfamilies that regulate cell growth and differentiation. TGF-β and other members of the family are evolutionally conserved secreted proteins that contain many different polypeptide morphogenesis factors and are involved in many cellular processes in both mature organisms and developing embryos.61
The TGF-β superfamily in mammals mainly includes three subtypes: TGF-β1, TGF-β2, and TGF-β3. TGF-β1 is mainly expressed in endothelial cells, hematopoietic cells, and connective tissues, TGF-β2 is mainly expressed in epithelial cells and nerve cells, and TGF-β3 is mainly expressed in mesenchymal cells.62 They bind to the TGF-β receptor (TGFBR) 2 and have similar biological activities, regulating cell proliferation, migration, differentiation, apoptosis, and exerting significant effects in the regulation of embryonic growth and development, inflammation and repair (including angiogenesis), and host resistance mechanisms.63,64 The TGF-β signaling pathway is considered to play a key role in the process of fibrosis, exerting its biological functions by activating the downstream Smad pathway. TGF-β1/Smad3 is the most important regulatory signaling pathway involved in tissue fibrosis in many diseases.
Zhang et al65 investigated the anti-fibrosis-associated cardioprotective effects of matrine on diabetic cardiomyopathy (DbCM). They administered matrine orally to rats with experimental DbCM and found that matrine inhibited the expression of TGF-β and the phosphorylation of R Smad (Smad2/3) and blocked the activation of TGF-β1/R Smad signal transduction to repress collagen production and deposition, leading to a marked recovery in left ventricular function and heart compliance. These results indicated that matrine has therapeutic effects against cardiac fibrosis by affecting TGF-β1/Smad signaling and exerting cardioprotective effects in DbCM. Hou et al66 found that matrine treatment significantly decreased the level of non-fasting blood glucose, improved the hemodynamic parameters of DbCM model rats, inhibited the expression levels of the inflammatory cytokines TNF-α and IL-6, and decreased ROS generation, MDA, TGF-β levels, and peroxisome proliferator activator receptor β (PPARβ) and PPARγ1 activity. Matrine administration also significantly inhibited p-ERK expression, and endogenic expression of p-ERK counteracted matrine-induced apoptosis of myocardial cells. These results suggested that matrine may serve as a potential anti-inflammatory and anti-apoptotic agent in the pathological process of DbCM by downregulating the TGF-β induced p-ERK signaling pathway. Ma et al67 demonstrated that matrine treatment resulted in reduced rates of atrial fibrillation (AF) inducibility and shorter AF duration in myocardial infarction (MI) rats. They also observed improved left atrial conduction velocity and homogeneity and decreased fibrosis positive areas and protein levels of type I collagen and type III collagen in the left atrium. In vitro, matrine inhibited cardiac fibroblast proliferation, migration, secretion, and differentiation to myofibroblasts, as well as the expression of TGFβ-1 and MMP-9. These data indicated that matrine may reduce atrial fibrosis through the TGF-β signaling pathway and thus decrease susceptibility to AF after MI.
Yu et al68 found that treating liver fibrosis model rats with matrine significantly reduced the expression of TGF-β1, enhanced the activity of hepatocyte growth factor (HGF), and alleviated liver tissue damage, cell necrosis and connective tissue hyperplasia in collect abbacy, and there is a trend of obvious improvement, which clearly indicates its inhibitory effect on liver fibrosis in rats. Li et al69 designed and synthesized a series of matrine derivatives, among which compound 3F displayed the strongest anti-fibrotic activity, significantly inhibited the fibroblast-to-myofibroblast transition and extracellular matrix (ECM) production of the human embryonic lung fibroblast (HELF) MRC-5 cell lines, as well as the fibroblasts migration induced by TGF-β1; while the cytoplasm-to-nuclear translocation of Smad2/3 and TGF-β1-induced upregulation of TGFβR1 were also inhibited by 3F. These data suggested that 3F may be a potential drug to treat idiopathic pulmonary fibrosis (PF) by inhibiting the TGFβ/Smad signaling pathway. Zhang et al70 construct a PF model of HELF induced by TGF-β1, and characterized the effect of ginkgo biloba leaf extract (GBLE), shenmai (S), and matrine on PF. The results showed that all three preparations significantly inhibited the proliferation and induced apoptosis of lung fibroblasts, suppressed the synthesis and secretion of collagen-I/III and α-smooth muscle actin (α-SMA), but promoted superoxide dismutase 3 (ECSOD) synthesis and secretion. Among them, matrine exhibited the strongest activity in reducing collagens synthesis, possibly by inhibiting the expression of collagen genes. These data indicated that GBLE, S, and matrine inhibited the pro-fibrotic effects of TGF-β1 by targeting different steps in TGF-β1-mediated fibrosis. Liu et al71 treated the pancreatic fibrosis model rats with matrine and found that the expression of TGF-β1, α-SMA, collagen I, Smad2, TGFβR1, and TGFβR2 was significantly reduced in the matrine treatment group, suggesting that matrine inhibited pancreatic fibrosis by modulating the TGF-β/Smad signaling pathway. Overall, these findings provided useful information for the further development of matrine-based anti-fibrotic agents.
Heterotopic ossification (HO) is a debilitating disease characterized by ectopic bone formation within extraskeletal tissues, and active TGF-β contributes to trauma-induced HO. Mao et al72 found that matrine significantly inhibited TGF-β-induced mesenchymal stem cells (MSCs) migration, alkaline phosphatase (ALP) activity and osteogenic differentiation, reduced ossification in achilles tendon puncture mice, and inhibited HO progression. Mechanistically, matrine inhibited TGF-β induced Smad2/3 phosphorylation and the transcription of RUNX family transcription factor 2 (Runx2), ALP, and osteocalcin (Ocn) after osteoinduction, indicating that matrine can inhibit the activation of TGF-β/Smad2/3 signaling pathway in HO, and is expected to be a candidate for HO treatment.
Moreover, matrine has been proven to be an effective medication for the treatment of eczema, but the under mechanism remains unclear. Zhang et al73 used a matrine derivative, oxymatrine to treat eczema guinea pigs, and showed that oxymatrine treatment alleviated the symptoms of eczema, inhibited the expression of pro-inflammatory factor proteins, increased Th1 and CD4+TGFβ+ levels, and regulated the expression levels of interferon gamma (IFN-γ) and TGF-β, indicating that matrine can mediate the Th1/Th2 balance to treat eczema by upregulating IFN-γ and downregulating TGF-β levels.
We have summarized the relevant studies on the pharmacological effects of matrine through the TGF-β signaling pathway in Table 2.
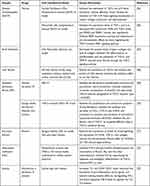 |
Table 2 The Relevant Studies on the Pharmacological Effects of Matrine Through the TGF-β Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the NF-κB Signaling Pathway
NF-κB is a family of key nuclear transcription factor proteins that includes C-REL, RELB, RelA/p65, NF-κB1 (p50/p105), and NF-κB2 (p52/p100). Mediation of the NF-κB signaling pathway affects a variety of biological processes including innate and adaptive immunity, inflammation, the stress response, differentiation, apoptosis, and tumorigenesis.74
When unstimulated, NF-κB binds to its inhibitor, IκB, forms a complex in the form of homo- or heterodimers, and remains inactive in the cytoplasm of almost all cell types.75 Only when the cell is stimulated or under stress, upstream signals directly activate IκB kinase (IKK) to promote phosphorylation of IκB, and then, the p-IκB leads to its own ubiquitination and is rapidly degraded and released by proteases, thereby releasing the inhibitory effect of IκB and dissociating NF-κB from the NF-κB-IκB complex. Mediated by nuclear localization signals, NF-κB is then translocated into the nucleus to bind to specific DNA sequences in the promoter regions of genes and promotes downstream target genes transcription and protein synthesis, participating in various pathophysiological reactions such as infection, inflammation, and the immune response.74,75
Zhang et al76 investigated the effect of matrine on lipopolysaccharide (LPS)-induced lung injury, and found that matrine significantly reduced the mortality of mice with LPS administration, improved LPS-induced lung histopathologic changes, alleviated pulmonary edema and lung vascular leakage, inhibited MPO and MDA activity, and reduced the production of inflammatory mediators such as TNF-α, IL-6, and high mobility group box 1 (HMGB1). In vitro, matrine reduced the production of ROS and inflammatory factors, which may be related to the inhibition of NF-κB. These data demonstrated that matrine exerts a protective effect against LPS-induced acute lung injury by inhibiting the inflammatory response, which may involve the suppression of ROS and tissue oxidative stress-mediated by the NF-κB signaling pathway.
Shao et al77 observed that matrine effectively inhibited the proliferation of three breast cancer cell lines: estrogen receptor (ER)-positive MCF7 cells, HER2-positive BT-474 cells, and highly metastatic MDA-MB-231 cells, and significantly reduced the expression of IKKβ, suggesting that matrine may be an effective candidate drug for the treatment of breast cancer by inhibiting NF-κB signaling pathway.
Yu et al78 demonstrated that matrine effectively inhibited the invasion of HCC SMMC-7721 cells and the expression of MMP-9 and NF-κB, and the use of NF-κB inhibitor significantly reduced MMP-9 expression, suggesting that matrine may downregulate MMP-9 by regulating the NF-κB signaling pathway, thereby inhibiting the invasion of HCC.
Studies by Huang et al79 showed that matrine significantly inhibited the migration and invasion ability of pancreatic cancer PAN-1 cells, decreased intracellular ROS levels, upregulated E-cadherin expression, and downregulated N-cadherin, Vimentin, MMP-2, and MMP-9 expression, as well as the levels of p-p65 and p-IκBα. These data indicated that matrine may inhibit the invasion and epithelial-to-mesenchymal transition (EMT) of pancreatic cancer cells through the ROS/NF-κB/MMPs pathway, further validating the anti-metastasis effect of matrine. Jung et al80 also evaluated the potential of matrine to affect tumor metastasis and found that matrine downregulated the phosphorylation and nuclear translocation of NF-κB-p65 in lung cancer (A549), prostate cancer (DU145), and pancreatic cancer (MIAPACA-2) cells, and effectively inhibited the cellular invasion and metastasis through significantly affecting the expression levels of C-X-C motif chemokine receptor 4 (CXCR4), MMP-2, MMP-9, and NF-κB, and may considered as a potential candidate for anti-tumor metastasis.
To date, the therapeutic efficacy of matrine on prostate cancer remains poorly understood. Li et al81 treated prostate cancer DU-145 and PC-3 cells with matrine, and found that cell proliferation, migration and invasion were significantly inhibited in a dose- and time-dependent manner, the levels of p65, IKK-α/β, IκB-α and corresponding phosphorylation were all decreased, suggesting matrine may exert an anti-tumor effect by regulating the NF-κB signaling pathway, and is expected to be a promising supplement for androgen-dependent prostate cancer drug. Huang et al82 explored the anti-metastatic effects of matrine on castration-resistant prostate cancer (CRPC) and found that matrine inhibited the growth of DU145 and PC3 cells time- and dose-dependently both in vitro and in vivo, and suppressed cell migration and invasive ability. In addition, the expression levels of MMP-2, MMP-9, NF-κB subunit p65 and p-p65 in both cell lines were significantly down-regulated, and it was finally demonstrated that matrine inhibited the migration and invasion of CRPC by downregulating MMP-2/9 through the NF-κB pathway. These data reveal a potential novel application of matrine in anti- CRPC metastatic therapy.
Luo et al83 investigated the anti-GC activity of matrine and found that matrine dose-dependently inhibited the GC cell line MNK45, the expression of NF-κB, X-linked inhibitor of apoptosis (XIAP), cellular inhibitor of apoptosis 1 (CIAP), and p-ERK were also markedly reduced under the action of matrine, indicating the matrine’s anti-tumor effect is achieved by modulation of these proteins, which may serve as an effective potential anti-GC agent.
Multidrug resistance is currently the major obstacle to cancer chemotherapy. Chen et al84 evaluated the reversal effect of matrine on chemoresistant leukemia K562/ADR cells and found that matrine enhanced the cytotoxicity of cancer medicines on K562/ADR cells and apoptotic rate induced by doxorubicin, and inhibited the expression of drug-exporting factor ATP binding cassette subfamily B member 1 (ABCB1) and the phosphorylation of NF-κB. These results indicated that matrine can downregulate the expression of ABCB1 by inhibiting NF-κB, thereby reducing drug transport and promoting the endogenous apoptosis pathway, which may be an effective sensitizer for anti-tumor drug resistance.
Yi et al85 evaluated the anti-metastatic effect of matrine on osteosarcoma and showed that matrine inhibited proliferation of osteosarcoma cells in vivo and in vitro, inhibited tumor cell metastasis in vitro, downregulated the expression of MMP-2 and MMP-9, and reduced nuclear translocation of p50 and p65, as well as phosphorylation of IκB-β and ERK 1/2. The results showed that matrine inhibited the invasion and proliferation of human osteosarcoma cells by downregulating the NF-κB signaling pathway.
Matrine has been used in anti-inflammatory and anticancer treatments for a long time. Jiang et al86 explored the potential application of matrine in the treatment of spinal cord injury (SCI) and found that matrine pretreatment partially attenuated LPS-induced loss of viability, apoptotic stimulation, and release of pro-inflammatory factors (IL-1β, IL-6, and TNF-α) in neuron-like PC12 cells, reversed LPS-reduced miR-9 levels. In addition, matrine also inhibited the activation of c-Jun-n-terminal kinase (JNK) and NF-κB pathways under LPS conditions, and miR-9 silencing attenuated the effects of matrine on these signal transductions. This study suggested that matrine can protect nerve cells from LPS-induced inflammatory damage by regulating miR-9 expression and activating JNK and NF-κB signaling pathways.
The signaling pathways induced by inflammatory mediator HMGB1 have been reported to play important roles in the pathogenesis of multiple sclerosis (MS). Matrine has the capacity to effectively inhibit MS animal model with experimental autoimmune encephalomyelitis (EAE), but its effect on HMGB1-induced signaling remains unclear. Chu et al87 showed that matrine treatment alleviated the severity of EAE, decreased inflammatory infiltration and demyelination, as well as the production of inflammatory factors in the CNS, including TNF-α, IL-6, and IL-1β, significantly reduced the expression of HMGB1 and toll like receptor 4 (TLR4) in the spinal cord, especially in astrocytes and microglia/infiltrating macrophages, but increased the level of IκB-α, confirming the direct inhibitory effect of matrine on HMGB1/TLR4/NF-κB signaling in macrophages. These findings demonstrated that matrine treatment alleviated the inflammatory demyelination and activation of astrocytes and microglia/macrophages in the CNS of EAE rats, and the mechanism may be closely related to the modulation of HMGB1/TLR4/NF-κB signaling pathway.
Previous studies have demonstrated that matrine can alleviate brain edema induced by focal cerebral ischemia. Xu et al88 found that matrine reduced infarction volume and improved neurological deficit in a dose-dependent manner in the model of permanent middle cerebral artery occlusion (pMCAO), and further observed that matrine increased the level of IκBα protein, blocked the translocation of NF-κB p65 from the cytoplasm to nucleus in ischemic cortex and injured neurons and astrocytes induced by oxygen-glucose deprivation (OGD) in vitro, and downregulated NF-κB p65 downstream pro-apoptotic genes p53 and/or c-Myc (MYC proto-oncogene, bHLH transcription factor). These results suggested that matrine directly protected neurons and astrocytes from focal cerebral ischemia injury by inhibiting the NF-κB signaling pathway.
Porcine circovirus associated diseases (PCVAD) is a multifactorial disease that is quite common in the world swine industry. Porcine reproductive respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) are the most common pathogens of PCVAD, there is currently no effective treatment. A previous study by Sun et al89 showed that matrine treatment inhibited PRRSV and PCV2 infection, significantly downregulated the expression of TLR3, TLR4, and TNF-α, and suppressed the expression of p-IκBα to a certain extent, indicating that the antiviral efficacy of matrine may be partly mediated by regulating the TLR3, 4/NF-κB/TNF-α signaling pathway. Although matrine is known to have dual effects of antiviral and anti-inflammatory, the specific molecular mechanism remains unclear. Sun et al90 established an inflammatory model by co-stimulating porcine alveolar macrophages (PAMs) with PRRSV 5′UTR RNA and LPS to study the specific anti-inflammatory mechanism of matrine, and found that matrine treatment strongly inhibited the expression and secretion of IL-1β, IL-6, IL-8, and TNF-α, inhibited the expression of MYD88 innate immune signal transduction adaptor (MyD88), NLR family pyrin domain containing 3 (NLRP3) and caspase-1 and the phosphorylation of IκBα, inhibited the speck formation of apoptosis-associated speck-like protein (ASC), and interfered the translocation of NF-κB from the cytoplasm to the nucleus. These results suggested that matrine played an important role in inflammatory response by affecting NF-κB and the NLRP3 inflammasome, and laid a foundation for exploring the clinical application of matrine in PRRSV disease.
Wang et al91 investigated the effect of matrine on n-methyl-N’-nitro-n-nitrosoguanidine (MNNG) induced gastric mucosal injury in rats, and found that matrine treatment increased the body weight, drinking water, and diet, significantly reduced the expression levels of VEGF-C, VEGFR3, BAX, caspase-3, TLR4, MyD88, and NF-κB p65, but increased the protein level of Bcl-2 in the gastric mucosa tissues, suggesting that matrine may reduce MNNG- induced gastric mucosa injury in rats by downregulating VEGF-C/VEGFR3 and NF-κB/TLR4 signaling pathways.
Elevated suppressor of cytokine signaling 3 (SOCS3) has been demonstrated to correlate with the severity of asthma. Sun et al92 studied the effect of matrine on the expression of SOCS3 in airway inflammation and found that in ovalbumin (OVA)-induced asthmatic mice, matrine treatment significantly inhibited aryl hydrocarbon receptor (AHR), inflammatory cells infiltration, goblet cell differentiation, and mucogenesis. Further, mechanistic studies showed that matrine downregulated SOCS3, IL-6 and adhesion molecules, abrogated the levels of IL-4 and IL-13, but increase the expression of IFN-γ by inhibiting NF-κB signaling in asthmatic mice and TNF-α -stimulated airway epithelial cells, thereby inhibiting airway inflammation.
RA is a chronic and highly prevalent autoimmune disease characterized by synovial joint hyperplasia and inflammation. Niu et al93 established a rat model of RA and found that matrine treatment significantly mitigated the inflammation and severity of RA. Mechanistically, matrine may reduce the level of Th1 cytokines (IFN-γ, TNF-α, IL-1β) but increase the level of Th2 cytokines (IL-4 and IL-10) by inhibiting the NF-κB signaling pathway in T cells, thereby modulated the imbalance of Th1 and Th2 cytokine responses in RA rats, suggesting that matrine may be a promising drug for RA intervention. Pu et al94 investigated the anti-arthritic effect of matrine on type II collagen-induced arthritis (CIA) in rats and found that matrine significantly suppressed inflammatory response and the destruction of synovial tissue, and inhibited the paw swelling, arthritis indices, and weight loss. In addition, matrine reduced the levels of TNF-α, IL-1β, IL-6, IL-8, IL-17A, MMP-2, MMP-3, and MMP-9, downregulated the expression of p-IκB, cytochrome c oxidase II (Cox-2), and nitric oxide synthase (iNOS), but upregulated the expression of IκB in the synovial tissues of CIA rats. These results suggested matrine possessed an anti-arthritic effect on CIA rats by inhibiting the release of pro-inflammatory cytokines and proteins that promote the NF-κB pathway. Zou et al95 explored the therapeutic efficacy of matrine derivative MASM on RA in CIA mice and on fibroblast-like synoviocytes (FLS) cells, and found that MASM significantly reduced the severity of arthritis in CIA mice. Additionally, MASM induced RA-FLS apoptosis and inhibited the expression of pro-inflammatory cytokines and matrix metalloproteinases (MMP-1, MMP-3, and MMP-13) by inhibiting the phosphorylation of MAPKs and the activation of NF-κB, suggesting that MASM may at least exert therapeutic effects on CIA by blocking the phosphorylation of MAPKs and the activation of NF-κB, and may serve as a potent drug for the treatment of RA.
IL-1β plays an important role in promoting osteoarthritis (OA) lesions, but the underlying anti-inflammatory mechanism of matrine in this process remains unclear. Lu et al96 found that matrine significantly inhibited IL-1β-induced chondrocytes apoptosis and the release of MMP-3 and MMP-13, increased the production of TIMP metallopeptidase inhibitor 1 (TIMP-1), and inhibited the phosphorylation of p38, ERK, JNK and the degradation of IκBα in chondrocytes. These results indicated that matrine played a therapeutic role in OA by inhibiting the activation of MAPK and NF-κB signaling pathways in human chondrocytes, thereby inhibiting IL-1β-induced matrix metalloproteinases.
Jiang et al97 investigated the protective effect of matrine on Staphylococcus aureus lipoteichoic acid (LTA)-induced endometritis, and found that matrine significantly relieved uterine injury in mice with endometritis, and dose-dependently reduced the expression of pro-inflammatory cytokines (TNFα and IL-1β). Besides, matrine inhibited the expression of TLR2 and the activation of its downstream NF-κB in LTA-stimulated bovine endometrial epithelial cells (bEECs). These findings suggested that the favorable effect of matrine on LTA-induced endometritis may be through the negative regulation of NF-κB signaling pathway mediated by TLR2, which may act as a potential anti-inflammatory agent.
Atherosclerosis is a chronic inflammatory disease associated with increased expression of adhesion molecules in vascular smooth muscle cells (VSMCs). Liu et al98 found that matrine inhibited the expression of vascular cell adhesion molecule-1 (VCAM 1) and intercellular adhesion molecule-1 (ICAM 1) in TNF-α stimulated human aortic smooth muscle cells (HASMCs), significantly inhibited the expression of NF-κB p65 and the activation of MAPKs, and matrine also suppressed the production of intracellular ROS. This study demonstrated that matrine inhibited the expression of VCAM 1 and ICAM 1 in TNF-α stimulated HASMCs by inhibiting the production of ROS and the activation of NF-κB and MAPK pathways, which may be used to prevent the progression of atherosclerotic lesions.
We have summarized the relevant studies on the pharmacological effects of matrine through the NF-κB signaling pathway in Table 3.
 |  |  |
Table 3 The Relevant Studies on the Pharmacological Effects of Matrine Through the NF-κB Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the Wnt/β-Catenin Signaling Pathway
The Wnt signaling pathway is a complex network of protein interactions that is highly conserved during species evolution. The Wnt gene was first cloned in mouse breast cancer cells and was named Integration 1 (Int1). Later studies found that the mouse Int1 gene was homologous to the Drosophila wingless gene WG, and the two were collectively named Wnt. Further studies showed that β-catenin is the key effector of the Wnt protein, controlling the classical Wnt/β -Catenin signaling pathway.99
In mammalian cells, the Wnt/β-catenin signaling pathway interacts with a series of cell surface-specific receptors through the ligand-protein Wnt, resulting in the phosphorylation of downstream protein kinases that inhibit the degradation of β-catenin. This results in the accumulation of stable β-catenin in the cytoplasm, which then enters the nucleus and combines with T-cell factor/lymphoid enhancing factor (TCF/LEF) family transcription factors to transmit growth-stimulating signals and initiate the transcription of downstream target genes. The Wnt/β-catenin signaling pathway plays an important role in development, stem cell differentiation, and homeostasis, but abnormal activation or interruption of this signaling is closely related to the occurrence of various types of diseases.100 Currently, the Wnt/β-catenin pathway has become one of the major targets for drug discovery and development.
Dai et al101 explored the effect of matrine on HCC cell proliferation and stemness, and found that matrine inhibited the proliferation and colony formation of HEPG2 and Huh7 cells in a time-concentration-dependent manner, and the mechanism may be to inhibit the activation of Wnt/β-catenin signaling pathway, and then interfere with the expression of tumor stem cell markers epithelial cell adhesion molecule (EPCAM), CD90 and CD133, thereby weakening the malignant biological characteristics such as infinite proliferation and differentiation of HCC cells, and playing an anti-tumor effect.
Eradication of cancer stem cell-like cells (CSCs) is of great significance for the treatment and prevention of cancer. Li et al102 focused on the possible roles of matrine on the self-renewal capacity of non-small cell lung cancer (NSCLC) CSCs and demonstrated that matrine inhibited the properties of CSCs, increased MicroRNA let-7b (Let-7b) levels in a Dicer1-dependent manner, and revealed that Let-7b directly targeted and reduced the expression of CCND1, thereby inactivating the CCND1/Wnt signaling pathway and inhibiting EMT. In addition, matrine enhanced the sensitivity of CSCs to 5-FU and inhibited the accumulation of CCND1 in 5-FU-induced tumor tissues. These results illustrated the role of matrine in inhibiting the properties of NSCLC CSCs and improving the therapeutic effect of 5-FU on NSCLC, and provided new insight for the molecular mechanism of matrine or combined 5-FU in interventing in the expansion of NSCLC CSCs, which is helpful to fulfill the anti-NSCLC effect of matrine. Another study by them103 explored the potential mechanism of matrine in regulating human breast cancer stem cells (BrCSCs) and found that matrine inhibited the differentiation and self-renewal of BrCSCs by down-regulating the expression of Lin-28 homolog A (Lin28A), a well-characterized suppressor of Let-7b, thereby inactivating the Wnt pathway in a Let-7b-dependent manner. Furthermore, matrine increased the sensitivity of BrCSCs to cisplatin inhibition of tumor expansion in vitro and in vivo. These data revealed a novel mechanism by which matrine induced breast cancer regression and chemosensitivity.
Xiao et al104 studied the effect of matrine on breast cancer and found that matrine inhibited the viability of breast cancer 4T1 and MCF-7 cells and induced apoptosis in a dose- and time-dependent manner. In addition, matrine suppressed the growth of 4T1 tumors, promoted apoptosis, inhibited vascular endothelial growth factor expression, and down-regulated the levels of major proteins of the Wnt/β-catenin signaling pathway in vivo, suggesting that matrine can effectively inhibit breast cancer growth by downregulating the Wnt/β-catenin signaling pathway, and may be a potential treatment for breast cancer.
Liu et al105 used matrine to stimulate colon cancer SW480 and SW620 cells and found that matrine upregulated the expression of miR-22, thereby obstructing Wnt/β-catenin and MEK/ERK pathways, significantly inhibiting cell viability, and inducing apoptosis and cell cycle arrest at G0/G1 phase, while suppression of miR-22 reversed the effect of matrine on colon cancer cell behaviors, revealing the antitumor activity of matrine in colon cancer cells.
Ma et al106 explored the mechanism of matrine inhibiting the migration and invasion of human pancreatic cancer cell, and demonstrated that matrine inhibited the proliferation of human pancreatic cancer HPAC and Capan-1 cells in a dose-effect manner within a certain concentration range, and markedly inhibited the cell migration and invasion. Moreover, the expression of matrix metallopeptidase 14 (MT1-MMP), MMP-2, MMP-9, Wnt, and β-Catenin significantly decreased upon matrine treatment. Analysis of MT1-MMP transcriptional activity revealed that matrine mediated downregulation of MT1-MMP expression through the Wnt signaling pathway. These findings indicated that matrine inhibited the migration and invasion of HPAC cells by downregulating the Wnt/β-Catenin signaling pathway.
Survivin has been reported to be overexpressed in HCC and promote the malignant progression of HCC. Yin et al107 established a Survivin-targeted drug screening platform, screened and identified a matrine derivative bearing a benzo-α-pyrone structure, WM-127, which was demonstrated to be a strong Survivin inhibitor that inhibited cell proliferation, induced cell cycle arrest and apoptosis of HCC cells, and showed low cytotoxicity in normal cells. Besides, WM-127 also suppressed the growth of HCC xenografted tumors in nude mice. Mechanistically, WM-127 may exert its anti-tumor effect by inhibiting the activity of the Survivin/β-catenin pathway and upregulating Bax expression. These data implied that WM-127 may be a promising chemotherapeutic drug for HCC with favorable safety profile.
Ni et al108 evaluated the effect of a novel matrine derivative WM130 on HCC CSCs, and demonstrated that WM130 significantly and preferentially inhibited hepatic CSCs, while inhibiting the expression of some stemness-related genes and HCC xenografts growth in vivo. Moreover, they indicated that WM130 in combination with Doxorubicin (DOX) exhibits a better inhibitory efficacy. Further mechanistic studies revealed that WM130 administration notably reduced GSK3β (Ser9) phosphorylation and decreased the expression level of β-catenin and its target gene CCND1, as well as the expression of hepatic stem cell biomarker EPCAM, suggesting that the inhibitory effect of WM130 on hepatic CSCs was mediated, at least partly, by inhibiting the Wnt/β-catenin signaling pathway. These findings prompted that WM130 may serve as a novel therapeutic candidate for HCC.
We have summarized the relevant studies on the pharmacological effects of matrine through the Wnt/β-catenin signaling pathway in Table 4.
 |
Table 4 The Relevant Studies on the Pharmacological Effects of Matrine Through the Wnt/β-Catenin Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the MAPK Signaling Pathways
MAPK belongs to a large family of conserved serine-threonine protein kinases that mediate the cascade of important signal transduction pathways from extracellular signals to intracellular reactions in eukaryotic cells. For example, MAPK is phosphorylated and activated by MAPK kinase (MAPKK), MAPKK is phosphorylated and activated by MAPKK kinase (MAPKKK), and MAPKKK is activated by interacting with small GTPase and/or other proteases, thereby linking MAPK with cell surface receptors and extracellular signals to jointly regulate various physiological and pathological processes, such as cell proliferation, differentiation, apoptosis, and the stress response.109
Four MAPK cascade signal transduction pathways have been identified in eukaryotic cells: extracellular signal-regulated protein kinase 1/2 (ERK1/2), JNK/stress-activated protein kinase (SAPK), p38 MAPK, and ERK5 signal transduction pathway. Erk1/2 was the earliest discovered classical Ras-Raf-MAPK signal transduction pathway, which participates in the signal transduction of various growth factors, cytokines, mitogens, and hormone receptors after activation and plays an important regulatory role in cell proliferation, growth, and differentiation. The JNK/SAPK and p38 MAPK signaling pathways are mainly responsible for signal transduction of various stress responses (such as UV, heat shock, and hypertonic pressure), chemotherapy drugs, and stimulation of inflammatory factors, and are involved in the regulation of apoptosis.110 Studies have shown that abnormal or excessive activation of the MAPK signaling pathway plays a significant role in the malignant transformation and evolution of cells.
Tan et al111 investigated the inhibitory effect of matrine on lung cancer cells and confirmed that matrine inhibited NSCLC cell proliferation and induced apoptosis by inhibiting Bcl-2 and activating caspase-3 and PARP. Additionally, matrine significantly increased the phosphorylation of p38, and inhibition of the p38 pathway partially prevented matrine-induced apoptosis. Furthermore, matrine stimulated ROS generation in a dose- and time-dependent manner. These findings indicated that matrine may be a promising NSCLC drug by activating the p38 pathway, inducing ROS production in non-small cell lung cancer cells, and leading to caspase-dependent apoptosis.
Yang et al112 explored the anticancer effect of matrine on human lymphoma cell line (U937) and found that matrine significantly suppressed the proliferation of U937 cell and induced apoptosis in vitro, inhibited the activity of ERK but increased the activities of p38 and JNK, suggesting matrine may promote apoptosis through the MAPK signaling pathway, and further provide experimental evidence for lymphoma chemotherapy and combination therapy.
Studies have shown that the MAPK pathway, a downstream signaling pathway abnormally activated by BCR/ABL tyrosine kinase, plays an important role in the occurrence and development of chronic myelogenous leukemia (CML). Ma et al113 studied the underlying molecular mechanism of the effect of matrine on CML K562 and HL-60 cells and demonstrated that matrine treatment significantly inhibited the expression of BCR/ABL in CML cells, downregulated the phosphorylation levels of MEK1, ERK1/2, and their upstream adaptor molecules Src homology 2 domain (SPH2) and Src homology 2 domain containing protein C (Shc), as well as the expression levels of BCL2-like 1 (Bcl-xL), CCND1, and c-Myc. Moreover, matrine significantly reduced the mortality of human leukemia cells-bearing mice. These results indicated that matrine inhibited the growth of CML cells by inhibiting the ERK/MAPK signaling pathway mediated by BCR/ABL, suggesting that matrine has a certain anti-leukemia activity.
MAPK/ERK has been found to be a crucial signaling pathway promoting endothelial cell proliferation, differentiation and survival. Lu et al114 studied the potential mechanism of matrine against lung cancer angiogenesis and found that matrine significantly inhibited the proliferation, migration, and p-ERK expression of HUVECs induced by conditioned medium of A549 human lung cancer cells, implying that suppressed MAPK/ERK signaling pathway is involved in the mechanism of matrine against angiogenesis in lung carcinoma, providing an experimental basis for the clinical application of matrine in the treatment of lung carcinoma.
A study by Ren et al115 explored the anti-metastatic effect of matrine on colorectal cancer (CRC) and found that matrine inhibited the proliferation, migration and invasion of CRC cells in vitro and in vivo. Additionally, matrine treatment significantly reduced the expression levels and proteinase activities of MMP-2 and MMP-9 in a dose-dependent manner, and decreased the phosphorylation level of p38. It was also found that the combination of p38 inhibitor SB203580 and matrine synergistically reduced CRC cell invasion and MMP-2/9 expression. These findings suggested that matrine may be involved in the inhibition of CRC cell migration and invasion by reducing the expression of MMP-2/9 through the p38 signaling pathway, and may serve as a potential CRC therapeutic drug.
Xin et al116 found that matrine significantly inhibited the viability, migration and invasion of ovarian cancer cells, and induced apoptosis. In addition, matrine treatment significantly upregulated the expression levels of p38MAPK, p-ERK/ERK, and p-JNK/JNK, while p38MAPK knockdown significantly downregulated the expression levels of p-ERK/ERK and p-JNK/JNK, promoting the viability, migration and invasion of ovarian cancer cells. Moreover, they also demonstrated that p38MAPK was involved in matrine-induced apoptosis of ovarian cancer cells by upregulating the expression of Fas cell surface death receptor (Fas) and caspase-8. This study showed that matrine significantly inhibited cell viability, migration and invasion, and induced apoptosis by up-regulating the p38 MAPK-mediated ERK/JNK signaling pathway, and in vivo experiments further verified these findings, which may provide a basis for evaluating the efficacy of matrine in the treatment of ovarian cancer.
Hai et al117 further studied the therapeutic potential of matrine on prostate cancer based on the cancer genome atlas (TCGA) database, and identified growth arrest and DNA damage inducible beta (GADD45B) as one of the major target genes of matrine. GADD45B is a tumor-suppressive gene involved in the regulation of cell cycle, DNA damage repair, cell survival, senescence, apoptosis, and other cellular processes. They found that matrine promoted the expression of GADD45B through the p38/JNK and ROS-GADD45B-p38 signaling pathways, and the higher level of GADD45B indicate the better survival of prostate cancer patients. These findings suggested that matrine can be used to treat prostate cancer patients and improve their survival.
A research by Ma et al118 investigated the effect of matrine against human osteosarcoma MG-63 cells through autophagy, and found that matrine significantly inhibited cell proliferation and induced cell apoptosis, and upregulated the expression of pro-apoptosis-related proteins Bax, LC3II, and p-ERK, and downregulated the expression of anti-apoptotic protein Bcl-2 in a time-dependent manner. Furthermore, matrine induced protective autophagy in MG-63 cells, and demonstrated for the first time that matrine-induced autophagy attenuated apoptosis through the ERK1/2 signaling pathway. This study indicated that matrine combined with autophagy inhibitors (including CHQ) or ERK signaling pathway inhibitors (including U0126) may be a promising therapeutic strategy for osteosarcoma.
Zhou et al119 studied the anti-inflammatory effect of matrine on oxidized low-density lipoprotein (ox-LDL)-exposed macrophages, and found that matrine significantly inhibited the inflammatory response of ox-LDL-induced macrophages, reduced the intracellular ROS levels. In addition, matrine reduced the relative phosphorylation of MAP Kinase Kinase (MKK) 3, MKK6, and p38 MAPK. These results suggested that matrine may inhibit ox-LDL-induced inflammation by inhibiting the activation of MKKs/p38 MAPK signaling pathway, providing evidence and clues for the potential application of matrine in the clinical treatment of atherosclerotic diseases.
AGEs have been reported to mediate diabetic vascular complications, trigger ROS overproduction, and induce apoptosis in aortic endothelium cells both in vitro and in vivo. Liu et al120 indicated that administration of matrine-type alkaloids (ie, matrine, oxymatrine and sophocarpine) suppressed intracellular ROS levels and endothelial cell apoptosis by restoring the phosphorylation of MKK3/6 and the activation of p38 MAPK/Nrf2/ antioxidant response element (ARE) in vitro and in vivo. These findings proved that matrine-type alkaloids have endothelial protective effects on ROS-mediated AGEs-induced apoptosis of aortic endothelial cells by targeting MKK3 and p38MAPK signaling, and provide clues for the potential clinical application of matrine-type alkaloids in the therapy of diabetic vascular complications.
Zhang et al121 explored the effect of matrine on cardiac fibrosis and found that matrine significantly reduced fibrosis reconstruction and cardiac dysfunction induced by aortic banding (AB) operation or continuous injection of isoproterenol (ISO) in mice, and significantly inhibited the proliferation, migration, collagen production and phenotypic transdifferentiation of cardiac fibroblasts. Mechanistically, matrine inhibited the activation of p38 both in vitro and in vivo, but overexpression of p38 eliminated the protective effect of matrine. It was also confirmed that the upregulation of RPS5 was associated with matrine-mediated p38 inhibition and fibrogenesis. What’s more, matrine can improve preexisting cardiac fibrosis in mice. From these results, it can be seen that matrine treatment attenuated cardiac fibrosis in mice by regulating the RPS5/p38 signaling pathway, and may be an effective drug for the treatment of pathological cardiac fibrosis.
Wu et al122 evaluated the anti-tumor effect of matrine in cervical cancer and found that matrine significantly inhibited the growth of cervical cancer cells by inducing apoptosis, and suppressed the invasion and migration ability of cervical cancer cells in a concentration-dependent manner in vitro, while in vivo by intraperitoneal (i.p.) injection of matrine significantly reduced the growth of xenograft tumor. Mechanistically, matrine reduced the expression and activity of the ECM factors, MMP-2 and MMP-9 by inhibiting the p38 signaling pathway. These findings suggested that matrine may suppress the growth and metastasis of cervical cancer by downregulating the p38 signaling pathway, providing a potential drug candidate for the treatment of cervical cancer.
MASM, a matrine derivative, has been reported to inhibit the activation of macrophages, dendritic cells, and hepatic stellate cells, and bind to RPS5. Xu et al123 evaluate the effect of MASM on murine-established lethal sepsis and found that MASM markedly attenuated LPS-induced release and expression of TNFα, IL-6, and NO/iNOS in murine peritoneal macrophages and RAW264.7 cells. Meanwhile, MASM inhibited LPS-induced activation of NF-κB and MAPK pathways. They also found that RPS5, a cellular target of MASM, inhibited LPS-induced inflammatory response, at least partially mediating the anti-inflammatory effects of MASM in vitro. Furthermore, MASM significantly improved overall survival in mice model of sepsis. The mechanism of action of MASM observed in this study may be related to the RPS5-mediated suppression of NF-κB and MAPK activation and the decrease in the expression of proinflammatory mediators, indicating that MASM may be further used in the treatment of sepsis. Li et al124 found a protective effect of MASM against rats with lethal total body irradiation (TBI), and that MASM pretreatment reduced the symptoms of radiation sickness and increased the survival of rats before or after lethal TBI, which may be mainly or partly by modulating multiple MAPK pathways. These results suggested that MASM has the potential to serve as an effective therapeutic or radioprotective agent to minimize radiation damages and improve the efficacy of combined oncology radiotherapy.
The maturation of dendritic cells (DCs) is a crucial step in the development of T-cell immune responses and immune tolerance. Xu et al125 investigated the inhibitory effect of MASM on LPS-induced maturation of mouse bone marrow-derived DCs and found that MASM suppressed the maturation of phenotypic and functional DCs, which was associated with the downregulation of NF-κB, MAPKs, and PI3K/AKT signaling pathways. These findings may provide new insight into the immunosuppressive effects of MASM.
We have summarized the relevant studies on the pharmacological effects of matrine through the MAPKs signaling pathway in Table 5.
 |
Table 5 The Relevant Studies on the Pharmacological Effects of Matrine Through the MAPK Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the Notch Signaling Pathway
The Notch gene was originally identified by Thomas H. Morgan et al in mutant fruit flies and is so named based on its dominant wing notching phenotype. The Notch signaling pathway is an evolutionarily highly conserved transduction system, but unlike TGF-β, Wnt, Hedgehog, and other signaling pathways, activation of canonical Notch signaling is generally initiated by a ligand-binding process to the Notch receptor between two adjacent cells.126
To date, four Notch receptors (Notches 1–4) expressed on the surface of cell membranes and five transmembrane ligands, including delta-like ligand (DLL) 1, 3, and 4, and jagged canonical Notch ligand 1 and 2 (Jagged 1/2), have been described in mammals. After activation, Notch receptors are proteolytically cleaved by two proteases, a disintegrin and metalloprotease (ADAM) and gamma-secretase (GS), followed by cytoplasmic release and nuclear translocation of the notch intracellular domain (NICD), and further binds to the transcription factor CSL and participates in the transcriptional modulation of downstream target genes.127 The Notch signaling pathway governs many cellular core processes including cell fate and plays a crucial regulatory role in diverse biological processes such as the development, differentiation, survival, and functions of many immune cell populations.
Contractile-synthetic phenotypic conversion of VSMCs results in the formation of atherosclerotic plaque through abnormal synthesis, secretion, and deposition of ECM. Liu et al128 investigated the effect and underlying mechanism of matrine on AGE-induced VSMC contractile-synthetic phenotypic conversion. They found that matrine restored AGE-induced contractile-synthetic phenotypic conversion by increasing intracellular MYH11 and smooth muscle α-actin (ACTA2) in HCSMCs, reduced AGE-mediated activation of Notch signaling, and downregulated the expression levels of NICD1, Hes family basic helix-loop-helix (bHLH) transcription factor 1 (HES1), collagen I, as well as collagen VIII and collagen secretion. Moreover, matrine treatment inhibited the expression of Dll4 without affecting other Notch ligands in HCSMCs exposed to AGEs. These results indicated that matrine blocked AGE-induced HCSMC phenotypic conversion by suppressing the activation of the Dll4-Notch signaling pathway. Zhao et al129 also carried out relevant studies and confirmed that matrine inhibited AGE-induced phenotypic transformation of HCSMCs in a concentration-dependent manner and reduced the expression levels of glucose-regulated protein 78 (GRP78), Dll4, NICD1, and HES1 and the phosphorylation level of protein kinase RNA-like ER kinase (PERK). This suggested that matrine suppressed AGE-induced HCSMC phenotypic conversion by attenuating endoplasmic reticulum stress PERK signaling-dependent Dll4-Notch signaling pathway activation. These studies provide a theoretical basis for the clinical application of matrine in the treatment of arteriosclerosis.
In vivo administration of matrine was observed to promote oval cell-mediated liver regeneration in rats, suggesting that matrine may affect the differentiation of hepatic progenitor cells. To further investigate the effect of matrine on the differentiation of rat hepatic progenitor WB-F344 cells, Yang et al130 collected rat serum after administering matrine and co-cultured WB-F344 cells with rat serum. Matrine serum was found to inhibit the proliferation of WB-F344 cells, downregulate Jagged1 and HES1, upregulate albumin (ALB), induce WB-F344 cells differentiation; and also found that Notch signaling pathway activator reversed the effects of matrine serum. These findings indicated that matrine may induce differentiation of hepatic progenitor cells by inhibiting the Notch/Jagged1/HES1 signaling pathway.
Shi et al131 found in HCC patients, the hyperactive Notch pathway was closely related to poor liver function, and confirmed that the Notch pathway could maintain stem cell properties of hepatic oval cells (HOCs). In addition, matrine inhibited the stem cell properties of HOCs, inhibited Notch1, Jagged1, HES1, and the HOC markers Alpha-fetoprotein (AFP), cytokeratin19 (CK-19), and C-Kit (KIT proto-oncogene, receptor tyrosine kinase) expression, but increased ALB secretion in mature hepatocytes. In the rat Solt-Farber precancerous lesion model, prophylactic matrine attenuated liver injury, downregulated the Notch pathway and the expression of HOC markers, and dose-dependently upregulated ALB. These suggested that matrine can induce HOCs to differentiate into hepatocytes by inhibiting the Notch signaling pathway and alleviate liver injury, providing a novel strategy for the treatment of severe liver damage and the prevention of HCC.
Shi et al132 established a rat model of drug-resistant hepatocyte and further explored the effect of matrine on the early changes of liver carcinogenesis. They indicated that matrine treatment significantly inhibited the development of precancerous lesions, including restoration of hepatic cord structure, reduction of hepatic edema and degeneration, and changes in AFP and ALB expression. In addition, the activation of Notch signaling pathway was detected in the model, while the application of high-dose matrine significantly reduced the expression levels of Notch pathway components Notch1 and HES1, indicating that matrine may prevent the early development of HCC-like lesions by suppressing the activation of Notch signaling pathway, and may be an effective chemopreventive agent for HCC.
We have summarized the relevant studies on the pharmacological effects of matrine through the Notch signaling pathway in Table 6.
 |
Table 6 The Relevant Studies on the Pharmacological Effects of Matrine Through the Notch Signaling Pathway |
Matrine Exerts Pharmacological Effects Through the JAK/STAT Signaling Pathway
The JAK/STAT signaling pathway is an evolutionarily conserved signaling cascade that is essential for numerous developmental and homeostatic processes. The pathway communicates information from extracellular chemical signals to receptors that permit their associated intracellular JAKs to phosphorylate one another. Trans-p-JAKs then phosphorylate and activate downstream STATs, which are transported into the nucleus, bind as dimers or as more complex oligomers to specific enhancer sequences in target genes, and regulate their transcription.133 There are three key parts of JAK/STAT signaling: JAKs, STATs, and tyrosine kinase-related receptors (which bind the chemical signals). These signaling pathways are involved in cell proliferation, differentiation, apoptosis, immune regulation, and hematopoiesis, as well as other cellular processes.134 Disrupted JAK/STAT signaling may lead to a variety of diseases, such as skin conditions, cancers, and disorders affecting the immune system.
Ma et al135 instilled bleomycin intratracheally in C57BL/6 mice to establish a PF model and then administered matrine intragastrically. They found that matrine treatment significantly relieved the severity of alveolitis and PF, inhibited the growth of pulmonary fibrocytes, and downregulated the abnormal expression of JAK, STAT1, and STAT3, showing that matrine exerts its anti-fibrotic effect by inhibiting the JAK-STAT signal transduction pathway, which may provide a new target for PF treatment.
Ma et al136 investigated the growth inhibitory effect of matrine on CML K562 cells and found that matrine significantly inhibited the phosphorylation levels of STAT3 and JAK2, inhibited the transcription and protein expression of downstream regulatory genes Bcl-xL, CCND1 and c-Myc of STAT3, and inhibited the IL-6 expression and secretion. Furthermore, matrine treatment decreased the upregulation of STAT3, JAK2, p-STAT3, and p-JAK2 protein in K562 cells after IL-6 pretreatment. These findings suggested that matrine exerts its anti-leukemia effect by interfering with the JAK/STAT3 signaling pathway, and inhibiting the expression of IL-6 may be the initial mechanism of matrine regulated JAK/STAT3 signaling. Lu et al,137 who belong to the same group as Ma et al, showed that matrine induced the expression of an killer cell lectin like receptor K1 (NKG2D) ligand UL16 binding protein 2 (ULBP2) in CML K562 cells and enhanced natural killer (NK) cell cytotoxicity, which is related to the inhibition of IL-6 expression, which in turn suppressed the activation of IL-6 receptor-mediated JAK/STAT3 signaling pathway. These studies provided useful evidence for further elucidating the underlying mechanism of matrine in the treatment of leukemia, as well as its clinical application.
Resistance to EGFR inhibitors is a major threat to patients with EGFR-mutant NSCLC. Chen et al138 demonstrated that matrine treatment reduced the expression of IL-6, inhibited the activation of JAK1/STAT3 signaling pathway, and restrained the growth of T790M EGFR-mutated NSCLC H1975 cells, while afatinib and matrine combination strengthened the inhibitory effects of afatinib, indicating that matrine enhanced the toxicity of afatinib toward H1975 cells by suppressing the activation of the IL-6/JAK1/STAT3 signaling pathway. Combination of matrine and afatinib in patients with T790M EGFR-mutant NSCLC may be a successful treatment strategy.
Excessive proliferation of FLS and intrinsic resistance to apoptosis are significant mechanisms for the occurrence of RA. Nevertheless, the effect of matrine on FLS in RA is unclear. Yang et al139 explored the effect of matrine in the CIA rat model and found that matrine significantly reduced the arthritis index (AI) and improved ankle pathology, inhibited the proliferation of FLS in CIA rats, induced G0/G1 cell cycle arrest and apoptosis. In addition, matrine treatment also suppressed the phosphorylation of JAK2, STAT1, and STAT3. It is suggested that matrine exerted anti-proliferative and pro-apoptotic effects on FLS by inhibiting the activation of JAK/STAT signaling pathway, and may serve as a novel RA therapeutic agent.
We have summarized the relevant studies on the pharmacological effects of matrine through the JAK/STAT signaling pathway in Table 7.
 |
Table 7 The Relevant Studies on the Pharmacological Effects of Matrine Through the JAK-STAT Signaling Pathway |
Matrine Exerts Pharmacological Effects Through Other Signaling Pathways
The AMP-activated protein kinase (AMPK) signaling pathway is a fuel sensor and regulator that promotes ATP-production and inhibits ATP-consuming pathways in various tissues, and also plays an important role in the regulation of cellular energy homeostasis.140,141 It is therefore a potential target in the development of new treatments for obesity, type II diabetes, metabolic syndrome, and cancer.142 Lu et al143 explored the cardioprotective effect of matrine on cardiomyocyte damage under hypoxia/reoxygenation and found that matrine significantly increased cardiomyocyte viability and inhibited apoptosis after hypoxia/reoxygenation. In addition, matrine administration upregulated Sirtuin 3 (Sirt3) and AMPK expression and activity in the presence of hypoxia/reoxygenation, where inhibition of the Sirt3/AMPK pathway abolished the cardioprotective effect of matrine in cardiomyocytes. These findings demonstrated that matrine reduced hypoxia/reoxygenation-mediated cardiomyocyte death by activating the Sirt3/AMPK pathway, which may provide novel insight into the treatment of myocardial ischemia-reperfusion injury. Xie et al144 found that matrine inhibited proliferation and induced apoptosis in hepatoma cells and stimulated autophagy in HCC HepG2 cells in an mTOR-independent manner. Further studies showed that autophagy induced by matrine was regulated by p53 inactivation through AMPK signal transduction, and inhibition of AMPK converted autophagy to apoptosis. These results suggested that the p53/AMPK signaling pathway is involved in matrine-induced autophagy, and pharmacological regulation of autophagy may improve the therapeutic effect of matrine against HCC.
Mitochondrial homeostasis is closely related to the progression of HCC through multiple mechanisms and is a potential tumor suppressor target in clinical practice. Cao et al145 investigated the effect of matrine on mitochondrial homeostasis in HCC HepG2 cells and showed that matrine treatment activated mitochondrial fission, which promoted mitochondrial dysfunction and initiated apoptotic pathways to inhibit cell proliferation. Moreover, they verified that the macrophage stimulating 1 (MST1)/JNK signaling pathway was necessary for matrine-modulated mitochondrial fission and that matrine-mediated mitochondrial dysfunction can be reversed by inhibiting this pathway. These results suggested that matrine has an anti-HCC effect via activating the mitochondrial division mediated by the MST1/JNK pathway. Yang et al146 established a rat oval cell-mediated liver regeneration model and found that matrine accelerated the recovery of hepatic function and decreased the expression of the OV6 protein (oval cell marker), recombination signal sequence-binding protein Jκ (RBP-Jκ), and HES1, suggesting that matrine promoted oval cell-mediated liver regeneration through downregulation of the RBP-Jκ/HES1 signaling pathway. These findings highlight new strategies for potential targets and treatment of HCC.
Lu et al147 studied the effect of matrine on lung adenocarcinoma A549 cells and found that matrine inhibited the proliferation and migration ability of A549 cells, which may be associated with the induction of p53 and p21 overexpression and the reduction of the expression of proliferating cell nuclear antigen (PCNA) and eukaryotic translation initiation factor 4E (eIF4E), indicating the p53/p21/PCNA/eIF4E signaling pathway may be a new discovery in the treatment of lung adenocarcinoma with matrine. Abnormalities in the c-Myc gene make the clinical manifestations of diffuse large B-cell lymphoma (DLBCL) more aggressive. Gu et al148 found that the growth inhibitory effect of matrine on human B lymphoma SU-DHL-16 cells could be rescued by overexpression of c-Myc via recombinant adenovirus infection, the ectopic expression of c-Myc protein rescued the decreased expression of CDK6, and matrine treatment downregulated the phosphorylation of calcium/calmodulin dependent protein kinase II gamma (CaMKIIγ), a critical protein kinase of c-Myc, confirming that matrine suppressed cell growth of DLBCL by inhibiting te CaMKIIγ/c-Myc/CDK6 signaling pathway. Studies suggest that the dysregulation of the JNK pathway and neurotrophic factors play a key role in the pathogenesis of neurobehavioral disorders (anxiety and depression). Khan et al149 observed that matrine treatment ameliorated anxiety- and depression-like emotional states in an acute burn injury mouse model by modulating JNK-mediated inflammation, oxidative stress, apoptosis, and brain-derived neurotrophic factor (BDNF)/VEGF signaling pathway, targeting the JNK and BDNF/VEGF signaling by matrine may represent a novel strategy for the treatment of neurobehavioral disorders.
We have summarized the relevant studies on the pharmacological effects of matrine through other signaling pathways in Table 8.
 |
Table 8 The Relevant Studies on the Pharmacological Effects of Matrine Through Other Signaling Pathways |
Conclusions
Researchers have carried out many beneficial studies on the roles and molecular mechanisms of matrine in the treatment of different pathological processes. Matrine acts on tissues and cells through multiple signaling pathways, exerting pharmacological functions such as anti-tumor, anti-inflammatory, and anti-fibrosis. Studies have reported that matrine has a strong inhibitory effect on various tumor cells, and its molecular mechanism lies in regulating cell cycle progression, promoting apoptosis, inhibiting tumor invasion and metastasis, inducing autophagy, reversing multidrug resistance in tumor cells, and regulating metabolism through mediating multiple cellular signaling pathways such as the PI3K/AKT/mTOR,13–43 NF-κB,77–85 Wnt/β-catenin,101–108 MAPKs,111–118 Notch,131,132 and JAK-STAT136–138 pathways. Non-coding RNAs also play an important role in these events,16,19,22,41,42,102,103,105 indicating that the anti-tumor mechanisms of matrine involve multi-pathways, multi-targets, and multi-systems. In addition, matrine has anti-inflammatory effects in a variety of organ system diseases such as those of the cardiovascular, cerebrovascular, liver, kidney, and immune systems. Its mechanism of action may be related to blocking inflammatory signaling pathways induced by PI3K/AKT/mTOR,44,47,49 NF-κB,76,86,87,90,92–95,97,98 and p38/MAPKs,119 ultimately inhibiting the release of inflammatory factors (IL-1β, IL-6, IL-8, TNF-α, etc.) and the expression of matrix metalloproteinases (MMP-1, MMP-2, MMP-3, MMP-9 and MMP-13, etc.), promoting the antioxidant-related Nrf2 signaling pathway to inhibit oxidative damage and improving the antioxidant capacity of the body. TGF-β is an important factor driving fibrosis, and the dysregulation of the TGF-β/Smad signaling pathway is the main pathogenesis of fibrosis in the liver, lung, kidney, and myocardium.63,64 The anti-fibrotic effect of matrine is mainly mediated through the inhibition of the TGF-β/Smad signaling pathway, promoting the activity of cell growth factors, reducing apoptosis and collagen synthesis, and inhibiting fibrosis progression.65,67–71 Additionally, matrine can also exert anti-fibrotic effects by inhibiting the activation of p38 and the JAK-STAT signal transduction pathway,121,135 providing a basis for the development of anti-fibrotic drugs. By blocking multiple signaling pathways such as NF-κB, PI3K/AKT, and MAPK, matrine inhibited osteoclast formation and may therefore be a promising drug for the treatment of osteoporosis and other related diseases.50,72 The antiviral effects of matrine are related to NF-κB and other signaling pathways,89 but its clinical antiviral activity still requires further exploration. Some studies have proved that matrine exerts antidepressant effects through different mechanisms,51,149 further expanding our understanding of the pharmacological actions of matrine.
Over the past few decades, matrine has attracted tremendous interest in the medical community due to its widespread biological activity and therapeutic effects. However, matrine has only moderate bioactivity and certain toxic and side effects, which limit its further application; but it is considered to be an ideal lead compound for further modification since its good solubility, intriguing structure and favorable safety. Recently, in order to ameliorate the bioactivity and extend its applicability, a series of matrine derivatives have been synthesized by focusing on the structural modifications such as the lactam ring alteration, D-ring variation and structural simplification, leading to the less toxic effects, better drug activity, and improved pharmacokinetic properties.150 Literature analysis found that these novel derivatives performed more significant effects in inhibiting tumor cell proliferation, invasion and metastasis,52–58,107 targeted killing tumor stem cells,108 treating osteoporosis,59,60 and anti-inflammatory,123 and the mechanisms may be related to the regulation of the PI3K/AKT/mTOR, NF-κB, and Survivin/β-catenin signaling pathways. Overall, novel modificatory strategies of matrine should be developed to explore more potent bioactive derivatives and further reveal their molecular mechanisms of action in the future.
Matrine interferes with multiple intracellular signaling pathways and regulates multiple target genes systematically, thus achieving anti-tumor and other pharmacological effects. These effects fully embody the advantages of the systemic, omnidirectional, multi-angle, and multi-target mechanisms of the treatment of diseases by traditional Chinese medicine preparations. However, current studies on the pharmacological mechanisms of matrine tend to focus on linear and single signaling pathways, and exploratory studies on the interaction network of different targets and signaling pathways remain rare. With the in-depth development of network pharmacology and holistic pharmacology, and the active use of gene chips and proteomics technology, the pharmacological mechanisms of matrine will be further clarified, and its signal transduction regulatory networks and synergistic mechanisms will be revealed. This will provide more theoretical basis for the design of effective drug targets at the molecular level, as well as theoretical support for the further expansion of the clinical application of matrine.
Acknowledgment
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20200936), the Natural Science Research Project of the Higher Educational Institutions of Jiangsu Province (20KJB320007), and the Yangzhou Science and Technology Planning Project (YZ2021082).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Li X, Tang Z, Wen L, et al. Matrine: a review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches. J Ethnopharmacol. 2021;269:113682. doi:10.1016/j.jep.2020.113682
2. Zhang H, Chen L, Sun X, et al. Matrine: a promising natural product with various pharmacological activities. Front Pharmacol. 2020;11:588. doi:10.3389/fphar.2020.00588
3. Rashid HU, Xu Y, Muhammad Y, et al. Research advances on anticancer activities of matrine and its derivatives: an updated overview. Eur J Med Chem. 2019;161:205–238. doi:10.1016/j.ejmech.2018.10.037
4. Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol. 2012;13(3):195–203. doi:10.1038/nrm3290
5. Fruman DA, Chiu H, Hopkins BD, et al. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi:10.1016/j.cell.2017.07.029
6. Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi:10.1016/S0960-9822(06)00122-9
7. Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi:10.1016/j.cell.2007.06.009
8. Fayard E, Xue G, Parcellier A, et al. Protein kinase B (PKB/Akt), a key mediator of the PI3K signaling pathway. Curr Top Microbiol Immunol. 2010;346:31–56. doi:10.1007/82_2010_58
9. Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. doi:10.1038/s41580-019-0199-y
10. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi:10.3389/fonc.2014.00064
11. LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34(31):3803–3815. doi:10.1200/JCO.2014.59.0018
12. Yang J, Nie J, Ma X, et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. doi:10.1186/s12943-019-0954-x
13. Niu H, Zhang Y, Wu B, et al. Matrine induces the apoptosis of lung cancer cells through downregulation of inhibitor of apoptosis proteins and the Akt signaling pathway. Oncol Rep. 2014;32(3):1087–1093. doi:10.3892/or.2014.3273
14. Wan Q, Du Z, Fang Z, et al. Matrine induces apoptosis and autophagy in human lung adenocarcinoma cells via upregulation of Cavin3 and suppression of PI3K/AKT pathway. J BUON. 2020;25(3):1512–1516.
15. Xie W, Lu J, Lu Q, et al. Matrine inhibits the proliferation and migration of lung cancer cells through regulation of the protein kinase B/glycogen synthase kinase-3 beta signaling pathways. Exp Ther Med. 2018;16(2):723–729. doi:10.3892/etm.2018.6266
16. Liao H, Zhao X, Qu J, et al. Matrine suppresses invasion and metastasis of NCI-H1299 cells by enhancing microRNA-133a expression. Int J Clin Exp Med. 2015;8(7):10714–10722.
17. Du J, Li J, Song D, et al. Matrine exerts antibreast cancer activity by mediating apoptosis and protective autophagy via the AKT/mTOR pathway in MCF7 cells. Mol Med Rep. 2020;22(5):3659–3666. doi:10.3892/mmr.2020.11449
18. Yu P, Liu Q, Liu K, et al. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology. 2009;59(3):219–229. doi:10.1007/s10616-009-9225-9
19. Li LQ, Li XL, Wang L, et al. Matrine inhibits breast cancer growth via miR-21/PTEN/Akt pathway in MCF-7 cells. Cell Physiol Biochem. 2012;30(3):631–641. doi:10.1159/000341444
20. Wang L, Gao C, Yao S, et al. Blocking autophagic flux enhances matrine-induced apoptosis in human hepatoma cells. Int J Mol Sci. 2013;14(12):23212–23230. doi:10.3390/ijms141223212
21. Wang Y, Zhang S, Liu J, et al. Matrine inhibits the invasive and migratory properties of human hepatocellular carcinoma by regulating epithelial-mesenchymal transition. Mol Med Rep. 2018;18(1):911–919. doi:10.3892/mmr.2018.9023
22. Chi G, Xu D, Zhang B, et al. Matrine induces apoptosis and autophagy of glioma cell line U251 by regulation of circRNA-104075/BCL-9. Chem Biol Interact. 2019;308:198–205. doi:10.1016/j.cbi.2019.05.030
23. Wang Z, Wu Y, Wang Y, et al. Matrine inhibits the invasive properties of human glioma cells by regulating epithelial to mesenchymal transition. Mol Med Rep. 2015;11(5):3682–3686. doi:10.3892/mmr.2015.3167
24. Zhou W, Wang J, Qi Q, et al. Matrine induces senescence of human glioblastoma cells through suppression of the IGF1/PI3K/AKT/p27 signaling pathway. Cancer Med. 2018;7(9):4729–4743. doi:10.1002/cam4.1720
25. Shen X, Huang J, Liu G, et al. Matrine inhibits neuroblastoma cell proliferation and migration by enhancing tribbles 3 expression. Oncol Res. 2018;26(7):1133–1142. doi:10.3727/096504018X15168461629558
26. Wu J, Hu G, Dong Y, et al. Matrine induces Akt/mTOR signalling inhibition-mediated autophagy and apoptosis in acute myeloid leukaemia cells. J Cell Mol Med. 2017;21(6):1171–1181. doi:10.1111/jcmm.13049
27. Hao Y, Zhang N, Wei N, et al. Matrine induces apoptosis in acute myeloid leukemia cells by inhibiting the PI3K/Akt/mTOR signaling pathway. Oncol Lett. 2019;18(3):2891–2896. doi:10.3892/ol.2019.10649
28. Zhang S, Zhang Y, Zhuang Y, et al. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One. 2012;7(10):e46853. doi:10.1371/journal.pone.0046853
29. Yang Y, Guo JX, Shao ZQ, et al. Matrine inhibits bladder cancer cell growth and invasion in vitro through PI3K/AKT signaling pathway: an experimental study. Asian Pac J Trop Med. 2017;10(5):515–519. doi:10.1016/j.apjtm.2017.05.009
30. Li L, Qi F, Wang K. Matrine restrains cell growth and metastasis by up-regulating LINC00472 in bladder carcinoma. Cancer Manag Res. 2020;12:1241–1251. doi:10.2147/CMAR.S224701
31. Liao XZ, Tao LT, Liu JH, et al. Matrine combined with cisplatin synergistically inhibited urothelial bladder cancer cells via down-regulating VEGF/PI3K/Akt signaling pathway. Cancer Cell Int. 2017;17:124. doi:10.1186/s12935-017-0495-6
32. Peng X, Zhou D, Wang X, et al. Matrine suppresses proliferation and invasion of SGC7901 cells through inactivation of PI3K/Akt/uPA pathway. Ann Clin Lab Sci. 2016;46(5):457–462.
33. Li Y, Zhang J, Ma H, et al. Protective role of autophagy in matrineinduced gastric cancer cell death. Int J Oncol. 2013;42(4):1417–1426. doi:10.3892/ijo.2013.1817
34. Bai S, Chen T, Yu X, et al. The specific killing effect of matrine on castration-resistant prostate cancer cells by targeting the Akt/FoxO3a signaling pathway. Oncol Rep. 2017;37(5):2819–2828. doi:10.3892/or.2017.5510
35. Li Q, Huang H, He Z, et al. Regulatory effects of antitumor agent matrine on FOXO and PI3K-AKT pathway in castration-resistant prostate cancer cells. Sci China Life Sci. 2018;61(5):550–558. doi:10.1007/s11427-016-9050-6
36. Zhang S, Cheng B, Li H, et al. Matrine inhibits proliferation and induces apoptosis of human colon cancer LoVo cells by inactivating Akt pathway. Mol Biol Rep. 2014;41(4):2101–2108. doi:10.1007/s11033-014-3059-z
37. Zhang X, Hou G, Liu A, et al. Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways. Cell Death Dis. 2019;10(10):770. doi:10.1038/s41419-019-2013-3
38. Jin H, Sun Y, Wang S, et al. Matrine activates PTEN to induce growth inhibition and apoptosis in V600EBRAF harboring melanoma cells. Int J Mol Sci. 2013;14(8):16040–16057. doi:10.3390/ijms140816040
39. Xu GP, Zhao W, Zhuang JP, et al. Matrine inhibits the growth and induces apoptosis of osteosarcoma cells in vitro by inactivating the Akt pathway. Tumour Biol. 2015;36(3):1653–1659. doi:10.1007/s13277-014-2764-5
40. Yu Q, Chen B, Zhang X, et al. Arsenic trioxide-enhanced, matrine-induced apoptosis in multiple myeloma cell lines. Planta Med. 2013;79(9):775–781. doi:10.1055/s-0032-1328554
41. Zhao L, Zhang X, Cui S. Matrine inhibits TPC-1 human thyroid cancer cells via the miR-21/PTEN/Akt pathway. Oncol Lett. 2018;16(3):2965–2970. doi:10.3892/ol.2018.9006
42. Li Q, Zhang S, Wang M, et al. Downregulated miR-21 mediates matrine-induced apoptosis via the PTEN/Akt signaling pathway in FTC-133 human follicular thyroid cancer cells. Oncol Lett. 2019;18(4):3553–3560. doi:10.3892/ol.2019.10693
43. Li L, Chen S, Sun Y, et al. Matrine inhibits the proliferation of pituitary tumor cells by decreasing Foxo3a phosphorylation and promoting Foxo3a nuclear localization. Exp Ther Med. 2019;17(5):3775–3780. doi:10.3892/etm.2019.7365
44. Xu G, Zhang W, Wang Z, et al. Matrine regulates H2O2-induced oxidative stress through long non-coding RNA HOTAIR/miR-106b-5p axis via AKT and STAT3 pathways. Biosci Rep. 2020;40(5). doi:10.1042/BSR20192560
45. Zhang S, Guo S, Gao XB, et al. Matrine attenuates high-fat diet-induced in vivo and ox-LDL-induced in vitro vascular injury by regulating the PKCα/eNOS and PI3K/Akt/eNOS pathways. J Cell Mol Med. 2019;23(4):2731–2743. doi:10.1111/jcmm.14180
46. Ma W, Xu J, Zhang Y, et al. Matrine pre-treatment suppresses AGEs- induced HCSMCs fibrotic responses by regulating Poldip2/mTOR pathway. Eur J Pharmacol. 2019;865:172746. doi:10.1016/j.ejphar.2019.172746
47. Liu X, Zhang X, Ma K, et al. Matrine alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of PI3K/Akt-mediated NF-κB inhibition and Keap1/Nrf2-dependent HO-1 induction. Cell Mol Biol. 2016;62(11):38–44.
48. Chen L, Chen L, Wan L, et al. Matrine improves skeletal muscle atrophy by inhibiting E3 ubiquitin ligases and activating the Akt/mTOR/FoxO3α signaling pathway in C2C12 myotubes and mice. Oncol Rep. 2019;42(2):479–494. doi:10.3892/or.2019.7205
49. Liu SQ, Zhang ML, Zhang HJ, et al. Matrine promotes oligodendrocyte development in CNS autoimmunity through the PI3K/Akt signaling pathway. Life Sci. 2017;180:36–41. doi:10.1016/j.lfs.2017.05.010
50. Chen X, Zhi X, Pan P, et al. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. FASEB J. 2017;31(11):4855–4865. doi:10.1096/fj.201700316R
51. Wu Z, You Z, Chen P, et al. Matrine exerts antidepressant-like effects on mice: role of the hippocampal PI3K/Akt/mTOR signaling. Int J Neuropsychopharmacol. 2018;21(8):764–776. doi:10.1093/ijnp/pyy028
52. Feng Y, Ying HY, Qu Y, et al. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell. 2016;7(9):662–672. doi:10.1007/s13238-016-0285-2
53. Sun X, Zhuo XB, Hu YP, et al. A novel matrine derivative WM622 inhibits hepatocellular carcinoma by inhibiting PI3K/AKT signaling pathways. Mol Cell Biochem. 2018;449(1–2):47–54. doi:10.1007/s11010-018-3341-9
54. Qian L, Liu Y, Xu Y, et al. Matrine derivative WM130 inhibits hepatocellular carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Cancer Lett. 2015;368(1):126–134. doi:10.1016/j.canlet.2015.07.035
55. Zou Y, Sarem M, Xiang S, et al. Autophagy inhibition enhances Matrine derivative MASM induced apoptosis in cancer cells via a mechanism involving reactive oxygen species-mediated PI3K/Akt/mTOR and Erk/p38 signaling. BMC Cancer. 2019;19(1):949. doi:10.1186/s12885-019-6199-7
56. Wu L, Wang G, Liu S, et al. Synthesis and biological evaluation of matrine derivatives containing benzo-α-pyrone structure as potent anti-lung cancer agents. Sci Rep. 2016;6:35918. doi:10.1038/srep35918
57. Zhou YJ, Guo YJ, Yang XL, et al. Anti-cervical cancer role of matrine, oxymatrine and sophora flavescens alkaloid gels and its mechanism. J Cancer. 2018;9(8):1357–1364. doi:10.7150/jca.22427
58. Xie M, Yi X, Wang R, et al. 14-Thienyl methylene matrine (YYJ18), the derivative from matrine, induces apoptosis of human nasopharyngeal carcinoma cells by targeting MAPK and PI3K/Akt pathways in vitro. Cell Physiol Biochem. 2014;33(5):1475–1483. doi:10.1159/000358712
59. Chen X, Zhi X, Cao L, et al. Matrine derivate MASM uncovers a novel function for ribosomal protein S5 in osteoclastogenesis and postmenopausal osteoporosis. Cell Death Dis. 2017;8(9):e3037. doi:10.1038/cddis.2017.394
60. Xin Z, Jin C, Chao L, et al. A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by targeting ribosomal protein S5. Front Pharmacol. 2018;9:22. doi:10.3389/fphar.2018.00022
61. Zhang Y, Alexander PB, Wang XF. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9(4):a022145. doi:10.1101/cshperspect.a022145
62. Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi:10.1016/j.cbi.2018.07.008
63. Vander Ark A, Cao J, Li X. TGF-β receptors: in and beyond TGF-β signaling. Cell Signal. 2018;52:112–120. doi:10.1016/j.cellsig.2018.09.002
64. Clark DA, Coker R. Transforming growth factor-beta (TGF-beta). Int J Biochem Cell Biol. 1998;30(3):293–298. doi:10.1016/S1357-2725(97)00128-3
65. Zhang Y, Cui L, Guan G, et al. Matrine suppresses cardiac fibrosis by inhibiting the TGF-β/Smad pathway in experimental diabetic cardiomyopathy. Mol Med Rep. 2018;17(1):1775–1781. doi:10.3892/mmr.2017.8054
66. Hou H, Zhang Q, Dong H, et al. Matrine improves diabetic cardiomyopathy through TGF-β-induced protein kinase RNA-like endoplasmic reticulum kinase signaling pathway. J Cell Biochem. 2019;120(8):13573–13582. doi:10.1002/jcb.28632
67. Ma J, Ma S, Yin C, et al. Matrine reduces susceptibility to postinfarct atrial fibrillation in rats due to antifibrotic properties. J Cardiovasc Electrophysiol. 2018;29(4):616–627. doi:10.1111/jce.13448
68. Yu J-L, Li J-H, Chengz R-G, et al. Effect of matrine on transforming growth factor β1 and hepatocyte growth factor in rat liver fibrosis model. Asian Pac J Trop Med. 2014;7(5):390–393. doi:10.1016/S1995-7645(14)60062-6
69. Li L, Ma L, Wang D, et al. Design and synthesis of matrine derivatives as novel anti-pulmonary fibrotic agents via repression of the TGF-β/Smad pathway. Molecules. 2019;24(6):1108.
70. Zhang X, Cai Y. Effects of Ginkgo biloba leaf extract, shenmai and matrine on a human embryonic lung fibroblast fibrosis model. Exp Ther Med. 2018;16(5):4289–4295. doi:10.3892/etm.2018.6698
71. Liu P, Zhu L, Zou G, et al. Matrine suppresses pancreatic fibrosis by regulating TGF-β/Smad signaling in rats. Yonsei Med J. 2019;60(1):79–87. doi:10.3349/ymj.2019.60.1.79
72. Mao D, Pan X, Rui Y, et al. Matrine attenuates heterotopic ossification by suppressing TGF-β induced mesenchymal stromal cell migration and osteogenic differentiation. Biomed Pharmacother. 2020;127:110152. doi:10.1016/j.biopha.2020.110152
73. Zhang L, Lin S, Zheng Y, et al. Matrine regulates Th1/Th2 balance to treat eczema by upregulating interferon-γ. J Nanosci Nanotechnol. 2020;20(6):3378–3386. doi:10.1166/jnn.2020.17417
74. Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1–2):37–57. doi:10.1016/j.cell.2016.12.012
75. Sacks D, Baxter B, Campbell BCV. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi:10.1177/1747493018778713
76. Zhang B, Liu ZY, Li YY, et al. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011;44(5):573–579. doi:10.1016/j.ejps.2011.09.020
77. Shao H, Yang B, Hu R, et al. Matrine effectively inhibits the proliferation of breast cancer cells through a mechanism related to the NF-κB signaling pathway. Oncol Lett. 2013;6(2):517–520. doi:10.3892/ol.2013.1399
78. Yu HB, Zhang HF, Li DY, et al. Matrine inhibits matrix metalloproteinase-9 expression and invasion of human hepatocellular carcinoma cells. J Asian Nat Prod Res. 2011;13(3):242–250. doi:10.1080/10286020.2010.551641
79. Huang M, Xin W. Matrine inhibiting pancreatic cells epithelial-mesenchymal transition and invasion through ROS/NF-κB/MMPs pathway. Life Sci. 2018;192:55–61. doi:10.1016/j.lfs.2017.11.024
80. Jung YY, Um JY, Narula AS, et al. Identification of matrine as a novel regulator of the CXCR4 signaling axis in tumor cells. Int J Mol Sci. 2020;21(13):4731. doi:10.3390/ijms21134731
81. Li Q, Lai Y, Wang C, et al. Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-κB signaling pathway. Oncol Rep. 2016;35(1):375–381. doi:10.3892/or.2015.4341
82. Huang H, Du T, Xu G, et al. Matrine suppresses invasion of castration-resistant prostate cancer cells by downregulating MMP-2/9 via NF-κB signaling pathway. Int J Oncol. 2017;50(2):640–648. doi:10.3892/ijo.2016.3805
83. Luo C, Zhong HJ, Zhu LM, et al. Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol Biol Rep. 2012;39(5):5459–5464. doi:10.1007/s11033-011-1346-5
84. Chen Z, Nishimura N, Okamoto T, et al. Molecular mechanism of matrine from sophora alopecuroides in the reversing effect of multi-anticancer drug resistance in K562/ADR cells. Biomed Res Int. 2019;2019:1269532. doi:10.1155/2019/1269532
85. Li Y, Zhang ZN, Zhao HM, et al. Matrine inhibits the invasive properties of human osteosarcoma cells by downregulating the ERK-NF-κB pathway. Anticancer Drugs. 2014;25(9):1035–1043. doi:10.1097/CAD.0000000000000136
86. Jiang J, Wang G. Matrine protects PC12 cells from lipopolysaccharide-evoked inflammatory injury via upregulation of miR-9. Pharm Biol. 2020;58(1):314–320. doi:10.1080/13880209.2020.1719165
87. Chu Y, Jing Y, Zhao X, et al. Modulation of the HMGB1/TLR4/NF-κB signaling pathway in the CNS by matrine in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2021;352:577480. doi:10.1016/j.jneuroim.2021.577480
88. Xu M, Yang L, Hong LZ, et al. Direct protection of neurons and astrocytes by matrine via inhibition of the NF-κB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain Res. 2012;1454:48–64. doi:10.1016/j.brainres.2012.03.020
89. Sun N, Sun P, Lv H, et al. Matrine displayed antiviral activity in porcine alveolar macrophages co-infected by porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Sci Rep. 2016;6:24401. doi:10.1038/srep24401
90. Sun P, Sun N, Yin W, et al. Matrine inhibits IL-1β secretion in primary porcine alveolar macrophages through the MyD88/NF-κB pathway and NLRP3 inflammasome. Vet Res. 2019;50(1):53. doi:10.1186/s13567-019-0671-x
91. Wang J, Liu Z, Li W, et al. Matrine alleviates N-methyl-N’-nitro-N-nitrosoguanidine (MNNG)-induced gastric mucosal damage in rats and its mechanism. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36(2):157–163.
92. Sun D, Wang J, Yang N, et al. Matrine suppresses airway inflammation by downregulating SOCS3 expression via inhibition of NF-κB signaling in airway epithelial cells and asthmatic mice. Biochem Biophys Res Commun. 2016;477(1):83–90. doi:10.1016/j.bbrc.2016.06.024
93. Niu Y, Dong Q, Li R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol Int. 2017;41(6):611–621. doi:10.1002/cbin.10763
94. Pu J, Fang FF, Li XQ, et al. Matrine exerts a strong anti-arthritic effect on type II collagen-induced arthritis in rats by inhibiting inflammatory responses. Int J Mol Sci. 2016;17(9):1410. doi:10.3390/ijms17091410
95. Zou Y, Li Q, Liu D, et al. Therapeutic effects of matrine derivate MASM in mice with collagen-induced arthritis and on fibroblast-like synoviocytes. Sci Rep. 2017;7(1):2454. doi:10.1038/s41598-017-02423-7
96. Lu S, Xiao X, Cheng M. Matrine inhibits IL-1β-induced expression of matrix metalloproteinases by suppressing the activation of MAPK and NF-κB in human chondrocytes in vitro. Int J Clin Exp Pathol. 2015;8(5):4764–4772.
97. Jiang K, Guo S, Yang J, et al. Matrine alleviates Staphylococcus aureus lipoteichoic acid-induced endometritis via suppression of TLR2-mediated NF-κB activation. Int Immunopharmacol. 2019;70:201–207. doi:10.1016/j.intimp.2019.02.033
98. Liu J, Zhang L, Ren Y, et al. Matrine inhibits the expression of adhesion molecules in activated vascular smooth muscle cells. Mol Med Rep. 2016;13(3):2313–2319. doi:10.3892/mmr.2016.4767
99. Dannheisig DP, Bachle J, Tasic J, et al. The Wnt/β-catenin pathway is activated as a novel nucleolar stress response. J Mol Biol. 2021;433(2):166719. doi:10.1016/j.jmb.2020.11.018
100. Astudillo P. Extracellular matrix stiffness and Wnt/β-catenin signaling in physiology and disease. Biochem Soc Trans. 2020;48(3):1187–1198. doi:10.1042/BST20200026
101. Dai M, Cai Z, Chen N, et al. Matrine suppresses stemness of hepatocellular carcinoma cells by regulating β-catenin signaling pathway. Nan Fang Yi Ke Da Xue Xue Bao. 2019;39(10):1239–1245. doi:10.12122/j.issn.1673-4254.2019.10.17
102. Li X, Wang M, Du N, et al. Matrine inhibitory effect on self-renewal and re-sensitization of 5-FU resistant NSCLC stem cells were through Let-7b dependent downregulation of CCND1. Cell Cycle. 2020;19(23):3249–3259. doi:10.1080/15384101.2020.1838791
103. Li X, Liang T, Chen SS, et al. Matrine suppression of self-renewal was dependent on regulation of LIN28A/Let-7 pathway in breast cancer stem cells. J Cell Biochem. 2020;121(3):2139–2149. doi:10.1002/jcb.29396
104. Xiao X, Ao M, Xu F, et al. Effect of matrine against breast cancer by downregulating the vascular endothelial growth factor via the Wnt/β-catenin pathway. Oncol Lett. 2018;15(2):1691–1697. doi:10.3892/ol.2017.7519
105. Liu J, Guo Y, Cao J. Matrine triggers colon cancer cell apoptosis and G0/G1 cell cycle arrest via mediation of microRNA-22. Phytother Res. 2020;34(7):1619–1628. doi:10.1002/ptr.6626
106. Ma Y, Zou F, Xiong J, et al. Effect of Matrine on HPAC cell migration by down-regulating the expression of MT1-MMP via Wnt signaling. Cancer Cell Int. 2015;15:59. doi:10.1186/s12935-015-0210-4
107. Yin H, Que R, Liu C, et al. Survivin-targeted drug screening platform identifies a matrine derivative WM-127 as a potential therapeutics against hepatocellular carcinoma. Cancer Lett. 2018;425:54–64. doi:10.1016/j.canlet.2018.03.044
108. Ni CX, Qi Y, Zhang J, et al. WM130 preferentially inhibits hepatic cancer stem-like cells by suppressing AKT/GSK3β/β-catenin signaling pathway. Oncotarget. 2016;7(48):79544–79556. doi:10.18632/oncotarget.12822
109. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi:10.1016/j.bbadis.2009.12.009
110. Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi:10.1038/nrc2694
111. Tan C, Qian X, Jia R, et al. Matrine induction of reactive oxygen species activates p38 leading to caspase-dependent cell apoptosis in non-small cell lung cancer cells. Oncol Rep. 2013;30(5):2529–2535. doi:10.3892/or.2013.2727
112. Yang Z, Mu J, Chen J, et al. Apoptosis of U937 cell line promoted by matrine through MAPK signal transduction pathway. Zhongguo Zhong Yao Za Zhi. 2009;34(12):1553–1556.
113. Ma L, Xu Z, Wang J, et al. Matrine inhibits BCR/ABL mediated ERK/MAPK pathway in human leukemia cells. Oncotarget. 2017;8(65):108880–108889. doi:10.18632/oncotarget.22353
114. Lu J, Luo Q, Cheng P, et al. The role of matrine and mitogen-ativated protein kinase/extracellular signal-regulated kinase signal transduction in the inhibition of the proliferation and migration of human umbilical veins endothelial cells induced by lung cancer cells. Zhongguo Fei Ai Za Zhi. 2009;12(7):747–752. doi:10.3779/j.issn.1009-3419.2009.07.002
115. Ren H, Zhang S, Ma H, et al. Matrine reduces the proliferation and invasion of colorectal cancer cells via reducing the activity of p38 signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2014;46(12):1049–1055. doi:10.1093/abbs/gmu101
116. Liang X, Ju J. Matrine inhibits ovarian cancer cell viability and promotes apoptosis by regulating the ERK/JNK signaling pathway via p38MAPK. Oncol Rep. 2021;45(5). doi:10.3892/or.2021.8033
117. Huang H, Wang Q, Du T, et al. Matrine inhibits the progression of prostate cancer by promoting expression of GADD45B. Prostate. 2018;78(5):327–335. doi:10.1002/pros.23469
118. Ma K, Huang MY, Guo YX, et al. Matrine-induced autophagy counteracts cell apoptosis via the ERK signaling pathway in osteosarcoma cells. Oncol Lett. 2016;12(3):1854–1860. doi:10.3892/ol.2016.4848
119. Zhou J, Ma W, Wang X, et al. Matrine suppresses reactive oxygen species (ROS)-mediated MKKs/p38-induced inflammation in oxidized low-density lipoprotein (ox-LDL)-stimulated macrophages. Med Sci Monit. 2019;25:4130–4136. doi:10.12659/MSM.917151
120. Liu Z, Lv Y, Zhang Y, et al. Matrine-type alkaloids inhibit advanced glycation end products induced reactive oxygen species-mediated apoptosis of aortic endothelial cells in vivo and in vitro by targeting MKK3 and p38MAPK signaling. J Am Heart Assoc. 2017;6(12). doi:10.1161/JAHA.117.007441
121. Zhang X, Hu C, Zhang N, et al. Matrine attenuates pathological cardiac fibrosis via RPS5/p38 in mice. Acta Pharmacol Sin. 2021;42(4):573–584. doi:10.1038/s41401-020-0473-8
122. Wu X, Zhou J, Cai D, et al. Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol Rep. 2017;38(2):1312–1320. doi:10.3892/or.2017.5787
123. Xu J, Wang KQ, Xu WH, et al. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal sepsis. J Infect Dis. 2016;214(11):1762–1772. doi:10.1093/infdis/jiw445
124. Li J, Xu J, Lu Y, et al. MASM, a matrine derivative, offers radioprotection by modulating lethal total-body irradiation-induced multiple signaling pathways in wistar rats. Molecules. 2016;21(5):649.
125. Xu J, Qi Y, Xu WH, et al. Matrine derivate MASM suppresses LPS-induced phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2016;36:59–66. doi:10.1016/j.intimp.2016.04.022
126. Siebel C, Lendahl U. Notch signaling in development, tissue homeostasis, and disease. Physiol Rev. 2017;97(4):1235–1294. doi:10.1152/physrev.00005.2017
127. Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci. 2009;66(10):1631–1646. doi:10.1007/s00018-009-8668-7
128. Liu Z, Wang Y, Zhu H, et al. Matrine blocks AGEs- induced HCSMCs phenotypic conversion via suppressing Dll4-Notch pathway. Eur J Pharmacol. 2018;835:126–131. doi:10.1016/j.ejphar.2018.07.051
129. Zhao L, Cai H, Tang Z, et al. Matrine suppresses advanced glycation end products-induced human coronary smooth muscle cells phenotype conversion by regulating endoplasmic reticulum stress-dependent Notch signaling. Eur J Pharmacol. 2020;882:173257. doi:10.1016/j.ejphar.2020.173257
130. Yang Z, Wang L, Wang X. Matrine induces the hepatic differentiation of WB-F344 rat hepatic progenitor cells and inhibits Jagged 1/HES1 signaling. Mol Med Rep. 2016;14(4):3841–3847. doi:10.3892/mmr.2016.5668
131. Shi J, Han G, Wang J, et al. Matrine promotes hepatic oval cells differentiation into hepatocytes and alleviates liver injury by suppression of Notch signalling pathway. Life Sci. 2020;261:118354. doi:10.1016/j.lfs.2020.118354
132. Shi J, Han X, Wang J, et al. Matrine prevents the early development of hepatocellular carcinoma like lesions in rat liver. Exp Ther Med. 2019. doi:10.3892/etm.2019.7875
133. Harrison DA. The Jak/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4(3):a011205–a011205. doi:10.1101/cshperspect.a011205
134. Muller P, Kuttenkeuler D, Gesellchen V, et al. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature. 2005;436(7052):871–875. doi:10.1038/nature03869
135. Ma X, Chen R, Liu X, et al. Effects of matrine on JAK-STAT signaling transduction pathways in bleomycin-induced pulmonary fibrosis. Afr J Tradit Complement Altern Med. 2013;10(3):442–448. doi:10.4314/ajtcam.v10i3.10
136. Ma L, Zhu Z, Sun X, et al. Growth inhibition effect of matrine on K562 cells mediated by IL-6/JAK/STAT3 signaling pathway. Zhonghua Xue Ye Xue Za Zhi. 2015;36(5):422–426. doi:10.3760/cma.j.issn.0253-2727.2015.05.015
137. Lu X, Zhu Z, Jiang L, et al. Matrine increases NKG2D ligand ULBP2 in K562 cells via inhibiting JAK/STAT3 pathway: a potential mechanism underlying the immunotherapy of matrine in leukemia. Am J Transl Res. 2015;7(10):1838–1849.
138. Chen SF, Zhang ZY, Zhang JL. Matrine increases the inhibitory effects of Afatinib on H1975 cells via the IL6/JAK1/STAT3 signaling pathway. Mol Med Rep. 2017;16(3):2733–2739. doi:10.3892/mmr.2017.6865
139. Yang Y, Dong Q, Li R. Matrine induces the apoptosis of fibroblast-like synoviocytes derived from rats with collagen-induced arthritis by suppressing the activation of the JAK/STAT signaling pathway. Int J Mol Med. 2017;39(2):307–316. doi:10.3892/ijmm.2016.2843
140. Tamargo-Gomez I, Marino G. AMPK: regulation of metabolic dynamics in the context of autophagy. Int J Mol Sci. 2018;19(12):3812. doi:10.3390/ijms19123812
141. Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27(2):299–313. doi:10.1016/j.cmet.2017.10.009
142. Li Y, Chen Y. AMPK and autophagy. Adv Exp Med Biol. 2019;1206:85–108.
143. Lu Q, Lin X, Wu J, et al. Matrine attenuates cardiomyocyte ischemia-reperfusion injury through activating AMPK/Sirt3 signaling pathway. J Recept Signal Transduct Res. 2020;1–6. doi:10.1080/10799893.2020.1863987
144. Xie SB, He XX, Yao SK. Matrine-induced autophagy regulated by p53 through AMP-activated protein kinase in human hepatoma cells. Int J Oncol. 2015;47(2):517–526. doi:10.3892/ijo.2015.3023
145. Cao J, Wei R, Yao S. Matrine has pro-apoptotic effects on liver cancer by triggering mitochondrial fission and activating Mst1-JNK signalling pathways. J Physiol Sci. 2019;69(2):185–198. doi:10.1007/s12576-018-0634-4
146. Yang ZY, Wang L, Hou YX, et al. Effects of matrine on oval cellmediated liver regeneration and expression of RBP-Jκ and HES1. Mol Med Rep. 2013;7(5):1533–1538. doi:10.3892/mmr.2013.1398
147. Lu Z, Xiao Y, Liu X, et al. Matrine reduces the proliferation of A549 cells via the p53/p21/PCNA/eIF4E signaling pathway. Mol Med Rep. 2017;15(5):2415–2422. doi:10.3892/mmr.2017.6331
148. Gu J, Wang X, Zhang L, et al. Matrine suppresses cell growth of diffuse large B-cell lymphoma via inhibiting CaMKIIγ/c-Myc/CDK6 signaling pathway. BMC Complement Med Ther. 2021;21(1):163. doi:10.1186/s12906-021-03315-0
149. Khan A, Shal B, Naveed M, et al. Matrine alleviates neurobehavioral alterations via modulation of JNK-mediated caspase-3 and BDNF/VEGF signaling in a mouse model of burn injury. Psychopharmacology (Berl). 2020;237(8):2327–2343. doi:10.1007/s00213-020-05537-5
150. Cai XH, Guo H, Xie B. Structural modifications of matrine-type alkaloids. Mini Rev Med Chem. 2018;18(9):730–744. doi:10.2174/1389557516666161104150334
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
