Back to Journals » Journal of Inflammation Research » Volume 16
Mastoparan M Suppressed NLRP3 Inflammasome Activation by Inhibiting MAPK/NF-κB and Oxidative Stress in Gouty Arthritis
Authors Yan Y , Yu L, Chen B, Cao C, Zhao H , Wang Q, Xie D, Xi Y, Zhang C, Cheng J
Received 20 September 2023
Accepted for publication 28 November 2023
Published 15 December 2023 Volume 2023:16 Pages 6179—6193
DOI https://doi.org/10.2147/JIR.S434587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yunbo Yan,1 Linqian Yu,1 Binyang Chen,1 Chang’an Cao,1 Hairong Zhao,1,2 Qiang Wang,1 De Xie,1 Yuemei Xi,1 Chenggui Zhang,2 Jidong Cheng1,3
1Department of Internal Medicine, Xiang’an Hospital of Xiamen University, School of Medicine, Xiamen University, Xiamen, People’s Republic of China; 2Yunnan Provincial Key Laboratory of Entomological Biopharmaceutical R&D, Dali University, Dali, People’s Republic of China; 3Xiamen Key Laboratory of Translational Medicine for Nucleic Acid Metabolism and Regulation, Xiamen, People’s Republic of China
Correspondence: Jidong Cheng; Chenggui Zhang, Email [email protected]; [email protected]
Background: Gouty arthritis is characterized by the accumulation of monosodium urate crystals (MSU) in the synovial joints and surrounding tissues. Mastoparan M (Mast-M) is a biologically active peptide composed of 14 amino acids, extracted from wasp venom. This study aims to assess the impact of Mast-M on in vitro and in vivo gouty arthritis induced by lipolyaccharide (LPS) plus MSU crystal stimulation.
Methods: PMA-differentiated THP-1 macrophages were pre-treated with Mast-M or left untreated, followed by stimulation with LPS and MSU crystals. Cell lysates were collected to assess the expression of the NLRP3 inflammasome, inflammatory signaling pathways, and oxidative stress. Furthermore, to evaluate the in vivo anti-inflammatory effect of Mast-M, an experimental acute gouty arthritis mouse model was established through intra-articular injection of MSU crystals.
Results: Mast-M treatment demonstrated significant inhibition of the phosphorylation of MAPKs/NF-κB signaling pathways and reduction in oxidative stress expression in LPS and MSU-induced THP-1 macrophages. This resulted in the suppression of downstream NLRP3 inflammasome activation and IL-1β release. In vivo, Mast-M effectively attenuated the inflammation induced by MSU in mice with gouty arthritis. Specifically, Mast-M reduced swelling in the paws, inhibited the infiltration of neutrophils and macrophages into periarticular tissue, and decreased the activation of the NLRP3 inflammasome and IL-1β production.
Conclusion: Mast-M significantly improves gouty arthritis, and its potential mechanism may be achieved by inhibiting the MAPK/NF-κB pathway and alleviating oxidative stress, thus suppressing the activation of NLRP3 inflammasomes.
Keywords: Mastoparan M, NLRP3, MAPKs, NF-κB, oxidative stress, gout
Introduction
Gouty arthritis is a painful and inflammatory disease caused by the accumulation of MSU crystals in joints and surrounding tissues.1 Numerous studies conducted in Asia, Europe, and North America have reported varying prevalence rates for gout, ranging from 0.6 to 2.9 cases per 1000 person-years. Among adults, the prevalence rates have been documented between 0.68% and 3.9%, with a notable increase in both incidence and prevalence over time.2 Currently, the main drugs used to treat gouty arthritis are nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.3 Due to the potential occurrence of adverse reactions and inconsistent treatment effectiveness associated with these medications,3,4 there is an urgent need to develop new drugs for the treatment of gouty arthritis.
Research findings have demonstrated the pivotal role of prolonged macrophage activation in the inflammatory response mechanism of gouty arthritis.5 On one hand, macrophages are triggered by pathogens such as lipopolysaccharide (LPS). By activating signaling pathways such as mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB), they mediate the expression of inflammatory genes such as TNF-α and cyclooxygenase-2 (COX-2).6,7 On the other hand, macrophages engulf and internalize MSU crystals, triggering the activation of the NLRP3 inflammasome.8 The activated NLRP3 inflammasome subsequently processes pro-IL-1β into active IL-1β, which plays a pivotal role in the inflammatory response and pain linked to gouty arthritis.9 Additionally, recent research has reported that MSU crystals can also activate the NLRP3 inflammasome during gout by promoting excessive production of reactive oxygen species (ROS).10–12
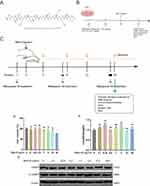
Mastoparan M (Mast-M) (C70H131N19O15) (Figure 1A) is one of the major active components of wasp venom, accounting for approximately 70–80% of the crude extract.13 Wasp venom is a complex mixture of various bioactive proteins and peptides, sharing similar pharmacological effects with bee venom.14 Previous research has shown that wasp venom can alleviate rheumatoid arthritis,15 and inhibit LPS-induced neuroinflammation in microglia through the inhibition of the NF-κB pathway.16 Notably, the compound Mast-M, derived from wasp venom, has garnered recognition for its potent anti-inflammatory, antioxidant, and antimicrobial properties.13,14,17,18 However, there is a lack of studies investigating the effect of Mast-M specifically on gouty arthritis. Based on the known anti-inflammatory and antioxidant activities of Mast-M, we hypothesized that it could be an effective treatment for gouty arthritis. Therefore, in this study, we utilized an arthritis mouse model and a macrophage inflammation model to investigate the potential mechanisms and therapeutic effects of Mast-M on gouty arthritis.
Methods
Materials and Reagents
NLRP3, Caspase 1, P38, P-P38, JNK, P-JNKP, ERK, P-ERK, IKK, P-IKK, IκB, P-IκB, P65, P-P65, caspase3, and cleaved caspase3 were acquired from Cell Signaling Technology (Danvers, MA). Nrf2, HO-1, SOD2, TNF-α, and GAPDH were obtained from Proteintech (Wuhan, China). COX-2, LY6G and β-actin were sourced from Abcolnal (Wuhan, China), while IL-1β and F4/80 were procured from Abcam (UK). Mastoparan M (Mast-M, purity >99%) was provided by the Shanghai Institute of Materia Medica (Shanghai, China). Colchicine (Col, purity >98%, C106738) was obtained from Aladdin Reagent (Shanghai, China). Lipopolysaccharide (LPS), phorbol 12-myristate 13-acetate (PMA), and uric acid (UA) were acquired from Sigma (USA). RPMI-1640 and fetal bovine serum (FBS) were sourced from Gibco (Shanghai, China).
Animals
C57BL/6 male mice, aged 6–8 weeks and weighing 23 ± 2g, were acquired from Xiamen University’s Laboratory Animal Center (Xiamen, China). Before commencing the experiments, a total of 5 mice were housed in each cage for 1 week, allowing them to acclimate to the standard laboratory conditions, including a 12/12-hour light-dark cycle and a temperature of 24 ± 2 °C. During this period, the mice were provided with ad libitum access to food and water. All animal experiments followed the guidelines of the Guide for the Protection and Use of Laboratory Animals and were ethically approved by the Ethics Committee of Xiamen University ((license no. XMULAC20200122).
Preparing MSU
MSU crystals were prepared following a previously described protocol with minor modifications.19 Briefly, 1g of UA and 0.5g of NaOH were dissolved in 100 mL of double-distilled water (ddH2O). The pH of the solution was adjusted to approximately 7.4 using HCl, ensuring that the sodium urate dissolved to the saturation point. The mixture was then allowed to crystallize overnight at 4°C. The MSU crystals were subsequently recovered by centrifugation at 3600 rpm for 5 minutes at 4°C. The crystals underwent two washes with ddH2O, followed by drying, autoclaving at 130°C for 30 minutes, and weighing under sterile conditions. Finally, the MSU crystals were suspended in PBS at a concentration of 20mg/mL.
Cell Culture and Viability Assay
The THP-1 cell line was procured from the American Type Cell Collection. These cells were cultivated in RPMI-1640 medium (Gibco, Shanghai) supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. To induce differentiation into adherent macrophages, THP-1 cells were incubated with 160 nM PMA for 24 hours. Aiming to evaluate the impact of Mast-M on THP-1 cells, a Cell Counting Kit-8 (CCK8) (Cat# K1018, APExBIO, USA) assay was performed as described.20
Murine Gouty Arthritis Model
To induce gouty arthritis, mice were anesthetized using isoflurane and then injected with 0.5 mg MSU in 25 μL PBS into the right hind paw. Control group mice were injected with sterile PBS 25ul intra-articular injection. 3 hours after the MSU injection, the joint swelling degree was measured with a digital caliper to determine whether the gouty arthritis model was established successfully (see Figure 1C for more details).
Joint Evaluation of Edema
The degree of paw edema was assessed by measuring the increase in paw thickness using a digital caliper. The calculation was performed by subtracting the initial measurement (taken before establishing the gout model) from the observed measurement at various time points. This difference in paw thickness served as an indicator of the extent of edema in the affected paw.
Drug Administration
The administration protocol was as follows: Mast-M (0.3mg/kg), colchicine (1mg/kg), or PBS (model) was injected intraperitoneally (i.p.) one hour before MSU injection, and then again at 5 and 23 hours after MSU injection. An hour after the final injection, the mice were euthanized, and tissue samples were collected for further analysis (see Figure 1C for more detailed information).
Measurement of Intracellular Reactive Oxygen Species (ROS)
To assess the production of reactive oxygen species (ROS) in THP-1 cells, a fluorescent probe called dichloro-dihydro-fluorescein diacetate (DCFH-DA) from Sigma was utilized, following the method described by Xie et al.21 A 6-well plate was used to seed THP-1 cells at a density of 2.0 x 10^5 cells per well. Following that, the cells were treated with DCFH-DA (10μmol/L) and incubated at 37°C for 30 minutes. Following the incubation, the cells were washed twice with PBS. Fluorescence microscopy was then employed to capture images of cells labeled with DCFH-DA in three random regions per sample, enabling the visualization and quantification of ROS levels.
Histopathological Analysis
The paw tissues from the feet were fixed using a 10% paraformaldehyde buffer and underwent decalcification with EDTA for 3 weeks. After decalcification, paraffin-embedded specimens were prepared. The paraffin specimens were then sectioned to a thickness of 5 μm. Hematoxylin and eosin staining was performed on these sections, allowing for the visualization of inflammatory infiltration in the joint tissues. The stained sections were observed under a light microscope at a magnification of 200X to assess the extent of inflammatory changes in the joint tissues.
Immunohistochemistry
Neutrophil and macrophage markers (anti-Ly6G and anti-F4/80 monoclonal antibodies) were utilized for immunohistochemical staining of mouse joints embedded in paraffin. The procedure involved dewaxing the sample sections in xylene, rehydrating them in varying concentrations of ethanol, and repairing the antigen using a pepsin antigen repair solution. Afterward, the slides were subjected to blocking with 10% goat serum at room temperature for 1 hour. This was followed by overnight incubation with the primary antibody at 4°C. The tissue sections were then exposed to an HRP-conjugated secondary antibody for 40 minutes, and subsequently stained with DAB and counterstained with hematoxylin. The expression of neutrophils and macrophages was observed under a 200x light microscope.
Enzyme-Linked Immunosorbent Assay (ELISA)
The analysis of IL-1β concentrations in hind paw tissue was conducted using ELISA kits (Neobioscience, Shenzhen) by the manufacturer’s instructions.
Quantitative PCR
Total RNA was extracted from cultured THP-1 cells and mouse tissues using Trizol reagent (Sangon, Biotech, Shanghai). Subsequently, the extracted RNA was reverse-transcribed into cDNA using a Tiangen Reverse Transcription Kit (Beijing). The mRNA expression levels were assessed using SYBR Green Master Mix (Yeason, Shanghai), with GAPDH serving as the endogenous control. The primer sequences employed for qRT-PCR analysis can be found in Table 1.
 |
Table 1 Primer Sequences |
Western Blotting
PMSF and phosphatase inhibitor (Topscience, China) was added to the RIPA buffer (SparkJade Biotechnology, China) for protein extraction from THP-1 cells and mouse tissues. And then the concentration of each sample was determined using the BCA assay kit (Beyotime, China). The protein separation was performed using Tris-glycine gels (Solarbio, China), followed by transfer onto polyvinylidene difluoride membranes (Millipore, USA). Following sealing with TBST containing 5% skim milk for 1 hour at room temperature, the membranes were subjected to overnight incubation at 4°C with primary antibodies (diluted 1:1000). Subsequently, they were further incubated with HRP-coupled secondary antibodies (diluted 1:3000) for 1 hour. Finally, blots were imaged in the imaging system (US Azure, C300) by using ECL Super Kit.
Statistical Analysis
Data analysis was conducted using GraphPad Prism 8 software. The results are presented as means ± standard error of the mean (SEM), and each experiment was performed three times. The data were analyzed using One-way ANOVA and Tukey’s multiple comparison tests, with statistical significance considered at P < 0.05.
Results
Mast-M Displays Low Cytotoxicity and Apoptosis to THP-1 Cells in vitro
To examine the cytotoxicity of Mast-M on THP-1 cells, we conducted a CCK8 assay to determine the minimum effective dose. The results revealed that the concentrations of Mast-M ranging from 0 to 10 μg/mL had no significant impact on cell viability when compared to the control group (Figure 1D). To evaluate the effect of Mast-M on apoptosis, we examined the ratio of cleaved caspase3/caspase3 using Western blot analysis and showed that Mast-M did not alter the ratio of cleaved caspase3/caspase3 at the protein level compared with the control group (Figure 1E and F). We selected Mast-M concentrations of 0.25, 0.5, and 1.0 μg/mL for subsequent in vitro experiments based on these findings.
Mast-M Attenuates the Activation of the NLRP3 Inflammasome
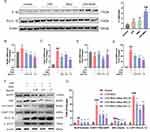
To establish a suitable in vitro gout model, we employed three different stimulations: LPS (200ng/mL) alone, MSU (200μg/mL) alone, and the combination of LPS (200ng/mL) plus MSU (200μg/mL) to induce THP-1 cells (refer to Figure 1B for more details). In the pathogenesis of gout, the cytokine IL-1β plays a crucial role.1,22,23 Clinical trials have demonstrated that biologics targeting IL-1β exhibit significant anti-inflammatory effects in inhibiting gout attacks.24,25 Therefore, we verify the success of inducing gout characteristics in our in vitro model by assessing the expression levels of IL-1β. As depicted in Figure 2A, the release of IL-1β was observed to be maximal only when LPS plus MSU were combined.
The activation of NLRP3 inflammasome induced by MSU is strongly correlated with the secretion of pro-inflammatory cytokines and the infiltration of inflammation in gouty arthritis.11,26 Consequently, we proceeded with qPCR and Western blot analyses to further study whether Mast-M could interfere with the activation of NLRP3 inflammasome in THP-1 cells induced by LPS plus MSU. The qPCR results showed that LPS plus MSU increased the expression NLRP3, Caspase 1, and IL-1β, but had no significant effect on the mRNA expression level of ASC (Figure 2B–E). However, pretreatment with Mast-M was able to reverse the overexpression of NLRP3, Caspase 1, and IL-1β. Next, we investigated the impact of Mast-M on the activation of NLRP3 inflammasome using Western blot analysis. Our results indicated that Mast-M could inhibit the activation of NLRP3, Caspase 1 (P20), ASC, and IL-1β (P17) (Figure 2F and G). Hence, Mast-M is capable of suppressing NLRP3 inflammasome activation in vitro.
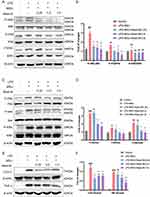
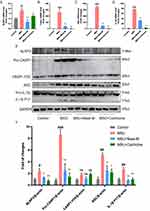
Mast-M Inhibits the Activation of MAPK and NF-κB Pathways
Numerous experimental studies have provided evidence supporting the significant roles of MAPK and NF-κB in the inflammatory stress response and NLRP3 inflammasome activation.27,28 Using the Western blot method, we investigated whether Mast-M can inhibit the activation of NLRP3 inflammasome by intervening in the MAPK and NF-κB pathways. We observed that Mast-M inhibited the upregulation of phosphorylated forms of P38 and JNK (P-P38/P38 and P-JNK/JNK, respectively) in THP-1 macrophages induced by LPS plus MSU (Figure 3A and B). However, Mast-M did not significantly affect the expression of phosphorylated ERK (P-ERK/ERK) (Figure 3A and B). Furthermore, Mast-M downregulated the overexpression of p-p65/p65, p-IκBα/ IκBα, p-IKK/IKK (Figure 3C and D). Additionally, considering the pivotal roles of COX-2 and TNF-α as inflammatory factors downstream of NF-κB in the inflammatory response,29 we investigated their protein expression levels. As expected, Mast-M demonstrated a significant inhibitory effect on the expression of COX-2 and TNF-α (Figure 3E and F). These data suggest that Mast-M may have the potential to impede the initial stage of NLRP3 inflammasome by obstructing MAPK and NF-κB pathways in vitro.
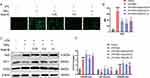
Mast-M Inhibits ROS Production and Restored the Antioxidant Capacities
The production of ROS serves as a crucial upstream signaling event in the activation of the NLRP3 inflammasome. The DCFH-DA fluorescent staining results demonstrated that the treatment with Mast-M markedly suppressed the production of ROS in macrophages primed with LPS plus MSU (Figure 4A). Additionally, we considered the nuclear factor-erythroid 2-related factor 2 (NRF2)-mediated antioxidant pathway, which is a crucial endogenous mechanism for combating oxidative stress. NRF2 stimulates the expression of downstream antioxidant enzymes such as heme oxygenase-1 (HO-1) and superoxide dismutase (SOD).30 Therefore, we examined the expression of NRF2, HO-1, and SOD2. The results revealed that Mast-M treatment enhanced the expression of NRF2 and SOD2 in LPS+MSU-stimulated cells, while also reversing the decrease in HO-1 expression (Figure 4B and C). Collectively, these findings suggest that Mast-M activates the NRF2/HO-1 antioxidant response, thereby protecting against oxidative stress.
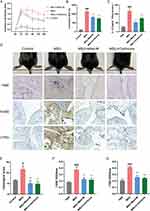
Mast-M Inhibited MSU-Induced Foot and Paw Swelling
To establish a gout arthritis model, we injected MSU crystals dissolved in PBS (0.5 mg/25 μL i.a.) into the right hind foot paw of mice, while the control group received a PBS injection (25 μL, i.a.). Mice injected with MSU crystals exhibited significant foot and paw swelling compared to the control group. However, treatment with Mast-M significantly inhibited the swelling of the foot and paw (Figure 5A–D). After 24 hours of model establishment, the mice were euthanized for subsequent experiments. Mast-M treatment effectively suppressed the excessive production of IL-1β in the edema tissue homogenates (Figure 5C). Hematoxylin and eosin (H&E) staining demonstrated that MSU administration led to increased infiltration of inflammatory cells, whereas Mast-M treatment significantly reduced the infiltration of inflammatory cells (Figure 5D and E). Immunohistochemistry (IHC) staining using a Ly6G antibody or an F4/80 antibody was conducted to assess the aggregation of neutrophils and macrophages in joint sections. The IHC results revealed that MSU crystals recruited a large number of neutrophils and macrophages to the synovial tissue. However, Mast-M and colchicine effectively reduced the accumulation of neutrophils and macrophages (Figure 5D–G). These findings indicate that Mast-M is effective in reducing swelling and decreasing inflammatory cell infiltration in mice with gouty arthritis.
Mast-M Inhibited NLRP3 Inflammasome Activation in the Gout Mouse Model
The qPCR results showed that Mast-M significantly downregulated the excessive expression of NLRP3, Caspase 1, ASC, and IL-1β induced by MSU crystals at the gene level in mouse paw joint tissues (Figure 6A–D). Similarly, the Western blot results also demonstrated that after treatment with Mast-M, the protein expression level of NLRP3, Caspase1, ASC, and IL-1β (Figure 6E and F) was suppressed. Hence, these results provide evidence that Mast-M effectively blocked the MSU-induced activation of NLRP3 inflammasomes in vivo.
Discussion
The deposition of MSU crystals serves as the clinicopathological basis of gouty arthritis.1 In this study, a mouse model of gouty arthritis was constructed by injecting MSU crystals into the hind paw of mice to study the therapeutic effect of Mast-M. Injected MSU crystals can cause inflammatory effusion of the synovial membrane, congestion, and swelling of periarticular tissues.31 Therefore, the degree of joint swelling can reflect the severity of acute gouty arthritis and is widely used to evaluate the effectiveness of the treatment of gouty arthritis.32 In the current study, we have shown that Mast-M has a therapeutic effect on MSU-induced gouty arthritis. Mast-M effectively alleviated MSU-induced joint swelling, reduced the production of inflammatory factors, and inhibited the aggregation of neutrophils and macrophages in the paw tissue of mice, similar to the effects observed with colchicine treatment. Moreover, in THP-1 cells, Mast-M pretreatment significantly inhibited the activation of LPS plus MSU-induced NLRP3 inflammasomes. Furthermore, Mast-M demonstrated inhibitory effects on MAPKs/NF-κB signaling and ROS activation. Through our study, we have revealed the potential of Mast-M as a treatment for gouty arthritis and have provided insights into its possible mechanisms of action.
Wasp venom is considered to be a natural toxin with powerful anti-inflammatory properties.33 Our previous study found that at lower concentrations, either wasp venom mixture or Mast-M alone had a notable suppressive effect on the generation of inflammatory cytokines, such as IL-1β and TNF-α.13,34 The findings from this study indicate that Mast-M might effectively suppress the inflammatory response in acute gouty arthritis by diminishing the production of the pro-inflammatory cytokine IL-1β and inhibiting the aggregation of neutrophils and macrophages. The evidence from the present study further deepens our understanding of the anti-inflammatory activity of Mast-M.
When MSU crystals act on macrophages, it triggers activation of NLRP3 inflammasome, which is composed of NLRP3/ASC/caspase1 and cleaves pro-IL-1β into mature IL-1β.8 IL-1β promotes neutrophil recruitment at sites of inflammation as well as activates peripheral nociceptive receptors, causing intense pain and subsequently triggering the initiation of gout inflammation.8,23 Hence, it becomes evident that the successful inhibition of NLRP3 inflammasome activation and the release of inflammatory factors are crucial for providing relief in cases of gout. Our data show that Mast-M significantly reduces the expression of NLRP3, ASC, caspase1, and IL-1β in both in vivo and in vitro models. These findings indicate that Mast-M holds the potential to alleviate gouty arthritis by inhibiting the activation of NLRP3 inflammasome.
The activation of both NF-κB and MAPK pathways can induce the activation of the NLRP3 inflammasome.35,36 In the NF-κB signaling pathway, NF-κB forms a complex with its inhibitor IκB-α, keeping it in an inactive state. However, upon stimulation by bacterial component LPS, IκB is phosphorylated by its specific kinase, IKK (IκB kinase). This phosphorylation event triggers the ubiquitination of IκB-α, leading to its degradation by the ubiquitin-proteasome system. Consequently, with the degradation of IκB-α, NF-κB is released and translocates to the cell nucleus. This process activates the transcription of downstream genes, including various inflammatory factors and immune-related molecules, such as NLRP3, IL-1β, COX-2, and TNF-α.7,37,38 On the other hand, MAPKs are considered essential regulators of inflammatory responses and innate immunity. They are closely associated with the NF-κB signaling pathway, participating in the activation of NF-κB, and they also have the ability to block the activation of the NLRP3 inflammasome.20,31,39,40 Therefore, the protective effect of dampening the inflammatory response can be achieved through the inhibition of both NF-κB and MAPK signaling pathways. In this study, Mast-M inhibited NLRP3 inflammasome priming by blocking the phosphorylation of P65, IκB-α, and IKK in the NF-κB signaling pathway. Additionally, Mast-M disrupted the MAPK signaling pathway by downregulating the P-P38/P38 and P-JNK/JNK ratios. These findings indicate that Mast-M inhibits the activation of the NLRP3 inflammasome by interfering with the NF-κB and MAPK signaling pathways.
According to recent research, ROS serves as a crucial signaling event triggering the NLRP3 inflammasome activation, and eliminating ROS can hinder NLRP3 inflammasome activation.41,42 Additionally, the Nrf2 signaling pathway promotes the expression of downstream antioxidant enzymes, such as HO-1, SOD2, glutathione, and NAD(P)H quinone oxidoreductase 1, to mitigate oxidative stress and act as negative regulators of the NLRP3 inflammasome.43,44 In our study, we observed that Mast-M reversed the induction of ROS by LPS + MSU in vitro, indicating its ability to mitigate oxidative stress. Moreover, Mast-M treatment promoted the expression of Nrf2, HO-1, and SOD2, which are important antioxidants. These findings suggest that Mast-M exerts its inhibitory effects on NLRP3 inflammasome activation by reducing ROS production and promoting the expression of antioxidant enzymes through the Nrf2 signaling pathway.
Next, we examined whether Mast-M positively impacted a gout mouse model. The results showed that Mast-M significantly inhibited paw swelling as well as IL-1β production, with effects close to those of the positive control drug colchicine. In addition, histological analysis showed that Mast-M significantly inhibited the infiltration of inflammatory cells into the joint tissue. Further analysis showed that Mast-M was able to inhibit the protein and transcript levels of NLRP3, ASC, Caspase1, and IL-1β. Based on these findings, Mast-M appears to have a therapeutic impact on mice with gout by hindering the activation of the NLRP3 inflammasome.
Together, our research results indicate that Mast-M effectively alleviates the inflammatory response and oxidative stress in THP-1 cells and a mouse model of gouty arthritis, providing experimental evidence for the traditional use of wasp venom in treating gouty arthritis. However, whether Mast-M can reverse the formation of tophi in chronic gouty arthritis and its potential impact on hyperuricemia remains unclear. Additionally, attention should be given to potential toxic side effects with the increasing dosage of Mast-M. Although no toxic side effects were observed in macrophages at concentrations of 0.25 μg/mL to 1.0 μg/mL, the therapeutic effects on certain indicators may weaken with higher doses. Further research is needed to assess the effective dosage range and safety of Mast-M in future studies.
Conclusion
In summary, Mast-M, at least in part, inhibits the activation of the NLRP3 inflammasome by blocking the MAPK/NF-κB signaling pathway and oxidative stress, thereby exhibiting a therapeutic effect in MSU-induced gouty arthritis (Figure 7). Overall, Mast-M demonstrates characteristics that make it a potential candidate for treating gouty arthritis.
 |
Figure 7 Schematic diagram showing the effect of Mast-M on LPS plus MSU-stimulated NLRP3 inflammasome activation in macrophages. |
Abbreviations
ASC, apoptosis-associated speck-like protein containing a CARD; CASP 1, caspase-1; COX-2, cyclooxygenase-2; IL-1β, interleukin - 1β; LPS, lipopolysaccharides; MAPK, mitogen-activated protein kinase; Mast-M, Mastoparan M; MSU, monosodium urate; NF-κB, nuclear factor – kappa B; NLRP3, Nod-Like Receptor Protein 3; NRF2, nuclear factor-erythroid 2-related factor 2; PMA, Phorbol 12-myristate 13-acetate; ROS, reactive oxygen species; SOD, superoxide dismutase.
Funding
This work was supported by the National Natural Science Foundation of China (No.82370895, No.81570772, No.82160798, and No.82260163).
Disclosure
The authors declare no conflicts of interest.
References
1. Dalbeth N, Choi HK, Khanna PP, et al. Gout. Nat Rev Dis Primers. 2019;5(1):69.
2. Dalbeth N, Gosling AL, Gaffo A, et al. Gout. Lancet. 2021;397(10287):1843–1855. doi:10.1016/S0140-6736(21)00569-9
3. Pillinger MH, Mandell BF. Therapeutic approaches in the treatment of gout. Semin Arthritis Rheum. 2020;50(3s):S24–s30. doi:10.1016/j.semarthrit.2020.04.010
4. Stamp LK, Farquhar H. Treatment advances in gout. Best practice & research. Clinical Rheumatol. 2021;35(4):101719. doi:10.1016/j.berh.2021.101719
5. Renaudin F, Orliaguet L, Castelli F, et al. Gout and pseudo-gout-related crystals promote GLUT1-mediated glycolysis that governs NLRP3 and interleukin-1β activation on macrophages. Ann Rheum Dis. 2020;79(11):1506–1514. doi:10.1136/annrheumdis-2020-217342
6. Ostareck DH, Ostareck-Lederer A. RNA-binding proteins in the control of LPS-induced macrophage response. Front Genet. 2019;10:31. doi:10.3389/fgene.2019.00031
7. Li Z, Pan H, Yang J, et al. Xuanfei Baidu formula alleviates impaired mitochondrial dynamics and activated NLRP3 inflammasome by repressing NF-κB and MAPK pathways in LPS-induced ALI and inflammation models. Phytomedicine. 2023;108:154545. doi:10.1016/j.phymed.2022.154545
8. Martinon F, Pétrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
9. Kingsbury SR, Conaghan PG, McDermott MF. The role of the NLRP3 inflammasome in gout. J Inflamm Res. 2011;4:39–49. doi:10.2147/JIR.S11330
10. Liu HJ, Pan -X-X, Liu B-Q, et al. Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J Neuroinflammation. 2017;14(1):74. doi:10.1186/s12974-017-0849-y
11. Cabău G, Crișan TO, Klück V, et al. Urate-induced immune programming: consequences for gouty arthritis and hyperuricemia. Immunol Rev. 2020;294(1):92–105. doi:10.1111/imr.12833
12. Wang Y, Zhu W, Lu D, et al. Tetrahydropalmatine attenuates MSU crystal-induced gouty arthritis by inhibiting ROS-mediated NLRP3 inflammasome activation. Int Immunopharmacol. 2021;100:108107. doi:10.1016/j.intimp.2021.108107
13. Wang M, Wu X-M, He M, et al. Mastoparan M extracted from Vespa magnifica alleviates neuronal death in global cerebral ischemia-reperfusion rat model. Iran J Basic Med Sci. 2022;25(3):320–329. doi:10.22038/IJBMS.2022.60745.13461
14. Moreno M, Giralt E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: melittin, apamin and mastoparan. Toxins. 2015;7(4):1126–1150. doi:10.3390/toxins7041126
15. Gao Y, Yu W-X, Duan X-M, et al. Wasp venom possesses potential therapeutic effect in experimental models of rheumatoid arthritis. Evid Based Complement Alternat Med. 2020;2020:6394625. doi:10.1155/2020/6394625
16. Yun HS, Oh J, Lim JS, et al. Anti-inflammatory effect of wasp venom in BV-2 microglial cells in comparison with bee venom. Insects. 2021;12(4):297. doi:10.3390/insects12040297
17. Xu X, Yang H, Yu H, et al. The mastoparanogen from wasp. Peptides. 2006;27(12):3053–3057. doi:10.1016/j.peptides.2006.09.003
18. Li ML, Liao R-W, Qiu J-W, et al. Antimicrobial activity of synthetic all-D mastoparan M. Int J Antimicrob Agents. 2000;13(3):203–208. doi:10.1016/S0924-8579(99)00127-2
19. Mansour AA, Raucci F, Saviano A, et al. Galectin-9 regulates monosodium urate crystal-induced gouty inflammation through the modulation of Treg/Th17 Ratio. Front Immunol. 2021;12:762016. doi:10.3389/fimmu.2021.762016
20. Zhao W, Ma L, Cai C, et al. Caffeine inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-Induced THP-1 Macrophages. Int J Biol Sci. 2019;15(8):1571–1581. doi:10.7150/ijbs.34211
21. Xie D, Zhao H, Lu J, et al. High uric acid induces liver fat accumulation via ROS/JNK/AP-1 signaling. Am J Physiol Endocrinol Metab. 2021;320(6):E1032–e1043. doi:10.1152/ajpendo.00518.2020
22. Wood DD, Ihrie EJ, Dinarello CA, et al. Isolation of an interleukin-1-like factor from human joint effusions. Arthritis Rheum. 1983;26(8):975–983. doi:10.1002/art.1780260806
23. Di Giovine FS, Malawista SE, Nuki G, et al. Interleukin 1 (IL 1) as a mediator of crystal arthritis. Stimulation of T cell and synovial fibroblast mitogenesis by urate crystal-induced IL 1. J Immunol. 1987;138(10):3213–3218. doi:10.4049/jimmunol.138.10.3213
24. Solomon DH, Glynn RJ, MacFadyen JG, et al. Relationship of interleukin-1β blockade with incident gout and serum uric acid levels: exploratory analysis of a randomized controlled trial. Ann Intern Med. 2018;169(8):535–542. doi:10.7326/M18-1167
25. Schlesinger N, Mysler E, Lin H-Y, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70(7):1264–1271. doi:10.1136/ard.2010.144063
26. Mei Y, Dong B, Geng Z, et al. Excess uric acid induces gouty nephropathy through crystal formation: a review of recent insights. Front Endocrinol. 2022;13:911968. doi:10.3389/fendo.2022.911968
27. An Y, Zhang H, Wang C, et al. Activation of ROS/MAPK s /NF- κ B/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB j. 2019;33(11):12515–12527. doi:10.1096/fj.201802805RR
28. Peng L, Wen L, Shi Q-F, et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020;11(11):978. doi:10.1038/s41419-020-03178-2
29. Fernando IPS, Dias MKHM, Madusanka DMD, et al. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int J Biol Macromol. 2021;168:620–630. doi:10.1016/j.ijbiomac.2020.11.115
30. Xiao Q, Piao R, Wang H, et al. Orientin-mediated Nrf2/HO-1 signal alleviates H(2)O(2)-induced oxidative damage via induction of JNK and PI3K/AKT activation. Int J Biol Macromol. 2018;118(Pt A):747–755. doi:10.1016/j.ijbiomac.2018.06.130
31. Lin X, Wang H, An X, et al. Baeckein E suppressed NLRP3 inflammasome activation through inhibiting both the priming and assembly procedure: implications for gout therapy. Phytomedicine. 2021;84:153521. doi:10.1016/j.phymed.2021.153521
32. Ouyang X, Li N-Z, Guo M-X, et al. Active flavonoids from lagotis brachystachya attenuate monosodium urate-induced gouty arthritis via inhibiting TLR4/MyD88/NF-κB Pathway and NLRP3 expression. Front Pharmacol. 2021;12:760331. doi:10.3389/fphar.2021.760331
33. Abd El-Wahed A, Yosri N, Sakr HH, et al. Wasp venom biochemical components and their potential in biological applications and nanotechnological interventions. Toxins. 2021;13(3):206. doi:10.3390/toxins13030206
34. Zhao H, Wang M, Huang X, et al. Wasp venom from Vespa magnifica acts as a neuroprotective agent to alleviate neuronal damage after stroke in rats. Pharm Biol. 2022;60(1):334–346. doi:10.1080/13880209.2022.2032207
35. Liu Z, Yao X, Jiang W, et al. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J Neuroinflammation. 2020;17(1):90. doi:10.1186/s12974-020-01751-2
36. Fann DY-W, Lim Y-A, Cheng Y-L, et al. Evidence that NF-κB and MAPK signaling promotes NLRP inflammasome activation in neurons following ischemic stroke. Mol Neurobiol. 2018;55(2):1082–1096. doi:10.1007/s12035-017-0394-9
37. Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223–244. doi:10.1038/cr.2011.13
38. Perkins ND. The diverse and complex roles of NF-κB subunits in cancer. Nat Rev Cancer. 2012;12(2):121–132.
39. Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13(9):679–692.
40. Wang M, Xu H, Chong Lee Shin OL, et al. Compound α-keto acid tablet supplementation alleviates chronic kidney disease progression via inhibition of the NF-kB and MAPK pathways. J Transl Med. 2019;17(1):122.
41. Yin C, Liu B, Wang P, et al. Eucalyptol alleviates inflammation and pain responses in a mouse model of gout arthritis. Br J Pharmacol. 2020;177(9):2042–2057. doi:10.1111/bph.14967
42. Abais JM, Xia M, Zhang Y, et al. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–1129. doi:10.1089/ars.2014.5994
43. Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–336. doi:10.1016/j.freeradbiomed.2015.05.036
44. Zhou Y, Gao C, Vong CT, et al. Rhein regulates redox-mediated activation of NLRP3 inflammasomes in intestinal inflammation through macrophage-activated crosstalk. Br J Pharmacol. 2022;179(9):1978–1997. doi:10.1111/bph.15773
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.