Back to Journals » Journal of Inflammation Research » Volume 15
Mast Cell Specific Receptor Mrgprb2 Regulating Experimental Colitis is Associated with the Microbiota-Gut-Brain Axis
Authors Shao M, Yuan F, Liu J, Luo H
Received 1 August 2022
Accepted for publication 20 October 2022
Published 9 November 2022 Volume 2022:15 Pages 6137—6151
DOI https://doi.org/10.2147/JIR.S383812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ming Shao,1,2,* Fangting Yuan,1,2,* Jingwen Liu,1,2 Hesheng Luo1,2
1Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, 430060, People’s Republic of China; 2Hubei Key Laboratory of Digestive Diseases, Wuhan, 430060, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hesheng Luo, Department of Gastroenterology, Renmin Hospital of Wuhan University, Wuhan, 430060, People’s Republic of China, Email [email protected]
Purpose: Ulcerative colitis (UC) patients have disturbances in the microbiota-gut-brain axis, and mast cells are important components of this axis. The mast cell-specific receptor Mrgprb2 has effects on host defense against bacterial infection and neurogenic inflammation, which may help mast cells act on the axis. This study analyzed how Mrgprb2 participates in the pathogenesis of UC by affecting the microbiota-gut-brain axis.
Materials and Methods: Mrgprb2 knockout (b2KO) mice and wild-type (WT) mice were fed 2% (w/v) dextran sulfate sodium (DSS) in drinking water for 7 days, which was then replaced with normal water for 14 days. This cycle was repeated three times. Feces were collected on Days 21, 42, and 63 for intestinal microbiota analysis, and mice were euthanized on Day 64. Hypothalamus, amygdala and colon tissues were removed and analyzed.
Results: Compared with WT mice, B2KO mice exhibited increased weight loss, colon shortening and colonic pathological damage after colitis induction. Analysis of the intestinal microbiota showed that b2KO mice with colitis had a significant decrease in the abundance and diversity, as well as an increase in Allobaculum and a decrease in norank_f__Muribaculaceae and Ileibacterium. In colon tissues, the expression of mucin 2 (MUC2) and junctional adhesion molecule A (JAM-A) in b2KO mice was reduced, and oxidative stress levels were higher. B2KO mice with colitis had higher corticotropin-releasing hormone (CRH), corticotropin-releasing hormone receptor 1 (CRHR1), neuropeptide Y (NPY) and brain-derived neurotrophic factor (BDNF) mRNA levels in hypothalamus tissues and glucocorticoid receptor mRNA levels in the amygdala.
Conclusion: In the microbiota-gut-brain axis, Mrgprb2 was involved in regulating the intestinal microbiota composition, intestinal barrier and oxidative stress levels, and was related to stress regulation, which might help to explain the pathogenesis of UC.
Keywords: ulcerative colitis, mast cells, Mrgprb2, microbiota-gut-brain axis
Introduction
UC is a chronic inflammatory disease with increasing global prevalence.1–3 The microbiota-gut-brain axis describes the complex interactions among the gut microbiota, gastrointestinal tract, and neuroendocrine pathways and the central, peripheral, and autonomic nervous systems, which are disordered in UC patients.4–6 In patients with active UC, specific components of the gut microbiota were found to be associated with depression and anxiety.7 The gut-brain bidirectional communication has been indicated as a possible basis for their association between depression/ anxiety and UC.4,8 Stress activates the brain-gut axis and results in mast cells activation and release of proinflammatory cytokines and other endocrine and humoral mediators.9 Mast cells are considered prototypical neuroimmune cells located at the host–environment interface near sensory nerves and are thought to play an important role in the microbiota-gut-brain axis.4,10
Recently, a Mas-related G protein-coupled receptor X2 (MRGPRX2) has attracted much attention and is thought to be related to UC.11 Human MRGPRX2 and its mouse ortholog Mrgprb2, which recognize cationic neuropeptides, antimicrobial peptides, and insect venom peptides, are selectively expressed on connective tissue mast cells except for in the dorsal root ganglia.12,13 MRGPRX2/Mrgprb2 contributes to host defense against bacterial infection and skin wound healing and is involved in the neurogenic inflammatory response,14 which may assist mast cells in playing an important role in the microbiota-gut-brain axis.
UC patients have decreased diversity and increased instability with respect to the composition of the gut microbiota.15 Active UC patients have reduced MUC2 synthesis and an inner mucus layer that is penetrable.16 Bacteria tend to penetrate the inner mucous layer abnormally.17,18 Epithelial cells secrete β-defensins after microbial infection, which induce mast cell chemotaxis and degranulation through MRGPRX2.12 Activation of mast cells by MRGPRX2 and Mrgprb2 promotes bacterial killing, and Mrgprb2-deficient mice are more susceptible to bacterial infection.19 Patients with UC are in a long-term state of stress. In response to stress, the hypothalamic–pituitary–adrenal axis (HPA) triggered by CRH is activated, and several peptides associated with stress regulation and energy metabolism, such as NPY and BDNF, are released.20–22 In UC, the intestinal nervous system interacts with mast cells via several mediators, such as tumor necrosis factor alpha (TNF-α), nerve growth factor (NGF), and substance P (SP), and they are actively involved in inflammation.23,24 SP activation of mast cells via Mrgprb2 mediates inflammatory mechanical and thermal hyperalgesia by facilitating immune cell migration and leading to the release of multiple cytokines and chemokines.25
In brain-gut axis, changes in corticotropin-releasing hormone, mast cell activity, autonomic nervous system neurotransmission, and intestinal barrier function affect the pathogenesis of colitis.9 As an important receptor on mast cells, MRGPRX2/Mrgprb2 plays an important role in host defense and neurogenic inflammation, which may assist mast cells to participate in the communication between the microbiota-gut-brain axis during the pathogenesis of colitis. At present, there is little research on MRGPRX2/Mrgprb2 and UC. In this study, we will analyze how mrgprb2 regulates the severity of experimental colitis by affecting the microbiota-gut-brain axis.
Materials and Methods
Animals
Six- to eight-week-old B2 KO C57BL/6J mice were purchased from GemPharmatech Co., Ltd. (Jiangsu, China) and were crossed with WT C57BL/6J mice purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) to obtain homozygous b2 KO male mice and WT mice. All mice were raised in an SPF environment in Renmin Hospital of Wuhan University Animal Experiment Center under a 12-h/12-h dark/light cycle with an ambient temperature of 22 ℃±1 ℃ and 50%±10% humidity. Animal experiments were approved by the Animal Care and Ethics Committee of Wuhan University (Approval No. 20210303) according to the guidelines of the Care and Use of Laboratory Animals published by the National Institutes of Health.
DSS-Induced Colitis
Eight-week-old male mice were randomly divided into 4 groups: the control group (n=8), DSS group (n=15), b2KO group (n=8), and b2KODSS group (n=15). In the DSS and b2KODSS groups, chronic colitis was induced by providing mice with drinking water containing 2% (wt/vol) DSS (36 to 50 kDa, MP Biomedicals, Santa Ana, CA, USA) for 7 days and normal water for 14 days, which was cycled three times. Mice in the control group and b2KO group were given normal drinking water without DSS and served as a negative control. Weight was recorded every three days, and feces were collected on Days 21, 42, and 63. Body weight, the stool consistency and hematochezia were recorded weekly to determine the disease activity index (DAI). At Day 64, the mice were anesthetized for blood collection, and then the mice were euthanized while unconscious by cervical dislocation. Their hypothalamus, amygdala and colon were removed to perform relevant biochemical tests.
Histologic Analysis
The colonic tissues were fixed in 4% paraformaldehyde, embedded in paraffin and sliced into 4-µm sections for hematoxylin-eosin (H&E) staining. Five visual fields were selected for each slice and evaluated in a blinded manner by an experienced pathologist according to published guidelines: (a) crypt distortion and loss (normal to severe, 0−3); (b) inflammatory cell infiltration (normal to intensive, 0−3); (c) muscle thickening (presence of significant muscle thickening, 0−3); (d) goblet cell depletion (absence and presence, 0−1); and (e) crypt abscess (absence and presence, 0−1).26
Total RNA Extraction and Quantitative Real-Time PCR
Total RNA was isolated from hypothalamus, amygdala and colon tissues by using TRI-Reagent (Service bio, Wuhan, China). The quality of the total RNA was determined by a Nano Drop 2000 UV‒Vis spectrophotometer (Thermo Scientific, Wilmington, USA). cDNA was synthesized using a Servicebio® RT First Strand cDNA Synthesis Kit (Service bio, Wuhan, China). Quantitative real-time PCR was performed on a CFX Opus Real-Time PCR System (Bio-Rad, California, USA) with SYBR Green qPCR Master Mix (None ROX) (Service Bio, Wuhan, China) according to the manufacturer’s directions. PCR amplification was performed with the following conditions: 95 ℃ for 10 min, followed by 40 cycles of 95 ℃ for 15 sec and 60 ℃ for 30sec. All samples were analyzed three times, and GAPDH was used as the reference gene. The results were analyzed by the 2-ΔΔCT method. The primers used in this experiment are listed in Table 1.
 |
Table 1 Details of the Primer Sequences Used in the Study |
Elisa Analysis
The concentration of adrenocorticotropic hormone (ACTH), corticosterone (CORT), TNF-ɑ, myeloperoxidase (MPO), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH) and histamine in serum and/or colon including were detected with ELISA kits (Multi sciences Biotech, Co., Ltd, Hangzhou, China) following instruction.
Analysis of the Composition of Intestinal Microbiota
Mouse feces were collected and stored at −80 ℃ after freezing in liquid nitrogen. The E.Z.N.A.® soil DNA kit (Omega Bio-Tek, GA, USA) was used to extract genomic DNA. The quality and integrity of the extracted DNA were assessed with 1% agarose gel electrophoresis, and the concentration and purity of DNA were determined by a NanoDrop 2000 UV‒Vis spectrophotometer (Thermo Scientific, Wilmington, USA). The hypervariable region V3-V4 of the bacterial 16S rRNA gene was amplified with the primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGA CTACHVGGGTWTCTAAT-3’) by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA). The PCR conditions for amplifying the 16S rDNA gene were as follows: initial denaturation at 95 ℃ for 3 min, followed by 27 cycles of denaturing at 95 ℃ for 30s, annealing at 55 ℃ for 30s and extension at 72 ℃ for 45s, and a single extension at 72 ℃ for 10 min, ending at 4 ℃. PCR products were sequenced on the Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, USA).
Short-Chain Fatty Acids (SCFAs) Profiling
One hundred milligrams of solid feces samples was accurately weighed, and the SCFAs were extracted using 900 µL of methanol and 100 µL of 2-ethylbutyric acid (1000 μg/mL) as internal standards. The mixture was homogenized at 50 Hz for 3 min at −10 ℃ with a mill bead and then subjected to ultrasound treatment at 40 kHz for 30 min in an ice bath. The sample was allowed to settle at −20 ℃ for 30 min, and 200 µL of supernatant was transferred to a 1.5-mL EP tube after centrifugation at 13,000 × g at 4 ℃ for 15 min. Finally, 50 mg of anhydrous sodium sulfate was added to the new tube followed by vortexing. After centrifugation at 13,000 × g at 4 ℃ for 15 min, the supernatant was carefully transferred to sample vials for analysis. Agilent 8890B-5977B GC/MSD gas chromatography (Agilent Technologies Inc. CA, UAS) was used as the analysis instrument.
Statistical Analysis
GraphPad Prism version 9.20 for Windows (GraphPad Software, San Diego, CA, USA) was used to analyze the experimental data. All the data are presented as the mean ± standard error of the mean. The Shapiro–Wilk test was used to test for the normal distribution of numerical data. Comparisons between pairs of groups were analyzed using one-way ANOVA followed by a post hoc Bonferroni comparison. Dunn’s nonparametric test was used for data not conforming to a normal distribution. Two-way ANOVA was used for grouping samples followed by a post hoc Bonferroni comparison. The Bray‒Curtis distance algorithm was used for principal coordinates analysis (PCoA), and ANOSIM was used to test the difference between groups. A P value < 0.05 was considered statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
Results
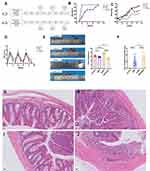
Effects of Mrgprb2 on mortality, weight loss, DAI, colon length, and histological scores in mice with colitis
The experimental design is shown in Figure 1A. The B2KODSS group showed a higher mortality rate at the first DSS feeding cycle, but at the end of the three cycles, the b2KODSS group and DSS group had the same mortality rate (Figure 1B). In addition, the b2KODSS group had greater body weight loss, higher DAI score, more shortening of colons and higher histological scores (Figure 1C–J).
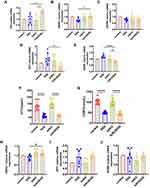
Effects of Mrgprb2 on the HPA axis and BDNF and NPY release in mice with colitis
The CRH mRNA expression level in the b2KODSS group was higher than that in the b2KO group and DSS group (Figure 2A). The mRNA expression level of CRHR1 in the hypothalamus of the b2KODSS group was higher than that of the DSS group, and there was no difference in the mRNA expression level of CRHR2 between the two groups (Figure 2B and C). The mRNA expression level of NPY in hypothalamus tissue in b2KODSS group was lower than that in DSS group (Figure 2D). The mRNA expression level of BDNF in b2KODSS group was lower than that in DSS group in hypothalamus (Figure 2E). The serum ACTH and CORT levels in the two DSS treatment groups were lower than those in the control group, and there was no difference in the serum ACTH and CORT levels between b2KODSS group and DSS group (Figure 2F and G). The mRNA expression level of glucocorticoid receptor (encoded by the NR3C1 gene) in b2KODSS group was higher than that in DSS group (Figure 2H). There was no difference in the mRNA expression levels of NPY and BDNF in the amygdala between the two groups (Figure 2I and J).
Effect of Mrgprb2 on Intestinal Microbiota Composition in Mice with Colitis
The alpha diversity and beta diversity of the samples were analyzed. The Sob index and Shannon index of the b2KODSS group decreased gradually (Figure 3A and B). The rarefaction curve plateaued, indicating that the sequencing depth was sufficient and the sequencing result was credible (Figure 3C). PCoA on Days 21, 42 and 63 showed gradual separation between the four groups, and the loss of Mrgprb2 and DSS treatment might be the reason for the separation (Figure 3D–F).
Figure 3 Continued. Figure 3 Continued.

A Venn diagram revealed that 159 operational taxonomic units (OTUs) coexisted in the four groups, and the b2KODSS group had the lowest number of OTUs on Day 63 (Figure 3G). We analyzed the species composition of the four groups at Days 21, 42, and 63 at the phylum and genus levels (Figure 3H and I). We compared the phylum level and genus level (Supplementary Figure 1A and B) of the two control groups and found that there was no significant difference between the two groups at the phylum level except for some fluctuation in the Bacteroidota content. At the genus level, the contents of norank_f__Muribaculaceae and Lachnospiraceae_NK4A136_group fluctuated between the two groups. The level of Dubosiella in the control group was significantly higher than that in the b2KO group on Day 63. Then, we compared the differences between the two DSS treatment groups at the phylum and genus levels (Supplementary Figure 1C and D). Although there were some fluctuations, the overall trend was that the content of Bacteroidota in the b2KODSS group was lower than that in the DSS group, while the content of Firmicutes was higher than that in the DSS group. At the genus level, the contents of norank_f__Muribaculaceae and Ileibacterium in the b2KODSS group were lower than those in the DSS group, while the content of Allobaculum was higher than that in the DSS group on Day 63. The distribution proportion of dominant species in each group and the distribution proportion of dominant species in different groups were reflected through the visual circle diagram on Day 63 (Figure 3J).
We also analyzed the changes in the composition of the DSS and b2KODSS groups over time. On Days 21, 42 and 63, the content of Bacteroidota in the DSS group gradually decreased, while that of Firmicutes gradually increased (Figure 3K). At the genus level, the content of norank_f__Muribaculaceae decreased while that of Ileibacterium increased (Figure 3L). On day 63 in b2KODSS group, the content of Firmicutes and Actinobacteriota increased significantly, while the content of Bacteroidota decreased (Figure 3M). At the genus level, norank_f__Muribaculaceae, Lachnospiraceae_NK4A136_group and Bacteroides in b2KODSS group were significantly reduced. The content of Allobaculum increased significantly. In addition, the levels of Dubosiella, Lactobacillus and Bifidobacterium were gradually increased (Figure 3N).
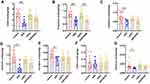
Effects of Mrgprb2 on SCFAs in Mice with Colitis
The levels of acetic acid, propanoic acid, isobutyric acid, butanoic acid, isovaleric acid and hexanoic acid were significantly lower in the DSS group than in the control group. The level of propanoic acid in the b2KODSS group was lower than that in the b2KO group (Figure 4A–G).
Effects of Mrgprb2 on Intestinal Barrier in Mice with Colitis
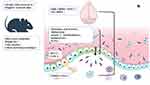
The mRNA expression levels of MUC2 and JAM-A in b2KODSS group were lower than those in DSS group (Figure 5A and B). The MUC2 expression level in both DSS treatment groups was lower than that in the two control groups. The JAM-A expression level in the DSS group was lower than that in the control group, and there was no statistically significant difference between the b2KO group and the b2KODSS group. In colon tissues, both DSS treatment groups had higher levels of TNF-ɑ, MPO, SOD, MDA, and GSH release than the two control groups (Figure 5C–G). The B2KODSS group showed lower levels of TNF-ɑ and MPO and higher levels of MDA and GSH than the DSS group. There was no difference in the mRNA levels of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) among the four groups (Figure 5H and I). In serum, there was no significant difference in TNF-ɑ between the b2KODSS group and the DSS group (Figure 5J). Histamine level and the mRNA expression level of mouse mast cell protease 6 (mMCP6) in the b2KODSS group were lower than in the DSS group, although the differences were not statistically significant. The mRNA expression level of mouse mast cell protease 4 (mMCP4) in b2KODSS group was lower than that in DSS group (Figure 5K–M). The complete mechanism diagram was shown in Figure 5N.
Figure 5 Continued.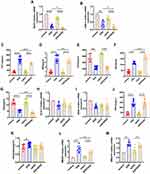
Discussion
Intestinal bacteria are mainly composed of four phyla, namely, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, and Firmicutes and Bacteroidetes are dominant in healthy adults.27 The microbiota changed in patients with UC, including reduced diversity and increased variability.28 We found that the abundance and diversity of intestinal microbiota in the b2KODSS group decreased gradually. The abundance of Allobaculum in mice is associated with a high-fat diet,29 and members of this genus can produce butyric acid.30 However, Allobaculum mucolyticum also plays an important role in the development of intestinal inflammation because it can secrete large amounts of mucin o-glycans that target carbohydrate-active enzymes (CAZymes), which can efficiently degrade and feed on intestinal mucin and facilitate bacterial colonization and degradation of the mucus layer.31 The beneficial probiotic Ileibacterium is correlated with serum lipid levels in atherosclerotic mice.32 As a kind of butyrate-producing bacteria, Lachnospiraceae_NK4A136_group is able to maintain gut barrier integrity in mice.33 Bacteroides are beneficial microbes for human health because they provide protection from pathogens and afford nutrients to other microbiota in the gut.34 Along with these adverse factors that might lead to intestinal homeostasis disorders, there was an increase in the growth of some probiotics. Dubosiella is positively correlated with the butyric acid level,35 and Lactobacillus, Bifidobacterium and Coriobacteriaceae_ UCG- 002 are beneficial probiotic genera.36 These bacteria increased as the disease progressed in the b2KODSS group and DSS group but were still lower than those in the b2KO and control groups. SCFAs can be passively taken up by epithelial cells and can provide 60–70% of their energy supply.37 UC patients had reduced SCFAs and SCFAs producing bacteria38,39 and in our study SCFAs levels were reduced in both DSS treatment groups. However, there was no significant difference in SCFAs between the two DSS-treated groups. At present, there is no research on Mrgprb2 and SCFAs. The connection between them requires further exploration.
The intestinal mucus barrier protects epithelial cells and the underlying immune system from bacterial contact and false activation.1 In mice and the human colon, an inner mucus layer formed by MUC2 separates bacteria from epithelial cells, and MUC2-deficient mice have bacteria that come into direct contact with epithelial cells and spontaneously develop colitis, which later develops into colon cancer.18 As an epithelial tight junction protein, JAM-A plays an important role in intestinal homeostasis by regulating epithelial permeability, inflammation, and proliferation.40 Our study found that the mRNA expression levels of MUC2 and JAM-A in b2KO mice were lower than those in WT mice after the induction of colitis. More researches were needed to see if this was related to the increase in bacteria that degrade intestinal mucus like Allobaculum. Damaged intestinal barrier allows bacteria to penetrate and reach the epithelium and induced inflammation.
Streptococcus pneumoniae released CSP-1 peptides to activated Mrgprb2 and MRGPRX2, causing mast cells to degranulate and release antimicrobial mediators such as ROS, TNF-a and PGD2.19 Cytokines and lipid mediators derived from mast cells recruit immune cells such as neutrophils and clear infections. B2KO mice had reduced secretion of cytokines from mast cells, expressed fewer MPO, and were more susceptible to bacterial infection.19,25 We found that b2KO mice with colitis had reduced TNF-a secretion, decreased MPO expression, and higher oxidative stress levels in the colon, which might suggest the role of Mrgprb2 in resistance to intestinal barrier damage and bacterial invasion present in colitis.
HPA axis plays an important role in modulating immune reactions in UC.41 CRH is a major regulator of the HPA axis and principal coordinator of the stress response while Glucocorticoid receptor (NR3C1) expression is considered as an indicator of stress hormone action within the brain.42,43 Mice deficient for CRHR1 display a severe impairment of stress-induced ACTH release from pituitary corticotropes, marked glucocorticoid deficiency, impaired stress response and significantly reduced anxiety-like behavior.44,45 CRHR2-mutant mice are hypersensitive to stress and display increased anxiety-like behaviour.46 CRH, CRHR, other neuropeptides such as SP, mast cells and other cells communicate as part of a local network,47 and SP can stimulate mast cells and also increase expression of functional CRHR1.48 As an endogenous agonist of Mrgprb2, SP activates mast cell and release multiple pro-in-flammatory cytokines and chemokines via Mrgprb2.25 We found that b2KODSS group had higher CRH, CRHR1 and NR3C1 expression level in b2KODSS group decreased significantly, which suggests that b2KO mice might be more susceptible to mood disorders after induction of colitis, and Mrgprb2 might be associated with the HPA axis. NPY promotes stress resilience, the ability to deal with stress.49,50 The expression of BDNF indicates stress-induced neuronal plasticity, and serves as a key transducer of antidepressant effects and is essential for maintaining energy and glucose balance in the hypothalamus.21,51,52 Our results showed that both NPY and BDNF levels were significantly reduced in the b2KODSS group, suggesting that b2KO mice might have a diminished ability to regulate stress.
Conclusion
In conclusion, Mrgprb2 was involved in intestinal microbiota, intestinal barrier and oxidative stress levels, and was related to stress regulation, which might help to explain the pathogenesis of UC.
Data Sharing Statement
The 16S rRNA gene sequences were deposited in the GenBank Sequence Read Archive (SRA) database (ID PRJNA853097).
Compliance with Ethical Standards
Animal experiments were approved by the Animal Care and Ethics Committee of Wuhan University (Approval No. 20210303)) and were performed according to the guidelines of the Care and Use of Laboratory Animals published by the National Institutes of Health.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74. doi:10.1038/s41572-020-0205-x
2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
3. Windsor JW, Kaplan GG. Evolving Epidemiology of IBD. Curr Gastroenterol Rep. 2019;21(8):40. doi:10.1007/s11894-019-0705-6
4. Gracie DJ, Hamlin PJ, Ford AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. 2019;4(8):632–642. doi:10.1016/S2468-1253(19)30089-5
5. Cryan JF, O’Riordan KJ, Cowan CSM, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi:10.1152/physrev.00018.2018
6. Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501. doi:10.1053/j.gastro.2020.10.066
7. Yuan X, Chen B, Duan Z, et al. Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes. 2021;13(1):1987779. doi:10.1080/19490976.2021.1987779
8. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology. 2013;144(1):36–49. doi:10.1053/j.gastro.2012.10.003
9. Brzozowski B, Mazur-Bialy A, Pajdo R, et al. Mechanisms by which stress affects the experimental and clinical inflammatory bowel disease (IBD): role of brain-gut axis. Curr Neuropharmacol. 2016;14(8):892–900. doi:10.2174/1570159X14666160404124127
10. Forsythe P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019;42(1):43–55. doi:10.1016/j.tins.2018.09.006
11. Chen E, Chuang LS, Giri M, et al. Inflamed ulcerative colitis regions associated with MRGPRX2-mediated mast cell degranulation and cell activation modules, defining a new therapeutic target. Gastroenterology. 2021;160(5):1709–1724. doi:10.1053/j.gastro.2020.12.076
12. Subramanian H, Gupta K, Ali H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J Allergy Clin Immunol. 2016;138(3):700–710. doi:10.1016/j.jaci.2016.04.051
13. Olivera A, Beaven MA, Metcalfe DD. Mast cells signal their importance in health and disease. J Allergy Clin Immunol. 2018;142(2):381–393. doi:10.1016/j.jaci.2018.01.034
14. Roy S, Chompunud Na Ayudhya C, Thapaliya M, Deepak V, Ali H. Multifaceted MRGPRX2: new insight into the role of mast cells in health and disease. J Allergy Clin Immunol. 2021;148(2):293–308. doi:10.1016/j.jaci.2021.03.049
15. Lee M, Chang EB. Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. 2021;160(2):524–537. doi:10.1053/j.gastro.2020.09.056
16. van der Post S, Jabbar KS, Birchenough G, et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut. 2019;68(12):2142–2151. doi:10.1136/gutjnl-2018-317571
17. Bergstrom K, Xia L. The barrier and beyond: roles of intestinal mucus and mucin-type O-glycosylation in resistance and tolerance defense strategies guiding host-microbe symbiosis. Gut Microbes. 2022;14(1):2052699. doi:10.1080/19490976.2022.2052699
18. Johansson ME, Gustafsson JK, Holmen-Larsson J, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63(2):281–291. doi:10.1136/gutjnl-2012-303207
19. Pundir P, Liu R, Vasavda C, et al. A connective tissue mast-cell-specific receptor detects bacterial quorum-sensing molecules and mediates antibacterial immunity. Cell Host Microbe. 2019;26(1):114–122 e118. doi:10.1016/j.chom.2019.06.003
20. Reber SO. Stress and animal models of inflammatory bowel disease--an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology. 2012;37(1):1–19. doi:10.1016/j.psyneuen.2011.05.014
21. Bjorkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi:10.1016/j.neuropharm.2015.10.034
22. Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32(5):645–659. doi:10.1007/s10571-011-9793-z
23. Boeckxstaens G. Mast cells and inflammatory bowel disease. Curr Opin Pharmacol. 2015;25:45–49. doi:10.1016/j.coph.2015.11.005
24. Wouters MM, Vicario M, Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–168. doi:10.1136/gutjnl-2015-309151
25. Green DP, Limjunyawong N, Gour N, Pundir P, Dong X. A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron. 2019;101(3):412–420 e413. doi:10.1016/j.neuron.2019.01.012
26. Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249.
27. Glassner KL, Abraham BP, Quigley EMM. The microbiome and inflammatory bowel disease. J Allergy Clin Immunol. 2020;145(1):16–27. doi:10.1016/j.jaci.2019.11.003
28. Halfvarson J, Brislawn CJ, Lamendella R, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):17004. doi:10.1038/nmicrobiol.2017.4
29. Zheng Z, Lyu W, Ren Y, et al. Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat diet. Front Nutr. 2021;8:690138. doi:10.3389/fnut.2021.690138
30. Balakrishnan B, Luckey D, Bodhke R, et al. Prevotella histicola protects from arthritis by expansion of allobaculum and augmenting butyrate production in humanized mice. Front Immunol. 2021;12:609644. doi:10.3389/fimmu.2021.609644
31. van Muijlwijk GH, van Mierlo G, Jansen P, et al. Identification of Allobaculum mucolyticum as a novel human intestinal mucin degrader. Gut Microbes. 2021;13(1):1966278. doi:10.1080/19490976.2021.1966278
32. Gu Y, Zhang Y, Li M, et al. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front Pharmacol. 2021;12:621339. doi:10.3389/fphar.2021.621339
33. Hu S, Wang J, Xu Y, et al. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019;10(3):1736–1746. doi:10.1039/C8FO02364F
34. Zafar H, Saier MH
35. Wan F, Han H, Zhong R, et al. Dihydroquercetin supplement alleviates colonic inflammation potentially through improved gut microbiota community in mice. Food Funct. 2021;12(22):11420–11434.
36. Wieers G, Belkhir L, Enaud R, et al. How probiotics affect the microbiota. Front Cell Infect Microbiol. 2019;9:454. doi:10.3389/fcimb.2019.00454
37. van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi:10.1016/j.tim.2021.02.001
38. Goncalves P, Araujo JR, Di Santo JP. A cross-talk between microbiota-derived short-chain fatty acids and the host mucosal immune system regulates intestinal homeostasis and inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(3):558–572. doi:10.1093/ibd/izx029
39. Imhann F, Vich Vila A, Bonder MJ, et al. Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–119. doi:10.1136/gutjnl-2016-312135
40. Fan S, Weight CM, Luissint AC, et al. Role of JAM-A tyrosine phosphorylation in epithelial barrier dysfunction during intestinal inflammation. Mol Biol Cell. 2019;30(5):566–578. doi:10.1091/mbc.E18-08-0531
41. Shibolet O, Alper R, Ilan Y, Weidenfeld J. Regulatory role of the pituitary-adrenal axis in experimental colitis: effect of adrenalectomy on the clinical course and the TH1/TH2 immune profile. Inflamm Bowel Dis. 2005;11(12):1053–1059. doi:10.1097/01.MIB.0000191610.97842.51
42. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873–904. doi:10.1152/physrev.00041.2006
43. Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33(1):67–84. doi:10.1016/j.yfrne.2011.08.002
44. Im E, Rhee SH, Park YS, Fiocchi C, Tache Y, Pothoulakis C. Corticotropin-releasing hormone family of peptides regulates intestinal angiogenesis. Gastroenterology. 2010;138(7):2457–2467. doi:10.1053/j.gastro.2010.02.055
45. Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19(2):162–166. doi:10.1038/520
46. Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–414. doi:10.1038/74263
47. Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;21(5):457–487. doi:10.1210/edrv.21.5.0410
48. Asadi S, Alysandratos KD, Angelidou A, et al. Substance P (SP) induces expression of functional corticotropin-releasing hormone receptor-1 (CRHR-1) in human mast cells. J Invest Dermatol. 2012;132(2):324–329. doi:10.1038/jid.2011.334
49. Webster EL, Torpy DJ, Elenkov IJ, Chrousos GP. Corticotropin-releasing hormone and inflammation. Ann N Y Acad Sci. 1998;840:21–32. doi:10.1111/j.1749-6632.1998.tb09545.x
50. Sajdyk TJ, Johnson PL, Leitermann RJ, et al. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28(4):893–903. doi:10.1523/JNEUROSCI.0659-07.2008
51. Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3(1):12–29. doi:10.2174/1874467211003010012
52. Ameroso D, Meng A, Chen S, Felsted J, Dulla CG, Rios M. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat Metab. 2022;4(5):627–643. doi:10.1038/s42255-022-00566-0
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.