Back to Journals » Infection and Drug Resistance » Volume 16
Massive Airway Hemorrhage in Severe COVID-19 and the Role of Endotracheal Tube Clamping
Authors Guo L , Liu Y, Zhang L, Li Q, Qiu H, Guo Y, Shi Q
Received 26 January 2023
Accepted for publication 14 April 2023
Published 21 April 2023 Volume 2023:16 Pages 2387—2393
DOI https://doi.org/10.2147/IDR.S378408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Litao Guo,1 Yu Liu,1 Lei Zhang,1 Qing Li,2 Haibo Qiu,2 Yaling Guo,3 Qindong Shi1
1Department of Critical Care Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Department of Critical Care Medicine, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, People’s Republic of China; 3Department of Infectious Diseases, Xi’an Eighth Hospital, Xi’an, People’s Republic of China
Correspondence: Qindong Shi, Department of Critical Care Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, No. 277 Road Yanta West, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel +86 029 85323186, Email [email protected]
Background: Venovenous extracorporeal membrane oxygenation (VV-ECMO) has been widely used in treating patients with coronavirus disease 2019 (COVID-19) with severe respiratory failure. However, there are few reports of the successful treatment of patients with massive airway hemorrhage in severe COVID-19 during VV-ECMO treatment.
Methods: We analyzed the treatment process of a patient with a massive airway hemorrhage in severe COVID-19, who underwent prolonged VV-ECMO treatment.
Results: A 59-year-old female patient was admitted to the intensive care unit after being confirmed to have severe acute respiratory syndrome coronavirus 2 infection with severe acute respiratory distress syndrome. VV-ECMO, mechanical ventilation, and prone ventilation were administered. Major airway hemorrhage occurred on day 14 of ECMO treatment; conventional management was ineffective. We provided complete VV-ECMO support, discontinued anticoagulation, disconnected the ventilator, clipped the tracheal intubation, and intervened to embolize the descending bronchial arteries. After the airway hemorrhage stopped, we administered cryotherapy under bronchoscopy, low-dose urokinase locally, and bronchoalveolar lavage in the airway to clear the blood clots. The patient’s condition gradually improved; she underwent ECMO weaning and decannulation after 88 days of VV-ECMO treatment, and the membrane oxygenator was changed out four times. She was successfully discharged after 182 days in hospital.
Conclusion: Massive airway hemorrhage in patients with severe COVID-19 and treated with ECMO is catastrophic. It is feasible to clamp the tracheal tube with the full support of ECMO. Notably, bronchoscopy with cryotherapy is effective for removing blood clots.
Keywords: coronavirus disease 2019, acute respiratory distress syndrome, mechanical ventilation, cryotherapy, airway hemorrhage, extracorporeal membrane oxygenation
Introduction
In 2019, the coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged.1,2 COVID-19 patients may present with severe acute respiratory distress syndrome (ARDS).3 Although extracorporeal membrane oxygenation (ECMO) has been widely used in treating COVID-19 complicated by severe ARDS,4 the 28-day mortality rate of severe COVID-19 patients in the intensive care unit can reach 61.5%.2 Furthermore, the mortality rate may be related to ECMO complications, with bleeding being common among COVID-19 patients.5 Since massive airway hemorrhage in patients with severe COVID-19 treated with ECMO is catastrophic and rare,6 there are few reports. We report a successfully treated case of a massive airway hemorrhage in severe COVID-19 during venovenous (VV)-ECMO treatment.
Case Study
A 59-year-old woman previously diagnosed with Sjögren’s syndrome, Hashimoto’s thyroiditis, and unvaccinated against SARS-CoV-2 developed a sore throat, dry cough, fatigue, and fever 2 days after COVID-19 exposure. Her polymerase chain reaction (PCR) test from a nasopharyngeal swab was positive for the SARS-CoV-2 delta variant. Chest computed tomography (CT) showed bilateral consolidation and ground-glass opacities in the lungs consistent with COVID-19 symptoms. The patient was diagnosed with COVID-19 (mild) and administered Abidol and traditional Chinese medicine antiviral therapy, as well as methylprednisolone 40 mg/day for 5 days.
ECMO Initiation
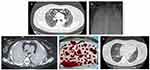
On day 4, oxygen support via high-flow nasal cannula was initiated. On day 14, the patient’s respiratory status worsened; ARDS and mediastinal emphysema followed. She was placed in the prone position and started on non-invasive mechanical ventilation, analgesia, and sedation. On day 28, her partial pressure of arterial oxygen-to-fractional inspired oxygen ratio was 91.25 mmHg, and partial pressure of carbon dioxide was 28 mmHg; repeat imaging revealed diffuse lung opacities and exacerbation of mediastinal emphysema (Figure 1A). VV-ECMO was initiated through the right femoral and internal jugular vein with the following settings: blood flow 3 L/min, sweep gas flow 2.6 L/min, and rotational speed 2800 rpm. Further, heparin was initiated (8 µ/kg/min) to achieve an activated partial thromboplastin time of 50–60 s and an activated clotting time of 160–200 s. On day 32, the patient’s breathing condition continued to deteriorate; the spontaneous breathing effort was very strong. To implement stricter lung protection, we performed orotracheal intubation and implemented invasive mechanical ventilation. She also received lung-protective ventilation (including prone positioning for 16 h/day), analgesia, sedation, and neuromuscular blockade.
Massive Airway Hemorrhage and Management
On day 42 (ECMO day 14), the patient developed a massive airway hemorrhage, which further led to tachycardia (132 beats/min), hypotension (87/45 mmHg), and anemia (hemoglobin level decreased from 10.3 g/dL to 7.3 g/dL). Coagulation profile: Activated partial thromboplastin time (APTT) 41.51 sec, thrombin time (TT) 16.79 sec, prothrombin time (PT) 12.89 sec, fibrinogen (FIB) 1.85g/L, D-dimer 0.96ug/mL. Accordingly, blood-product transfusion, fluid replacement, vasopressors administration, and heparin discontinuation became necessary. Hemostasis was uncontrolled, despite intratracheal administration of 0.01% adrenaline, tranexamic acid, thrombin, and fiberoptic bronchoscopy. The endotracheal tube (ET) was clamped, and the ventilator was disconnected. We provided complete VV-ECMO support, ECMO blood flow was increased to 4.5–5.2 L/min, oxygen concentration 100% (Arterial blood gas: pH 7.44, arterial partial pressure of oxygen [PO2] 104 mmHg, partial pressure of carbon dioxide [PCO2] 41.9 mmHg, and bicarbonate [HCO3−] 27.3 mmol/L), and bilateral bronchial-artery angiography showed thickening and tortuosity of peripheral vessels of a left and right common trunk bronchial artery, increased and disordered distal vessels, no contrast agent extravasation, and blind embolization was performed. Chest CT showed bilateral consolidation and airway hemorrhage (Figure 1B). Fiberoptic bronchoscopy following ET clamping revealed tracheal and bronchial obstruction by thrombi and fibrous tissues. Bronchoscopic cryotherapy was performed to remove the thrombi in the trachea, bilateral main bronchi, and segmental bronchi. On day 45, the airway hemorrhage recurred after the endotracheal tube was opened, which was managed with hemostatic drugs and ET reclamping. Repeat bronchial arteriography revealed recanalization of the left and right bronchial artery branches, however, contrast extravasation remains absent; hence, blind re-embolization was performed.
On day 47, bleeding cessation was noted; the patient’s artificial airway was established, and mechanical ventilation started. Blood clot removal via bronchoscopic cryotherapy was repeated by gradually advancing from the trachea to each segmental bronchus (Figure 1C). More distal clots in the bronchioles and alveoli were dissolved by local application of urokinase (1,000 U per site) and removed via saline bronchial irrigation. The patient was placed under deep sedation and ultra-lung-protective ventilation. Titrate optimal positive end-expiratory pressure, monitor pulmonary dynamic compliance, set mechanical ventilation-related parameters, and monitor respiratory mechanics (Table 1). Heparin was resumed on day 50. Heparin was initiated (3–5u/kg/min) to achieve an APTT of 40–50 s and an activated clotting time of 140–160 s.
 |
Table 1 Ventilation Parameters and Respiratory Mechanics |
Improvement and Follow-Up
On day 68, a repeat chest CT revealed complete removal of the pulmonary blood clots. The tidal volume was titrated to 4.5 mL/kg, while ECMO, lung-protective ventilation, analgesia, and sedation were continued.
On day 104, the patient’s sensorium improved. ECMO and mechanical ventilation were withdrawn on days 115 (prolonged ECMO for 88 days) and 120 (mechanical ventilation for 90 days) (Figure 2), respectively. In the 88 days, the membrane oxygenator was changed out four times because of gas exchange failure, apparent thrombus formation on the membrane, and acute D-dimer increases associated with massive clot formation around the hollow-fiber bundles in the oxygenator. A repeat chest CT scan showed significant improvement (Figure 1D). She was ambulatory with assistance and could perform her rehabilitation exercises. Monitoring of indicators revealed no abnormalities related to liver, kidney, and heart function during treatment.
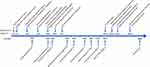 |
Figure 2 Timeline of ECMO and respiratory support events. |
During her illness, the patient developed pulmonary Aspergillus and Achromobacter xylosoxidans infections and was administered voriconazole and meropenem anti-infective treatment; the infections eventually resolved. Strict feeding procedures were adopted for nutritional therapy. Traditional Chinese medicine was administered during COVID-19 treatment. Nasopharyngeal PCR testing on day 38 of treatment was negative for SARS-CoV-2.
The patient was discharged 182 days after admission and was independent and ambulatory at her follow-up 2 months later.
Discussion
Treatment of COVID-19 complicated with ARDS using VV-ECMO improves patient outcomes.4,5 The median duration of ECMO therapy is 13.9 days.7 With improvements in ECMO technology and patient management, prolonged ECMO treatment has become more common.8 Patients with severe COVID-19 who require ECMO treatment have a longer disease course. There have been multiple case reports of prolonged ECMO used for successfully treating patients with severe COVID-19, with some ECMO durations lasting 111 days.9,10 Here, our patient was successfully treated with extended ECMO for 88 days. When massive airway hemorrhage occurred, we disconnected the ventilator and provided complete VV-ECMO support, but not VA-ECMO. The patient’s vital signs remained stable, and the pulse oxygen saturation was 95%–100%. Therefore, increasing the blood flow rate of VV-ECMO at 5.0 L/min can fully support patients, and prolonged ECMO treatment may improve the success rate of patients with severe COVID-19. Combined with the treatment process of this patient, we summarized the management process of massive airway hemorrhage associated with ECMO.
Although prolonged ECMO treatment is beneficial for patients with severe COVID-19, the complication rate of ECMO is related to the treatment duration. Bleeding, thrombosis, and infection are common ECMO complications.3,5,9 During ECMO, anticoagulation is essential to ensure smooth treatment progress and prevent thrombosis. The bleeding complications of ECMO are usually related to anticoagulation and include gastrointestinal bleeding, craniocerebral hemorrhage, and airway hemorrhage. In COVID-19, inflammation, vascular endothelial injury, and coagulation disorders lead to the coexistence of thrombosis and bleeding risk; if anticoagulation is used in COVID-19 treatment, the bleeding risk may further increase.11,12 Studies have shown that the incidence of major bleeding in patients with COVID-19 treated with ECMO is 30.9%–42%,13,14 and airway bleeding accounts for 26% of the bleeding instances.14 ECMO complicated with airway bleeding is more common with anticoagulation overdose or bronchial artery rupture bleeding. Further, vascular endothelial injury and changes in coagulation mechanism in COVID-19 aggravate the risk and severity of airway bleeding.9,14 The cause of massive airway hemorrhage in the patient was considered related to these factors: first, SARS-CoV-2 may cause pulmonary vascular endothelial injury.9,14 Second, pulmonary Aspergillus fumigatus infection (cultured as Aspergillus fumigatus in bronchoalveolar lavage fluid) was confirmed before massive airway hemorrhage occurred, and Aspergillus infection may erode pulmonary vessels and cause bleeding. Third, before airway bleeding, although APTT (41.51 sec, normal 28–43.5 sec) was not significantly prolonged, the levels of FIB (1.85 g/L, normal 2–4 g/L) and platelet (85.00 × 109/L, normal 125–350 × 109/L) were low, and heparin anticoagulation for ECMO increased the risk of bleeding. Fourth, the patient was found to have malformation of the peripheral vessels of the bronchial artery during interventional therapy. Bleeding complications of ECMO are directly related to anticoagulation. For patients at high risk of bleeding, APPT can be targeted at a low level, and frequency of coagulation monitoring increased to prevent excessive fluctuations in APTT values. Monitoring of coagulation parameters is only one aspect of prevention of bleeding, and bleeding complications of ECMO are associated with many factors. As discussed earlier, the causes of airway bleeding in our patient were multifaceted and require attention during treatment.
Massive airway hemorrhage is defined as airway blood (>300 mL/day) that cannot be controlled by conventional treatments such as bronchoscopy with cold saline lavage, diluted epinephrine lavage, and selective lung isolation. Massive airway hemorrhage during ECMO therapy, although rare, is a serious condition and has potentially fatal complications.6,14 The patient’s condition deteriorated rapidly after COVID-19 diagnosis, which was considered related to being unvaccinated against SARS-CoV-2 and underlying diseases. The patient was finally diagnosed with severe COVID-19 combined with ARDS and provided ECMO treatment. She developed a massive airway hemorrhage on day 14 of ECMO treatment. We stopped the heparin infusion after the airway hemorrhage, and routine treatments such as aggressive cold saline lavage with bronchoscopy, topical 0.01% epinephrine lavage in the airway, and tranexamic acid were administered; however, the outcomes were poor. Therefore, we clipped the ET to stop bleeding with full ECMO support, which can control massive airway hemorrhage when conventional methods are ineffective.6,15 Interventional embolization of bronchial arteries is a minimally invasive procedure that can control bleeding from the source.15
Overall, the immediate clinical success rate of bronchial artery embolization, defined as complete cessation of hemoptysis, varies from 70% to 99%.16–18 However, recurrence rate remains high, ranging from 10% to 57%, due to incomplete initial embolization, recanalization of previously embolized arteries, and recruitment of new collaterals.16 However, not all bleeding sites can be found. Currently, empiric bronchial artery embolization is effective in achieving bleeding control, which has become the treatment of choice for recurrent and massive airway hemoptysis.15,17 No major complication involving vital or functional prognosis, related to BAE was noted, thus it is safe and effective.17,18 Bronchoscopic cryotherapy is a safe and feasible technique to remove blood clots in the trachea and bronchi after bleeding cessation.19 In our patient, airway clots were cleared after multiple cryotherapy sessions. However, for the removal in distal bronchi and alveoli, a small dose of urokinase should be used locally to dissolve blood clots, which can further be removed gradually by repeated bronchoalveolar lavage. In our case, the blood clot was completely removed by the aforementioned method, and the patient’s airway gradually opened, leading to complete recovery.
Conclusion
Massive airway hemorrhage in patients with severe COVID-19 treated with ECMO is catastrophic. Strict monitoring of anticoagulation can help reduce major bleeding events. ET clipping with complete ECMO support is feasible after a massive airway hemorrhage, and early interventional radiology to embolize any potential bleeding source can prevent rebleeding. Bronchoscopy with cryotherapy is effective for removing blood clots.
Data Sharing Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Ethics Approval and Informed Consent
The First Affiliated Hospital of Xi’an Jiaotong University Ethics committee has approved case report. All procedures were performed in accordance with the Declaration of Helsinki.
Consent for Publication
Written informed consent has been provided by the patient to have the case details and any accompanying images published.
Acknowledgments
We are very thankful to the patient and all medical staff involved in the treatment of patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Key Research and Development Program of Shaanxi Province of China (No. 2021SF-056).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ghweil AA, Hassan MH, Khodeary A, et al. Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA quarantine hospital’s patients, Egypt: a Retrospective Study. Infect Drug Resist. 2020;13:2375–2383. doi:10.2147/IDR.S263489
2. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi:10.1016/S2213-2600(20)30079-5
3. Bemtgen X, Zotzmann V, Benk C, et al. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J Thromb Thrombolysis. 2021;51(2):301–307. doi:10.1007/s11239-020-02217-1
4. Whebell S, Zhang J, Lewis R, et al. Survival benefit of extracorporeal membrane oxygenation in severe COVID-19: a multi-centre-matched cohort study. Intensive Care Med. 2022;48(4):467–478. doi:10.1007/s00134-022-06645-w
5. Trejnowska E, Drobiński D, Knapik P, et al. Extracorporeal membrane oxygenation for severe COVID-19-associated acute respiratory distress syndrome in Poland: a multicenter cohort study. Crit Care. 2022;26(1):97. doi:10.1186/s13054-022-03959-5
6. Pitcher HT, Harrison MA, Shaw C, Cowan SW, Hirose H, Cavarocchi N. Management considerations of massive hemoptysis while on extracorporeal membrane oxygenation. Perfusion. 2016;31(8):653–658. doi:10.1177/0267659116651484
7. Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the extracorporeal life support organization registry. Lancet. 2020;396:1071–1078. doi:10.1016/S0140-6736(20)32008-0
8. Yoshiyasu N, Sato M, Anraku M, Ichiba S, Nakajima J. Lung transplant after long-term veno-venous extracorporeal membrane oxygenation: a case report. J Cardiothorac Surg. 2021;16(1):246. doi:10.1186/s13019-021-01614-8
9. Fitzgerald AL, Vachharajani HH, Davidson BP, Kruit NJ, Eslick AT. The prolonged use of VV ECMO support in COVID-19: a case report. J Crit Care Med. 2020;6(4):224–230. doi:10.2478/jccm-2020-0034
10. Xu Z, Xu Y, Liu D, et al. Case report: prolonged VV-ECMO (111 days) support in a patient with severe COVID-19. Front Med. 2021;8:681548. doi:10.3389/fmed.2021.681548
11. Stillfried SV, Bülow RD, Rhrig R, Meybohm P, Boor P; for the German Registry of COVID-19 Autopsies (DeRegCOVID), DeRegCOVID Collaborators. Intracranial hemorrhage in COVID-19 patients during extracorporeal membrane oxygenation for acute respiratory failure: a nationwide register study report. Critical Care. 2022;26:83. doi:10.1186/s13054-022-03945-x
12. Coppola A, Annunziata A, Gioia MR, Fiorentino G. Bleeding events in COVID-19: the other side of the coin? Monaldi Arch Chest Dis. 2021;91(3). doi:10.4081/monaldi.2021.1739
13. Schmidt M, Fisser C, Martucci G, et al. Tracheostomy management in patients with severe acute respiratory distress syndrome receiving extracorporeal membrane oxygenation: an International Multicenter Retrospective Study. Crit Care. 2021;25:238. doi:10.1186/s13054-021-03649-8
14. Arachchillage DJ, Rajakaruna I, Scott I, et al. Impact of major bleeding and thrombosis on 180‐day survival in patients with severe COVID‐19 supported with veno‐venous extracorporeal membrane oxygenation in the United Kingdom: a multicentre observational study. Br J Haematol. 2022;196(3):566–576. doi:10.1111/bjh.17870
15. Harrison M, Cowan S, Cavarocchi N, Hirose H. Massive haemoptysis on veno-arterial extracorporeal membrane oxygenation. Eur J Cardiothorac Surg. 2012;42(3):587–589. doi:10.1093/ejcts/ezs156
16. Panda A, Bhalla AS, Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23:307–317. doi:10.5152/dir.2017.16454
17. Abid N, Loukil M, Mokni A, Badri I, Bouzaidi K, Ghrairi H. Outcomes of bronchial artery embolization for the management of hemoptysis. Tunis Med. 2021;99(2):264–268.
18. Lu GD, Yan HT, Zhang JX, Liu S, Shi HB, Zu QQ. Bronchial artery embolization for the management of frequent hemoptysis caused by bronchiectasis. BMC Pulm Med. 2022;22(1):394. doi:10.1186/s12890-022-02198-2
19. Green A, Puri N, Kouch M, et al. Cryotherapy: a safe approach to airway hemorrhage during VV-ECMO. J Investig Med High Impact Case Rep. 2022;10:23247096221074590. doi:10.1177/23247096221074590
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.