Back to Journals » OncoTargets and Therapy » Volume 17
MAP2K1 K57N Conferred an Acquired Resistance to Furmonertinib, Dabrafenib and Trametinib Combined Therapy in Advanced Lung Adenocarcinoma with EGFR Mutation and BRAF V600E
Received 14 December 2023
Accepted for publication 20 March 2024
Published 9 April 2024 Volume 2024:17 Pages 307—312
DOI https://doi.org/10.2147/OTT.S454902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Nagashree Seetharamu
Xiang Tan,* Zuotao Wu,* Mingwu Chen
Department of Thoracic Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingwu Chen, Department of Cardio-Thoracic Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530021, People’s Republic of China, Email [email protected]
Abstract: Previous case reports have demonstrated the effectiveness of combination therapy involving EGFR TKI, BRAF inhibitor dabrafenib, and MEK inhibitor trametinib in metastatic non-small-cell lung cancer (NSCLC) patients with acquired BRAF V600E and EGFR mutations. However, the current literature does not provide any reports on the presence of resistant mutations in response to the administration of three-drug combination therapy. Exploring the resistance mechanism of targeted therapy is helpful to optimize the subsequent treatment strategy of patients. Herein, we report a case of a patient with advanced EGFR positive lung adenocarcinoma harboring an acquired BRAF V600E mutation who responded to the combination of furmonertinib, dabrafenib, and trametinib therapy. Unexpectedly, a MAP2K1 K57N acquired mutation was identified by NGS (Next-generation sequencing) analysis of re-biopsy tumor tissue after the patient was resistant to three-drug therapy. As far as we know, this is the first report demonstrating that the efficacy of using combination of furmonertinib and BRAF/MEK inhibitors and the MAP2K1 K57N mutation serves as a resistant mechanism to the three-drug therapy. This novel finding not only revealed a new resistant mutation but also had important implications for the identification of effective patients to EGFR/BRAF/MEK combination therapy.
Keywords: MAP2K1, furmonertinib, lung adenocarcinoma, EGFR, mutation
Introduction
Approximately 40–60% of Chinese patient with NSCLC harbor EGFR somatic mutations who can benefit from EGFR TKIs therapy. Unfortunately, most patients who initially respond to TKIs eventually develop acquired resistance within 1–2 years of treatment.1 Clarifying the potential drug resistance mechanism provides an important theoretical basis for overcoming the resistance of TKIs. BRAF V600E has been recognized as a causal factor for osimertinib resistance in 3% of lung cancer cases, both in first- and second-line therapy.2 The Food and Drug Administration (FDA) approved the combination of dabrafenib and trametinib for the treatment of people with nearly any type of advanced solid tumor that has BRAF V600E. The BRAF protein signals can be effectively inhibited by dabrafenib, while the MEK protein signals can be inhibited by trametinib. Several case reports have demonstrated that NSCLC patients harboring EGFR TKIs sensitive mutation and acquired BRAF V600E can benefit from osimertinib in combination with dabrafenib and trametinib.3–5 These findings have made us curious to probe whether a novel third-generation EGFR tyrosine-kinase inhibitor furmonertinib combined with BRAF- and MEK- inhibitors are effective against advanced NSCLC harboring EGFR mutation and the acquired resistance mutation BRAF V600E.
Case Report
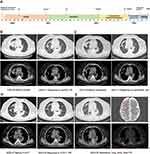
A 71-year-old Chinese female was diagnosed with stage IV adenocarcinoma of the right lung in July 2020 (Figure 1A). NGS analysis of tumor tissue revealed the presence of an EGFR exon 19 deletion (EGFR E746_A750del) mutation. Icotinib is a highly selective first-generation EGFR TKI which is approved by National Medical Products Administration (NMPA) for use in China as first-line monotherapy in patients with NSCLC with somatic EGFR mutations.6 The patient received three cycles of icotinib treatment, and reexamination via computed tomography (CT) indicated clinical benefit (Figure 1B). However, approximately 12 months later, follow-up CT imaging revealed slow progressive disease (PD) and the patient experienced severe adverse effects, such as diarrhea and rash. Therefore, the patient received rebiopsy and the EGFR T790M mutation was identified. The administration of osimertinib was initiated for the patient in June 2021 and demonstrated favorable tolerability (Figure 1C). However, a CT scan after one year of treatment revealed the presence of progressive lesions in the right lung (Figure 1D). Subsequent NGS analysis (YuceOne® Pro+, Yucebio Ltd., Shenzhen, China) of soft tissue metastasis identified EGFR E746_A750del, EGFR T790M, TP53 R156Afs*14 and acquired BRAF V600E mutations (Figure 2B). Consequently, in August 2022, the patient underwent a combination therapy comprising furmonertinib, dabrafenib, and trametinib. The CT scans after 2 months of treatment showed obvious shrinkage in the pulmonary lesions, which contributed to a partial response (PR) in February 2023 (Figure 1D). However, progressive tumor progression was observed on follow-up images in March 2023, and a puncture genetic test of the right lower lung mass revealed the disappearance of the BRAF V600E and the presence of the MAP2K1 K57N (Figure 2). Considering that the patient refuses to undergo any chemotherapy or combined chemotherapy protocols, and the genetic test showed that EGFR-TKIs resistance mutation BRAF V600E disappeared, we advised the patient to receive double-dose furmonertinib after three-drug therapy resistance. Cause of some studies suggest that high-dose furmonertinib may be effectively for NSCLC patients who are resistant to osimertinib,7 or those with brain metastases/leptomeningeal metastases,8,9 we advised the patient to receive double-dose furmonertinib after three-drug therapy resistance. Regrettably, the patient exhibited an unsatisfactory response to monotherapy, and the patient was readmitted to the hospital in May 2023 due to exacerbated symptoms of cough, chills, and fever. Subsequent CT scans revealed the presence of secondary malignant tumors in both the brain and bone (Figure 1E). Genetic testing of ctDNA (YuceOne® Pro ctDNA, Yucebio Ltd., Shenzhen, China) revealed the presence of EGFR E746_A750del, MAP2K1 K57N, and TP53 R156Afs*14 mutations in May 2023 (Figure 2). The patient was subsequently treated with anlotinib. Unfortunately, the management of anlotinib was ineffective and the patient passed away in July 2023.
Methods
Patient
The patient provided written informed consent for tissue biopsies, blood collection, NGS analysis, treatment, and publication of this report.
Clinical Testing
Molecular testing was conducted by the CAP certified WiHealth Medical Laboratory (Shenzhen, China), and performed using laboratory assays that were independently validated. Patient’s tumor tissue was taken from biopsies for genomic profiling using YuceOne® Pro+ (Yucebio Ltd., Shenzhen, China) panel. For ctDNA analysis, YuceOne® Pro ctDNA (Yucebio Ltd., Shenzhen, China) panel was utilized. The reference genome is hg19. Genomic DNAs were isolated from tumor specimens and blood and extracted using the GeneRead DNA FFPE Kit (Qiagen) and Qiagen DNA blood mini kit (Qiagen). Then, extracted DNAs were amplified, purified, and analyzed using NGS panel on the MGI-T7 platform. The research conducted in this study involving patient data has obtained appropriate approval and adhered to all ethical regulations pertaining to the utilization of such data.
Discussion
In the past decade, targeted therapy has significantly revolutionized the treatment of NSCLC. Considering factors such as age, chemotherapy tolerance, treatment duration, and increased incidence of adverse effects, EGFR TKIs has emerged as the preferred modality for EGFR mutation-positive NSCLC. However, almost all lung adenocarcinoma patients with EGFR mutations who respond to EGFR TKIs ultimately develop resistance to these agents, and the acquired drug resistance mechanism to EGFR TKIs has been one of the most urgent clinical challenges to be solved.
Our case report elucidates the mechanism of resistance to multi-line targeted therapies in the patient through continuous genetic testing, especially highlighting a potential acquired resistance mutation MAP2K1 K57N to furmonertinib, dabrafenib, and trametinib combined therapy in advanced lung adenocarcinoma with EGFR mutation and BRAF V600E. Although we attempt to overcome resistance of three-drug therapy and ultimately failed, our findings can still serve as a valuable reference for subsequent medication of similar patients.
BRAF V600E is a well-established oncogenic driver in NSCLC, which is a known acquired resistance mechanism to the third-generation EGFR TKI osimertinib.2 Clinical studies have reported favorable antitumor effects in patients with EGFR mutation-positive NSCLC and acquired BRAF V600E mutation when treated with the combination of dabrafenib, trametinib, and osimertinib.3–5 In this case, we attempted to utilize the combination of furmonertinib (a third-generation EGFR TKI approved in China), dabrafenib, and trametinib as a novel approach to overcome BRAF V600E-mediated resistance to osimertinib for the first time. The patient achieved progression-free survival (PFS) of 8 months, that suggest the usefulness of this novel combined treatment.
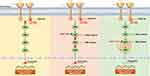
It is noteworthy that our patient exhibited a MAP2K1 K57N mutation, as determined by genetic testing, subsequent to receiving a three-drug combination therapy and displayed slow progression of the disease. This phenomenon suggests that the activating mutation MAP2K1 K57N may serve as a potential mechanism of resistance to this triple regimen. MAP2K1 (mitogen-activated protein kinase kinase 1), also known as MEK1, plays a crucial role in the RAS/MAPK signaling cascade pathway by transmitting extracellular chemical signals to the cell nucleus. This pathway tightly regulates various cellular processes, including proliferation, differentiation, migration, and apoptosis. Residues F53, Q56, and K57 of MAP2K1 are located at the C-terminus of the α-helix, which is in close contact with the N-terminus of the protein. These mutations may significantly alter the helical structure in this region and disrupt regulation of kinase function, as they form important interface connections with other parts of the kinase domain.10 Preclinical data indicate that the substitution of p.K57 results in the constitutive activation of MAP2K1 and the downstream RAS/MAPK signaling pathway.11 The prevalence of MAP2K1 mutations in tumors is low (1–2%), and studies show that MAP2K1 mutation may be a targeted resistance mechanism in multiple cancers, such as anti-EGFR monoclonal antibody resistance in colorectal cancer (CRC) and RAF inhibitors resistance in melanoma.12–14 MAP2K1 K57N mutation confers enhanced functionality to MAP2K1, as evidenced by increased levels of autophosphorylation and Erk phosphorylation.11 We propose that the acquired MAP2K1 K57N mutation induces hyperactivation of MAP2K1 or disrupts the binding between the MEK inhibitor trametinib and MAP2K1, resulting in reactivation of the MAPK pathway and subsequent ERK signaling (Figure 3). That’s why the three-drug therapy is difficult to inhibit the activation of the MAPK pathway when MAP2K1 K57N occurs and causing the patient’s tumor to continue to differentiate and grow, eventually leading to disease progression. Nicoś et al first reported that MEK1 gene mutations can be detected in central nervous system (CNS) metastases of NSCLC. The brain is often a common site for NSCLC metastasis, as well as a frequent location for the development of secondary drug resistance.15 Mizuno et al reported that MAP2K1 K57N was identified as a RAF-regulated mutation, and suggesting its potential association with resistance to RAF and MEK inhibitors in vitro.16 This study further supports our conjecture that MAP2K1 K57N is a secondary drug resistance mutation.
In addition, it is notable that neither ctDNA nor biopsy NGS can detect BRAF V600E mutation after the patient’s disease has progressed. Cheng et al reported that a 67-year-old male with lung adenocarcinoma exhibited a mixed response in multiple progressing sites after oral administration of trametinib for 14 weeks, including an increase in the size of the inguinal lymph node metastasis. Repeat biopsy of the inguinal lymph node metastasis and tumor NGS (FoundationOne CDx, Foundation Medicine) did not reveal any clear acquired resistance mechanisms. Despite initially experiencing rapid clinical benefit, the duration of response was ultimately limited. Further research is needed to investigate resistance mechanisms to MEK1 inhibitor monotherapy, and optimal targeted treatment strategies for MAP2K1-mutant NSCLC may require combination approaches involving drugs targeting components of the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway and/or other pathways.17 Considering that the loss of EGFR T790M mutation serves as a mechanism for Osimertinib resistance, it is worth investigating whether the loss of BRAF V600E could contribute to resistance against BRAF and MEK inhibitors. However, tumor heterogeneity cannot be avoided through tissue puncture, this hypothesis needs to be confirmed by further experiments.
NSCLC patients with EGFR mutation, who strongly prefer to avoid chemotherapy, would face limited options for subsequent-line systemic therapy after occurs resistance mutation. We attempted to use high dose furmonertinib to overcome our patient who were resistance to furmonertinib, dabrafenib and trametinib combined therapy, but unfortunately failed. We speculate that the presence of MAP2K1 K57N mutation may make furmonertinib monotherapy ineffective in NSCLC patients with EGFR sensitive mutations via activating MAPK downstream signaling pathways. This finding provides hints for selecting drugs for similar patients in the future.
Overall, our case firstly identified MAP2K1 K57N as a novel resistance mechanism to furmonertinib, dabrafenib and trametinib combined therapy in NSCLC with EGFR mutation and BRAF V600E, which may provide a new perspective to assess the drug-resistant state and the continued study of MAPK2k1 and ERK activation as a potential therapeutic target. Subsequent treatment showed that the patient with MAP2K1 K57N probably cannot benefit from high dose furmonertinib monotherapy which suggested that clinical needed to develop more effective drugs for multi-drug resistant lung adenocarcinoma patients who resist to receive chemotherapy in the future.
Ethics Approval and Consent to Participate
The authors confirm that written informed consent was obtained from the patients, and institutional approval was granted for the publication of data and images. The research on gene analysis received ethical approval (2024-E126-01).
Consent for Publication
All authors approved the publication of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jackman D, Pao W, Riely GJ, et al. Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non–small-cell lung cancer. J Clin Oncol. 2010;28(2):357. doi:10.1200/JCO.2009.24.7049
2. Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo MJB. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–737. doi:10.1038/s41416-019-0573-8
3. Mauclet C, Collard P, Ghaye B, Hoton D, Nana FAJLC. Tumor response to EGFR/BRAF/MEK co-inhibition in a patient with EGFR mutated lung adenocarcinoma developing a BRAFV600 mutation as an acquired resistance mechanism. Lung Cancer. 2021;159:42–44. doi:10.1016/j.lungcan.2021.06.025
4. Ribeiro M, Knebel FH, Bettoni F, et al. Impressive response to dabrafenib, trametinib, and osimertinib in a metastatic EGFR-mutant/BRAF V600E lung adenocarcinoma patient. NPJ Precis Oncol. 2021;5(1):5. doi:10.1038/s41698-021-00149-4
5. Huang Y, Gan J, Guo K, Deng Y, Fang Wjjo TO. Acquired BRAF V600E mutation mediated resistance to osimertinib and responded to osimertinib, dabrafenib, and trametinib combination therapy. J Thorac Oncol. 2019;14(10):e236–e7. doi:10.1016/j.jtho.2019.05.040
6. Shi YK, Zhang L, Liu XQ, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind Phase 3 non-inferiority trial. Lancet Oncol. 2013;14(10):953–961. doi:10.1016/S1470-2045(13)70355-3
7. Cheng D, Tang S, Li D, et al. Successful salvage therapy using high-dose furmonertinib (AST2818) for non–small-cell lung cancer after Osimertinib resistance: a case report. Anticancer Drugs. 2022;33:768. doi:10.1097/CAD.0000000000001368
8. Hu X, Zhang S, Ma Z, et al. Central nervous system efficacy of furmonertinib (AST2818) in patients with EGFR T790M mutated non-small cell lung cancer: a pooled analysis from two Phase 2 studies. BMC Med. 2023;21(1):1–11. doi:10.1186/s12916-023-02865-z
9. Xu Z, Hao X, Wang Q, Yang K, Li J, Xing PJB. Intracranial efficacy and safety of furmonertinib 160 mg with or without anti-angiogenic agent in advanced NSCLC patients with BM/LM as salvage therapy. BMC Cancer. 2023;23(1):206. doi:10.1186/s12885-023-10676-x
10. Arcila ME, Drilon A, Sylvester BE, et al. MAP2K1 (MEK1) mutations define a distinct subset of lung adenocarcinoma associated with smoking. Clin Cancer Res. 2015;21(8):1935–1943. doi:10.1158/1078-0432.CCR-14-2124
11. Marks JL, Gong YX, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68(14):5524–5528. doi:10.1158/0008-5472.CAN-08-0099
12. Chuang J, Wang C, Guo Y, Valenzuela V, Wu J, Fakih MJC. MAP2K1 mutations in advanced colorectal cancer predict poor response to anti-EGFR therapy and to vertical targeting of MAPK pathway. Clin Colorectal Cancer. 2021;20(1):72–78. doi:10.1016/j.clcc.2020.12.003
13. Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 2014;4(1):94–109. doi:10.1158/2159-8290.CD-13-0617
14. Mauri G, Patelli G, Gori V, et al. Case Report: MAP2K1 K57N mutation is associated with primary resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer. Front Oncol. 2022;12:1030232. doi:10.3389/fonc.2022.1030232
15. Nicos M, Krawczyk P, Jarosz B, et al. Sensitive methods for screening of the MEK1 gene mutations in patients with central nervous system metastases of non-small cell lung cancer. Clin Transl Oncol. 2016;18(10):1039–1043. doi:10.1007/s12094-016-1483-3
16. Mizuno S, Ikegami M, Koyama T, et al. High-throughput functional evaluation of MAP2K1 variants in cancer. Mol Cancer Ther. 2023;22(2):227–239. doi:10.1158/1535-7163.MCT-22-0302
17. Cheng ML, Lee JK, Kumar R, et al. Response to MEK inhibitor therapy in MAP2K1 (MEK1) K57N non-small-cell lung cancer and genomic landscape of MAP2K1 mutations in non-small-cell lung cancer. Jco Precis Oncol. 2022;3:6.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.