Back to Journals » Cancer Management and Research » Volume 12
Managing Ipilimumab-Induced Hypophysitis: Challenges and Current Therapeutic Strategies
Authors Tsoli M, Kaltsas G, Angelousi A, Alexandraki K, Randeva H , Kassi E
Received 30 June 2020
Accepted for publication 19 August 2020
Published 2 October 2020 Volume 2020:12 Pages 9551—9561
DOI https://doi.org/10.2147/CMAR.S224791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Bilikere Dwarakanath
Marina Tsoli,1 Gregory Kaltsas,1 Anna Angelousi,2 Krystallenia Alexandraki,1 Harpal Randeva,3 Eva Kassi1
1First Department of Propaedeutic and Internal Medicine, Laiko University Hospital, National and Kapodistrian University of Athens, Athens, Greece; 2First Department of Internal Medicine, Laiko University Hospital, National and Kapodistrian University of Athens, Athens, Greece; 3Warwick Medical School, University of Warwick, Coventry CV4 7AL, UK
Correspondence: Marina Tsoli
First Department of Propaedeutic and Internal Medicine, Laiko University Hospital, National and Kapodistrian University of Athens, Agiou Thoma 17, Athens 11527, Greece
Tel +30 6972692962
Fax +30 2132061794
Email [email protected]
Abstract: Over the past years, progress has been made in cancer immunotherapy following the development of immune checkpoint inhibitors (ICI) that have been proved effective in the management of many malignancies. Ipilimumab, a monoclonal antibody against cytotoxic T-lymphocyte antigen-4 (CTLA-4), has been approved for the treatment of advanced melanoma but has been associated with the development of several endocrine immune-related adverse events (irAEs). Hypophysitis is the most common endocrine irAE related to ipilimumab with a reported incidence ranging from 1.8% to 17%. The mechanism underlying ipilimumab-induced hypophysitis implicates immune, inflammatory and genetic factors, but there are still some points that are not well understood and remain to be elucidated. The diagnosis is based mainly on clinical, biochemical and imaging data. The majority of patients display multiple hormone deficiencies that may recover or persist for a prolonged period of time with corticotroph deficiency usually being permanent. Immune-related hypopituitarism is treated with replacement of deficient hormones while in severe forms of hypophysitis treatment with high-dose glucocorticoids may be required. Proper evaluation and registration of patients in clinical trials and further investigation are needed to precisely clarify the pathophysiology of the ICI-related hypophysitis, define predictive factors and ameliorate the management and outcome of the disease.
Keywords: immune checkpoint inhibitors, hypopituitarism, immune-related adverse effects
Introduction
Recent advances in the field of immune modulation and immune response to cancer have led to the development of immunotherapy for the treatment of solid and/or haematological malignancies.1 Over the past years, immune checkpoint inhibitors (ICI) targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4) or programmed cell death protein 1/ligand 1 (PD-1/PD-L1) have been proven to be effective in various cancer types.
Ipilimumab is a human monoclonal antibody directed against CTLA-4 (anti-CTLA-4 Ab), a receptor expressed on antigen-stimulated T-cells that suppress the immune response after T-cell/antigen interaction. Therefore, ipilimumab blocks CTLA-4, restores T-cell activation and proliferation, and potentiates the anti-tumor T-cell response.2,3 It has been approved in 2011 by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of advanced (metastatic or unresectable) melanoma while multiple studies have evaluated the efficacy of ipilimumab in other solid tumors such as prostate cancer, small-cell lung cancer, ovarian cancer, gastric cancer and bladder cancer.4–6
Although ipilimumab has shown considerable activity in patients with a variety of malignancies particularly in cases where conventional treatment has failed, it is associated with clinically significant side effects. The most common side effects observed with ICI are immune-related adverse events (irAEs) and may involve the skin, the gastrointestinal tract, the liver and the endocrine system.7,8 The incidence of endocrine adverse events associated with ICI has been found to be 4–20% and include hypophysitis, thyroid dysfunction, insulin-dependent diabetes mellitus, primary adrenal insufficiency while acute primary hypoparathyroidism has also been reported.9–13
Hypophysitis refers to inflammation of the pituitary gland and is categorized, according to histopathology, as lymphocytic, granulomatous, xanthomatous, necrotizing and IgG4 plasmacytic hypophysitis and/or its etiology to primary and secondary that is related to systemic diseases, infections or pharmacological agents.14,15 It is a relatively rare disease with an annual incidence of 1/9,000,000 while lymphocytic hypophysitis is the most common form and constitutes approximately 71.8% of all cases of primary hypophysitis.15,16 However, following the development of the new immune checkpoint therapies, its frequency has significantly increased. Given the increasing use of ICIs in patients with various cancer types and the potential life-threatening nature of hypophysitis if not been early recognized and properly treated, it is critical for clinicians to be aware of the clinical manifestations, diagnosis and management of ICI-related hypophysitis.
In this review, we will provide information on the current knowledge on hypophysitis associated to the treatment with ipilimumab in respect to its epidemiology, pathophysiology, clinical presentation and treatment.
Prevalence and Epidemiology of Ipilimumab-Induced Hypophysitis
Hypophysitis is the most common endocrine adverse event associated with ICI. A recent review reported 451 cases of ICI-related endocrinopathies and 222 cases of hypophysitis and anterior hypopituitarism. Of these cases, 200 were treated with ipilimumab.1 The incidence of hypophysitis after treatment with ipilimumab has been reported to range from 1.8% to 17%. The significant variation of incidence between studies is attributed mainly to the fact that the incidence is dependent on the dose of the administered drug and on the use of adjuvant treatment.9,17–19 In patients treated with low-dose ipilimumab (<3mg/kg) the incidence of hypophysitis was 1.8–3.3% while patients who were treated with ipilimumab doses greater than 3mg/kg developed hypophysitis in 4.9–17% of cases.17,18 Other factors affecting the reported incidence of ipilimumab-induced hypophysitis are the lack of a precise and consistent definition of hypophysitis in different studies and the duration of follow-up that ranged from few months up to several years (Table 1).16,20
 |
Table 1 Reported Prevalence of Ipilimumab-Induced Hypophysitis |
Compared to patients who receive ipilimumab, those who receive treatment with anti-PD-1 or anti-PD-L1 agents are significantly less likely to develop hypophysitis. In a meta-analysis of 34 studies, it has been reported that the incidence of hypophysitis was 0.4% with anti-PD-1 and <0.1% with anti-PD-L1 therapy.21 The results of initial studies evaluating the incidence of hypophysitis in patients receiving treatment with the combination of ipilimumab and anti-PD-1 agents were conflicting. Some studies reported an increased incidence of hypophysitis with the combination treatment compared to monotherapy with ipilimumab while others found that the combined therapy did not influence the incidence of hypophysitis.22,23 Overall, the combination of ipilimumab with the anti-PD-1 agent nivolumab is considered to be associated with an increased risk of hypophysitis compared to ipilimumab alone (RR=1.94 [95% CI: 1.7–3.5]).24 A recent meta-analysis that included 8 randomized controlled trials (RCTs) with 2716 patients reported that the incidence and severity of irAEs, including hypophysitis, were drug and dose dependent.25 Specifically, the risk of hypophysitis appeared to be related to the use of CTLA-4 antibodies (ipilimumab) while the combination of nivolumab 3 mg/kg plus ipilimumab 1 mg/kg significantly increased the total 3–5 grade irAEs. A recent study by our group reported for the first time that sequential treatment with anti-CTLA-4 and anti-PD1 agents increased the risk of developing hypophysitis to a level as high as that of combination therapy.26 Furthermore, high rates of hypophysitis (grade 3 or 4 toxicity) have been reported in patients receiving adjuvant treatment with prostate cancer cell vaccine or bevacizumab.27,28
In contrast, no increased incidence of hypophysitis was observed with the combination of ipilimumab with chemotherapeutic agents such as carboplatin, dacarbazine, paclitaxel and fotemustine or targeted agents including vemurafenib and dabrafenib compared to monotherapy with ipilimumab.29–31 Of note, it has been observed that hypophysitis was rarely reported in patients that have been treated with cytotoxic chemotherapy or brain radiotherapy before receiving ipilimumab.9,32,33
In contrast to idiopathic hypophysitis which is more common in females, the incidence of ipilimumab-related hypophysitis is higher in men.1,9,22 Faje et al observed that male gender and older age were risk factors for development of hypophysitis in 154 patients with melanoma treated with ipilimumab.6 A possible explanation for this male predominance could be the fact that ipilimumab is mainly used as treatment for melanoma that occurs at higher rates in men than in women. However, the incidence of ipilimumab-related hypophysitis seems to be higher in men even after taking this into account.6,34
The median time to onset of symptoms of hypophysitis after initiation of treatment with ipilimumab is 9 weeks while there are cases of hypophysitis diagnosed 19 months after first ipilimumab infusion suggesting a long-term monitoring is required in such cases.14,22 The combination of CTLA-4 with anti-PD-1 agents is associated with earlier development of hypophysitis.35
Pathophysiology
The precise mechanism by which ipilimumab causes hypophysitis remains largely unknown. Recent in vitro and in vivo studies have suggested that immune and genetic factors are implicated and a combination of inflammatory and immune mechanisms result to tissue damage.16,36,37
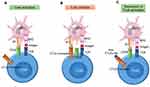
CTLA-4 acts as a negative regulator of the B7 and CD28 co-stimulation axis. In an immune response, activated T-cells upregulate the expression and the translocation of CTLA-4 in the plasma membrane. CTLA-4 binds with high-affinity B7 and can compete with CD28 to further inhibit T-cell activity. As a result, anti-CTLA-4 Abs, such as ipilimumab, bind to CTLA-4 and facilitate the B7 binding to CD28 and the up-regulation of T-cell activity (Figure 1).16,33
Recent in vitro and animal studies have suggested a potential role for Ab-dependent cell-mediated cytotoxicity (ADCC) and the complement pathway in the pathogenesis of ipilimumab-related hypophysitis.38,39 It has been observed that ipilimumab activates ADCC while repeated injections of CTLA-4 blocking Ab into mice resulted to lymphocytic infiltration of the pituitary gland and development of circulating pituitary Abs inducing a murine model of hypophysitis.37 In addition, it has been observed that CTLA-4 is expressed in murine and human pituitary gland. It is important to note that a distinct infiltration with mononuclear cells was observed in the pituitary gland but not in other organs suggesting that the mechanism of pituitary toxicity related to ipilimumab may be unique to this gland. Iwama et al studied 20 patients with negative Abs at baseline that received treatment with ipilimumab and found that pituitary Abs developed in the 7 patients with hypophysitis but not in the 13 without it.37 The Abs recognized predominately thyrotroph cells while anti-corticotroph and anti-gonadotroph Abs were also detected. CTLA-4 expressed in pituitary cells may be a direct target for anti-CTLA-4 Abs resulting in activation of ADCC by direct binding to pituitary cells.37 In particular, it is thought that the early events are likely to be inflammatory type II hypersensitivity reactions. Upon administration of the CTLA-4 Ab, it binds to the CTLA-4 antigen in the pituitary, inducing activation of the classical complement cascade that damages the pituitary cells recruiting macrophages and other inflammatory cells leading to phagocytosis and enhanced antigen presentation. However, later events are considered to involve type IV hypersensitivity reactions characterized by infiltration with lymphocytes.36,37
Genetic factors predisposing to ICI-related hypophysitis could also be implicated. It has been shown that polymorphisms in the CTLA-4 gene are associated with an increased incidence of autoimmune disorders including hypophysitis.16 Since most polymorphisms do not change the amino-acid sequencing of the CTLA-4 protein, it has been postulated that they do not alter the binding affinity of anti-CTLA-4 Abs to CLTA-4 but may alter the expression level of CTLA-4, making patients more or less prone to CTLA-4-related hypophysitis.10
A recent study demonstrated that a number of autoantibodies such as anti-guanine nucleotide-binding protein G(olf) subunit alpha (anti-GNAL) and anti-integral membrane protein 2B (anti-ITM2B) are associated with the development of ICI-related hypophysitis.40 Anti-GNAL autoantibody has the potential to act as a predictive or as on-treatment biomarker while anti-ITM2B may act as on-treatment biomarker of ICI-related hypophysitis. However, larger studies are required for these Abs to enable early detection, close monitoring and proper treatment of ICI-related hypophysitis.
Clinical Presentation and Diagnosis
The clinical manifestations of ipilimumab-induced hypophysitis are typically non-specific and relate either to pituitary enlargement and sellar compression or to hormonal disturbances. Headache and fatigue are the most common symptoms while other common manifestations include nausea, anorexia, weight loss, and hyponatremia. Less frequently reported symptoms include hallucinations, confusion, memory loss, insomnia and temperature intolerance.10,16 In contrast to other forms of autoimmune hypophysitis, visual field defects are rare in ipilimumab-related hypophysitis as the degree of pituitary enlargement is usually mild.20,41 In some cases, hypophysitis may present with symptoms and signs of adrenal crisis, including nausea, vomiting, hypotension, disorientation, electrolyte disturbance and shock.42 It is important to recognize that hypophysitis and the underlying illness may manifest with overlapping symptoms and laboratory results and the diagnosis may be significantly delayed.
The majority of patients with ipilimumab-induced hypophysitis display deficiency of multiple pituitary hormones. Central hypothyroidism is characterized by low- or low-normal free thyroxine (fT4) in the setting of an inappropriately low or normal thyroid-stimulating hormone (TSH).20,43 A recent study reported that a TSH fall ≥80% may be an early marker of ipilimumab-induced hypophysitis and serial TSH measurements during treatment with ipilimumab may comprise an inexpensive tool to expedite the diagnosis.44 Of interest, Siddiqui et al recently reported that FT4 decline, TSH index and standardized TSH index were more valuable predictors of ipilimumab-induced hypophysitis than TSH decline.45 It is important to note that the discrimination between central hypothyroidism, euthyroid sick syndrome and the effect of treatment with high-dose steroids may be difficult. The clinical context and the comparison with baseline thyroid function tests prior to the administration of ipilimumab may be helpful in the differential diagnosis.10,22,46
Secondary adrenal insufficiency is a frequent manifestation and confers significantly to the morbidity and mortality of ICI-related hypophysitis.10 In fact, recent case series report that adrenocorticotropic deficiency is the most common hormonal insufficiency observed in patients with ICI-related hypophysitis.26,47 It is characterized by a low- or low-normal early morning cortisol level in the setting of an inappropriately low or normal adrenocorticotropin hormone (ACTH). Hypogonadotropic hypogonadism is also common while the prevalence of growth hormone (GH) deficiency is unclear due to lack of confirmation of GH deficiency in most studies.10,20 Prolactin levels may be elevated but often are low in these cases. Diabetes insipidus (DI) occurring in ipilimumab-related hypophysitis has been reported in some cases.48,49
At the beginning of immunotherapy with ipilimumab and before each subsequent dose, it is recommended to perform biochemical and hormonal evaluation that includes glucose, electrolytes, thyroid function tests (fT4/T3 and TSH) and cortisol measurement (Figure 2).19,50 It is important to note that measurement of both fT4 and T3 is required since primary thyroid dysfunction is also common after treatment with ipilimumab, especially in cases of treatment with the combination of ipilimumab and nivolumab, and would be especially useful in the differential diagnosis of different causes of thyrotoxicosis (Grave’s disease from immune-related destructive thyroiditis).10,42 Dynamic function testing (for corticotroph and growth hormone axes deficiency) may be required in case of suspicious baseline blood tests in order to confirm or exclude pituitary dysfunction.43,51 The treating physicians should manage their patients with particular caution during the first months after ipilimumab initiation when the incidence of hypophysitis is significantly higher.
 |
Figure 2 Proposed algorithm for the management of ipilimumab-induced hypophysitis. |
Gadolinium-enhanced pituitary magnetic resonance imaging (MRI) is the modality of choice for assessing pituitary pathology and excluding the presence of sellar metastatic lesions. The most frequent neuroimaging finding in patients with ipilimumab-induced hypophysitis is slight to moderate enlargement more often with hypointensity on T1 weighted images and/or heterogeneous enhancement of the pituitary. Pituitary stalk thickening may also be observed in some cases.22,52,53 It has been shown that imaging findings may precede the clinical and biochemical manifestations by several weeks.6 However, pituitary enlargement can be quite mild and may not be detected without comparison with previous images.34 Caturegli et al reported normal MRI findings in 23% of cases with hypophysitis related to treatment with anti-CTLA-4 antibodies.36 In addition, Faje et al have observed radiographic resolution of MRI findings of hypophysitis in 40 days after diagnosis and another group has reported reduction in pituitary size in one week.20,54 Subsequent atrophy of the gland and empty sella may also be observed. Thus, a normal pituitary MRI does not exclude the diagnosis of hypophysitis.
The diagnosis is generally based on the clinical manifestations as well as on the biochemistry and neuroimaging findings after treatment with ipilimumab (Figure 2). The resolution of the pituitary enlargement after the administration of glucocorticoids supports the inflammatory/autoimmune nature of the pituitary mass20,33 while the persistence of pituitary enlargement two months after the diagnosis may suggest an alternate pathology such as metastatic disease.6 In order to confirm the diagnosis, it is not mandatory to perform a pituitary biopsy unless there is suspicion of other pituitary pathologies or metastatic lesions.55
Treatment
The management of ipilimumab-induced hypophysitis involves primarily the replacement of deficient hormones and/or treatment with high-dose glucocorticoids while discontinuation of ipilimumab may also be required in severe cases, at least temporarily. It depends on the severity of symptoms and hormonal deficits in relation to the underlying disease. Table 2 shows the grading of ICI-related hypophysitis according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03.56
 |
Table 2 Toxicity Grading of ICI-Induced Hypophysitis According to CTCAE |
Hormone Replacement
Glucocorticoid and thyroid hormone replacement should be instituted early after the diagnosis according to clinical practice guidelines on hormone replacement in hypopituitarism.43 It is important to note that in case of concomitant central hypothyroidism and hypoadrenalism, glucocorticoid replacement should be started before thyroid hormone initiation, as thyroid hormone replacement in case of untreated adrenal insufficiency may result to symptomatic deterioration and lead to adrenal crisis.57 In case of severe symptoms suggestive of adrenal crisis, treatment with intravenous glucocorticoids is recommended, even prior to the results of diagnostic tests.43 Specifically, 100 mg hydrocortisone hemisuccinate should be administered via intravenous, intramuscular or subcutaneous injection followed by continuous perfusion of 100 mg delivered over 24 h. After clinical and biochemical improvement, treatment is switched to oral hydrocortisone at a dose of 60 mg/24 h, progressively reduced to the final replacement dose.58 The daily replacement dose of hydrocortisone should be 15–20 mg/day divided in 2–3 doses. Patients should be provided with a Steroid Emergency Card, education regarding ‘sick day rules’ and a hydrocortisone emergency injection kit as per Society for Endocrinology guidance.59,60
Sex hormone replacement may be considered if hypogonadism persists long term and in case it is not contraindicated.20 GH replacement is contraindicated in patients with active malignancy and the assessment of GH axis is in general not useful.61 Despite the fact that DI is rare in ipilimumab-induced hypophysitis, close monitoring is required for the development of DI after starting glucocorticoid replacement since adrenal insufficiency may mask the presence of DI.43 Administration of desmopressin using individualized therapeutic schedules is suggested.
High-Dose Corticosteroids
Most authors have reported the use of high-dose systemic steroids in patients with severe forms (grade 3 or 4) of ipilimumab-induced hypophysitis.6,62,63 The suggested dosage is prednisolone 1mg/kg/day or equivalent with subsequent tapering to a physiological replacement dose of hydrocortisone or prednisolone. However, there are no compelling data to support this management approach as there is no prospective study comparing normal replacement with high-dose corticosteroids in patients with ICI-induced hypophysitis. In addition, it has been shown in retrospective studies that the use of high-dose steroids did not alter the course of the disease.34,52 Indeed, Min et al found in a retrospective cohort study that the use of high-dose steroids did not appear to improve the frequency or time to resolution of pituitary dysfunction nor affected the overall survival.
There are also some concerns regarding the impact of treatment with high-dose corticosteroids on the anti-tumor effect of ipilimumab. It has initially been observed that the median duration of response to ipilimumab was shorter in patients who received treatment with systemic steroids, although there was no statistically significant effect on the overall duration of clinical response and survival was not decreased in those patients receiving high-dose corticosteroids.34,64 Additionally, the function of T-cells does not seem to be inhibited by glucocorticoids and in particular fibrosarcoma cell growth inhibition by anti-CTLA-4 Ab was not affected by high doses of dexamethasone.20,64,65 However, in a recent study of 98 patients with ipilimumab-induced hypophysitis, it was shown that the administration of high-dose glucocorticoids may negatively affect the antitumor efficacy of ICIs as it is associated with lower overall survival compared to the administration of low-dose glucocorticoid therapy.66 Of note, the radiologic and endocrinologic outcomes and symptom resolution did not differ significantly between patients who received high or low-dose glucocorticoids.
Hence, according to recent guidelines, it is recommended that treatment with high-dose corticosteroids be reserved for cases with significant hyponatremia, severe headaches that do not respond to normal analgesics and visual field defects or cranial nerve palsies.6,58,67
Ipilimumab Discontinuation
The decision to continue or interrupt the treatment with ipilimumab depends on the severity of hypophysitis. Several studies suggest continuation of immunotherapy and close monitoring in mild forms (grade 1) while for the higher toxicity grades it is recommended to discontinue ipilimumab and resume it in patients that display resolution of hypophysitis to grade 1 and receive less than 7.5 mg of prednisolone or its equivalent daily.10,16 However, Min et al showed that in patients with ipilimumab-induced hypophysitis the discontinuation of ipilimumab did not appear to affect the outcome of hypophysitis.34 Indeed, the frequency and median time to resolution, based on laboratory testing, did not differ significantly between patients who interrupted or continued the treatment with ipilimumab while resolution of pituitary hormone axes was observed in a subset of patients who received prolonged ipilimumab treatment. In addition, a large sized study suggested the continuation of immunotherapy along with appropriate pituitary hormone replacement.52 Hence, the majority of experts agree that in patients with a potentially life-threatening malignancy, the clinical benefit of immunotherapy seems to outweigh the risk and according to recent guidelines, it is recommended to delay treatment with ICI in the acute phase of hypophysitis and resume it once patient is clinically stable on appropriate hormonal replacement therapy.58,67 In addition, the development of hypophysitis after treatment with an ICI is not considered a contraindication of therapy with another ICI while in patients with a history of pituitary pathology close monitoring and if required, adjustment of replacement therapy is recommended as there are no published data so far on the risk of hypophysitis in these patients.58
Longitudinal Outcomes and Follow-Up
Current guidelines on ICI-related hypophysitis recommend follow-up at each appointment for 3 months, then every 3 months for 6 months and bi-annually thereafter as well as a repeat MRI in 3 months in order to detect resolution of the disease, complications or relapse.58,68,69
In addition, it is suggested to periodically re-evaluate the patients for pituitary hormone deficiencies after the resolution of ipilimumab-induced hypophysitis.6
The proportion of patients with ipilimumab-induced hypophysitis that recovers pituitary function varies significantly and pituitary dysfunction may persist for a prolonged period of time even after treatment with high-dose steroids. This variation may be attributed to differences in follow-up, in frequency of hormonal evaluation, in strategies of weaning patients of hormone replacement as well as in the complicated way of estimating thyrotroph and gonadotroph dysfunction in patients with long-standing malignancies. In total, only in 25% of patients pituitary function recovery has been observed while a recent study involving patients receiving treatment with anti-CTLA-4 Abs for over 2.5 years reported a long-term hormonal replacement requirement in 86.6% of patients.52 Recovery of pituitary-thyroid axis has been observed in 37–50% of cases while recovery from central hypogonadism appears to be the most common and being reported in 57% of affected men.48,70,71 In contrast, adrenal recovery has rarely been reported and the majority of studies have shown that corticotroph deficiency persists in almost all patients.34,48,52 The time of recovery of the pituitary hormone axes is unpredictable. One study reported that the median time to recovery of the thyrotroph and gonadotroph axis was 13 and 10 weeks, respectively.49 Resolution of imaging findings is observed in the majority of cases with a duration of time that varies from 2 to 27 weeks.6,49
Conclusion
Advances in the field of cancer biology have led to the development of novel immunomodulatory molecules that are widely used as treatment of an increasing number of solid and hematologic malignancies. The use of these agents, particularly ipilimumab, has been associated with the development of hypophysitis in a significant subset of patients. The incidence of ipilimumab-induced hypophysitis varies significantly due to the heterogeneity between studies while the pathophysiology of this adverse event is still poorly elucidated. Further investigation is required to clarify the mechanism sustaining ICI-induced hypophysitis in order to identify predictive factors and enable the development of appropriate strategies of prevention or management. Corticotroph, thyrotroph and gonadotroph axes are the most commonly involved with corticotroph deficiency usually being permanent. With a growing number of patients treated with ICI, evaluation and reporting of endocrine irAEs in clinical trials would increase our knowledge regarding the incidence and clinical implications of these conditions. These endocrinopathies may be life-threatening and it is important for treating physicians to be aware of their clinical manifestations, diagnosis and management, allowing the optimal management and improving the outcome of ipilimumab-induced hypophysitis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tan MH, Iyengar R, Mizokami-Stout K, et al. Spectrum of immune checkpoint inhibitors-induced endocrinopathies in cancer patients: a scoping review of case reports. Clin Diabetes Endocrinol. 2019;5:1. doi:10.1186/s40842-018-0073-4
2. Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7(4):445–450. doi:10.1016/S1074-7613(00)80366-0
3. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi:10.3389/fimmu.2014.00520
4. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi:10.1056/NEJMoa1003466
5. Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter Phase I/II study. Ann Oncol. 2013;24(7):1813–1821. doi:10.1093/annonc/mdt107
6. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078–4085. doi:10.1210/jc.2014-2306
7. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi:10.1200/JCO.2014.59.4358
8. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473–486. doi:10.1038/nrclinonc.2016.58
9. Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98(4):1361–1375. doi:10.1210/jc.2012-4075
10. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17–65.
11. Torino F, Barnabei A, Paragliola RM, Marchetti P, Salvatori R, Corsello SM. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol. 2013;169(6):R153–164. doi:10.1530/EJE-13-0434
12. Bai X, Lin X, Zheng K, et al. Mapping endocrine toxicity spectrum of immune checkpoint inhibitors: a disproportionality analysis using the WHO adverse drug reaction database, VigiBase. Endocrine. 2020. doi:10.1007/s12020-020-02355-9
13. Ntali G, Kassi E, Alevizaki M. Endocrine sequelae of immune checkpoint inhibitors. Hormones. 2017;16(4):341–350. doi:10.14310/horm.2002.1754
14. Angelousi A, Alexandraki KI, Tsoli M, Kaltsas G, Kassi E. Hypophysitis (including IgG4 and immunotherapy). Neuroendocrinology. 2020. doi:10.1159/000506903
15. Fukuoka H. Hypophysitis. Endocrinol Metab Clin North Am. 2015;44(1):143–149. doi:10.1016/j.ecl.2014.10.011
16. Angelousi A, Chatzellis E, Kaltsas G. New molecular, biological, and immunological agents inducing hypophysitis. Neuroendocrinology. 2018;106(1):89–100. doi:10.1159/000480086
17. Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi:10.1200/JCO.2005.06.205
18. Maker AV, Yang JC, Sherry RM, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29(4):455–463. doi:10.1097/01.cji.0000208259.73167.58
19. Elia G, Ferrari SM, Galdiero MR, et al. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract Res Clin Endocrinol Metab. 2019;101370.
20. Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19(1):82–92. doi:10.1007/s11102-015-0671-4
21. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–182. doi:10.1001/jamaoncol.2017.3064
22. Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21(2):371–381. doi:10.1530/ERC-13-0499
23. Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi:10.1056/NEJMoa1302369
24. Shang YH, Zhang Y, Li JH, Li P, Zhang X. Risk of endocrine adverse events in cancer patients treated with PD-1 inhibitors: a systematic review and meta-analysis. Immunotherapy. 2017;9(3):261–272. doi:10.2217/imt-2016-0147
25. Da L, Teng Y, Wang N, et al. Organ-specific immune-related adverse events associated with immune checkpoint inhibitor monotherapy versus combination therapy in cancer: a meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:1671. doi:10.3389/fphar.2019.01671
26. Kassi E, Angelousi A, Asonitis N, et al. Endocrine-related adverse events associated with immune-checkpoint inhibitors in patients with melanoma. Cancer Med. 2019;8(15):6585–6594. doi:10.1002/cam4.2533
27. Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a Phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi:10.1016/S1470-2045(12)70006-2
28. Hodi FS, Lawrence D, Lezcano C, et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2(7):632–642. doi:10.1158/2326-6066.CIR-14-0053
29. Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm Phase 2 trial. Lancet Oncol. 2012;13(9):879–886. doi:10.1016/S1470-2045(12)70324-8
30. Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter Phase II study. J Clin Oncol. 2012;30(17):2046–2054. doi:10.1200/JCO.2011.38.4032
31. Haanen JB, Thienen H, Blank CU. Toxicity patterns with immunomodulating antibodies and their combinations. Semin Oncol. 2015;42(3):423–428. doi:10.1053/j.seminoncol.2015.02.011
32. Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi:10.1093/annonc/mds213
33. Joshi MN, Whitelaw BC, Palomar MT, Wu Y, Carroll PV. Immune checkpoint inhibitor-related hypophysitis and endocrine dysfunction: clinical review. Clin Endocrinol (Oxf). 2016;85(3):331–339. doi:10.1111/cen.13063
34. Min L, Hodi FS, Giobbie-Hurder A, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21(4):749–755. doi:10.1158/1078-0432.CCR-14-2353
35. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi:10.1056/NEJMoa1504030
36. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. doi:10.1016/j.ajpath.2016.08.020
37. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra245. doi:10.1126/scitranslmed.3008002
38. Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140–6145. doi:10.1073/pnas.1417320112
39. Laurent S, Queirolo P, Boero S, et al. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-alpha production. J Transl Med. 2013;11:108. doi:10.1186/1479-5876-11-108
40. Tahir SA, Gao J, Miura Y, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A. 2019;116(44):22246–22251. doi:10.1073/pnas.1908079116
41. Albarel F, Castinetti F, Brue T. Management of endocrine disease: immune check point inhibitors-induced hypophysitis. Eur J Endocrinol. 2019;181(3):R107–R118. doi:10.1530/EJE-19-0169
42. Del Rivero J, Cordes LM, Klubo-Gwiezdzinska J, Madan RA, Nieman LK, Gulley JL. Endocrine-related adverse events related to immune checkpoint inhibitors: proposed algorithms for management. Oncologist. 2020;25(4):290–300. doi:10.1634/theoncologist.2018-0470
43. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888–3921.
44. De Sousa SMC, Sheriff N, Tran CH, et al. Fall in thyroid stimulating hormone (TSH) may be an early marker of ipilimumab-induced hypophysitis. Pituitary. 2018;21(3):274–282. doi:10.1007/s11102-018-0866-6
45. Siddiqui MS, Lai ZM, Spain L, et al. Predicting development of ipilimumab-induced hypophysitis: utility of T4 and TSH index but not TSH. J Endocrinol Invest. 2020. doi:10.1007/s40618-020-01297-3
46. Adler SM, Wartofsky L. The nonthyroidal illness syndrome. Endocrinol Metab Clin North Am. 2007;36(3):657–672, vi. doi:10.1016/j.ecl.2007.04.007
47. Garon-Czmil J, Petitpain N, Rouby F, et al. Immune check point inhibitors-induced hypophysitis: a retrospective analysis of the French Pharmacovigilance database. Sci Rep. 2019;9(1):19419. doi:10.1038/s41598-019-56026-5
48. Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13(1):29–38. doi:10.1007/s11102-009-0193-z
49. Min L, Vaidya A, Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012;18(3):351–355. doi:10.4158/EP11273.OR
50. Castillero F, Castillo-Fernandez O, Jimenez-Jimenez G, Fallas-Ramirez J, Peralta-Alvarez MP, Arrieta O. Cancer immunotherapy-associated hypophysitis. Future Oncol. 2019;15(27):3159–3169. doi:10.2217/fon-2019-0101
51. Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245–4253. doi:10.1210/jc.2008-0710
52. Albarel F, Gaudy C, Castinetti F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172(2):195–204. doi:10.1530/EJE-14-0845
53. Rodrigues BT, Otty Z, Sangla K, Shenoy VV. Ipilimumab-induced autoimmune hypophysitis: a differential for sellar mass lesions. Endocrinol Diabetes Metabol Case Rep. 2014;2014:140098. doi:10.1530/EDM-14-0098
54. Majchel D, Korytkowski MT. Anticytotoxic T-lymphocyte antigen-4 induced autoimmune hypophysitis: a case report and literature review. Case Rep Endocrinol. 2015;2015:570293. doi:10.1155/2015/570293
55. Di Dalmazi G, Ippolito S, Lupi I, Caturegli P. Hypophysitis induced by immune checkpoint inhibitors: a 10-year assessment. Expert Rev Endocrinol Metab. 2019;14(6):381–398. doi:10.1080/17446651.2019.1701434
56. National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events (CTCAE). Bethesda M, ed. U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute; 2009.
57. Osman IA, Leslie P. Addison’s disease. Adrenal insufficiency should be excluded before thyroxine replacement is started. BMJ. 1996;313(7054):427. doi:10.1136/bmj.313.7054.427
58. Castinetti F, Albarel F, Archambeaud F, et al. French endocrine society guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. 2019;26(2):G1–G18. doi:10.1530/ERC-18-0320
59. Cooksley T, Knight T, Gupta A, Higham C, Lorigan P, Adam S. Emergency ambulatory outpatient management of immune-mediated hypophysitis. Support Care Cancer. 2020;28(9):3995–3999. doi:10.1007/s00520-020-05581-z
60. Arlt W; Society for Endocrinology Clinical C. Society For Endocrinology Endocrine Emergency Guidance: emergency management of acute adrenal insufficiency (adrenal crisis) in adult patients. Endocr Connect. 2016;5(5):G1–G3. doi:10.1530/EC-16-0054
61. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S. Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587–1609. doi:10.1210/jc.2011-0179
62. Marlier J, Cocquyt V, Brochez L, Van Belle S, Kruse V. Ipilimumab, not just another anti-cancer therapy: hypophysitis as side effect illustrated by four case-reports. Endocrine. 2014;47(3):878–883. doi:10.1007/s12020-014-0199-9
63. Della Vittoria Scarpati G, Fusciello C, Perri F, et al. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. Onco Targets Ther. 2014;7:203–209. doi:10.2147/OTT.S57335
64. Downey SG, Klapper JA, Smith FO, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13(22):6681–6688. doi:10.1158/1078-0432.CCR-07-0187
65. Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28(6):517–524. doi:10.1097/01.cji.0000177999.95831.7b
66. Faje AT, Lawrence D, Flaherty K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–3714. doi:10.1002/cncr.31629
67. Higham CE, Olsson-Brown A, Carroll P, et al. Society for endocrinology endocrine emergency guidance: acute management of the endocrine complications of checkpoint inhibitor therapy. Endocr Connect. 2018;7(7):G1–G7. doi:10.1530/EC-18-0068
68. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society Of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2018;14(4):247–249. doi:10.1200/JOP.18.00005
69. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv142. doi:10.1093/annonc/mdx225
70. Blansfield JA, Beck KE, Tran K, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28(6):593–598. doi:10.1097/01.cji.0000178913.41256.06
71. Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol. 2012;167(1):1–5. doi:10.1530/EJE-12-0167
72. Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at memorial sloan kettering cancer center. J Clin Oncol. 2015;33(28):3193–3198. doi:10.1200/JCO.2015.60.8448
73. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, Phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi:10.1016/S1470-2045(15)70122-1
74. Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. doi:10.1097/CJI.0b013e3181eec14c
75. Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. doi:10.1097/CJI.0b013e318156e47e
76. Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446–6453. doi:10.1158/1078-0432.CCR-09-1339
77. Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–1775. doi:10.1002/cncr.24951
78. Snyders T, Chakos D, Swami U, et al. Ipilimumab-induced hypophysitis, a single academic center experience. Pituitary. 2019;22(5):488–496. doi:10.1007/s11102-019-00978-4
79. NCI. National Cancer Institute Common Terminology Criteria for Adverse Events (CCTAE) v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. 2017 Accessed November 8, 2018.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.