Back to Journals » Cancer Management and Research » Volume 14
Management of Treatment-Related Infectious Complications in High-Risk Hemato-Oncological Patients via Telemedicine
Authors Hradská K , Popková T , Skořupová M, Mihályová J, Jelínek T, Lančová J, Schellong N, Hájek R
Received 17 December 2021
Accepted for publication 31 March 2022
Published 4 May 2022 Volume 2022:14 Pages 1655—1661
DOI https://doi.org/10.2147/CMAR.S348923
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Katarína Hradská,1,2 Tereza Popková,1,2 Michaela Skořupová,1,2 Jana Mihályová,2 Tomáš Jelínek,2 Jana Lančová,3 Norbert Schellong,3 Roman Hájek1,2
1Faculty of Science, University of Ostrava, Ostrava, Czech Republic; 2Department of Haematooncology, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, Ostrava, Czech Republic; 3National Monitoring Center, Ostrava, Czech Republic
Correspondence: Katarína Hradská, Department of Haematooncology, University Hospital Ostrava and Faculty of Medicine, University of Ostrava, 17. listopadu 1790, Ostrava, 70852, Czech Republic, Tel +420 597 37 2092, Fax +420 597 37 2092, Email [email protected]
Background: Infectious complications, especially febrile neutropenia, in hemato-oncological patients are associated with considerable morbidity, mortality and expenses. Remote monitoring of physiological functions and thus early detection of adverse events via telemedicine could improve the safety of these high-risk patients and save financial resources by shortening the time-to-antibiotics.
Methods: Patients undergoing active cancer treatment in high risk of acquiring severe infection are selected and enrolled in this project. Each patient receives a digital blood pressure monitor, an infrared thermometer and a mobile hub (cell phone). In the comfort of their homes, patients measure their blood pressure/pulse and body temperature regularly or whenever they feel unwell. The obtained data are encrypted and forwarded via the mobile hub to the password-protected portal. The values registered outside the set-up range trigger the alarms, which are immediately sent to the designated physician who can check the portal in real-time from any device with an Internet connection, contact the patient, if need be, and initiate the anti-infective therapy almost instantly after the first symptoms occur.
Results: Fifty hemato-oncological patients were recruited between March 1, 2018 and August 1, 2020. Two hundred ninety-seven alarms of body temperature were registered and checked by the physician and patients were contacted in 18.5% of the cases (55/297). Among these 55 events, 13 required medical assistance, which makes it approximately one-quarter of all conducted telephone interventions (23.4%) and neither septic shock nor death due to treatment-related toxicity occurred.
Conclusion: Telemedicine seems like a useful tool to improve the safety of high risk hemato-oncological patients when treatment-related infectious complications are concerned.
Keywords: telemedicine, patient monitoring, hematologic neoplasms, chemotherapy-induced febrile neutropenia, cancer, vulnerable populations
Introduction
Telemedicine, eHealth or telehealth can be described as the secure and cost-effective utilization of information communication technologies in support of health and health-related fields, including health-care services, health surveillance, health information, education and research.1 Another feasible and more simple definition takes telemedicine as a tool to deliver health care to populations with limited access to care by using telecommunications technology.2
Initially, the concept was developed by the National Aeronautics and Space Administration (NASA) in the 1970s to assist in the care of astronauts in space and then was gradually implemented in health care systems worldwide.2 One of the most important advantages that telemedicine brings to the field is the possibility of the remote management of long-term chronic conditions such as diabetes, heart failure, asthma, chronic obstructive pulmonary disease and last but not least, cancer, including hemato-oncological diagnoses.3
One of the most dangerous side effects of anti-cancer chemotherapy is the development of neutropenia, which can be complicated by life-threatening infection. Febrile neutropenia (FN) is defined as a fever greater than 38.0°C sustained for longer than 1 hour in patients with absolute neutrophil count (ANC) of <0.5 × 109/L, or expected to fall below 0.5 × 109/L.4,5 Hemato-oncological patients are at greater risk with typically longer periods of neutropenia compared to solid oncology and it is estimated that more than 80% of these patients will develop fever during at least one cycle of chemotherapy with associated neutropenia.5 Rapid clinical assessment and administration of broad-spectrum antibiotics is of utmost importance in preventing serious morbidity and mortality which can reach as high as 20–30% and 10%, respectively.4,6 According to European Society for Medical Oncology (ESMO) guidelines, the first administration of therapy should be given in the hospital within 1 hour from the admission of a patient with FN.4 A prospective cohort study conducted by Rosa et al set the threshold even lower, at 30 minutes, and proved that each hour of delay in the time to antibiotics raised the 28-day mortality risk by 18%.7
In our project, we have tried to use telemedicine, in particular the remote monitoring of body temperature, blood pressure and pulse, to improve the safety of our high-risk patients undergoing active treatment for hematological malignancies with focus on the early detection and resolution of febrile neutropenia and prevention of serious and expensive complications such as septic shock. Several studies have been conducted and protocols created to reduce the time to antibiotics in the hospital setting.6–8 However, we aim to shorten the time to the adequate therapy in the “pre-hospital period” – to record and promptly manage the onset of clinical symptoms.
Methods
This is a prospective project targeting hemato-oncological patients in high risk of developing severe infectious complications, including febrile neutropenia, in the Department of Haematooncology, University Hospital Ostrava, Czech Republic. It started on March 1, 2018 and will continue until December 31, 2022. During the course of the project, we aim to recruit 100 participants. Eligible patients are those with expected longer periods of neutropenia due to diagnosis or as a result of cancer treatment including 1) acute leukemia (myeloid, promyelocytic or lymphoblastic) – during active treatment; 2) malignant lymphoma (Hodgkin and non-Hodgkin) - intensive regimens, salvage regimens, 3 months following autologous stem cell transplantation (ASCT), after CAR (chimeric antigen receptor)-T cell therapy; 3) multiple myeloma - intensive regimens, regimens including anti-CD38 antibodies, 3 months following ASCT; 4) chronic lymphocytic leukemia – regimens with fludarabine or obinutuzumab in the first line of therapy; 5) myelodysplastic syndrome – with associated neutropenia; and 6) others – e.g. very severe aplastic anemia after administration of antithymocyte immunoglobulin. We also enrolled immunocompromised patients throughout all hematological diagnoses undergoing active treatment with no particular danger of developing febrile neutropenia, but with history of late-detected severe infectious complications.
Each potential patient is selected and approached by the designated doctor who performs the initial interview and gets the patient´s written consent. This usually happens early after the beginning of cancer treatment either during hospitalization at the ward or during regular check-up in the outpatient unit. The next step is within the competence of the designated nurse who educates the patient about the utilization of measuring devices, a schedule of measurements and hands out the devices. In the comfort of their homes, patients take their blood pressure once daily in the morning (between 6 am and 10 am) and body temperature twice a day – in the morning and in the evening (between 6 am and 10 am and between 5 pm and 9 pm) and additionally, also whenever they feel unwell.
Contactless measuring is provided by a digital blood pressure monitor (devices used: MD2020, MD4781, Beurer BM 85) and an infrared thermometer (device used: Rycom JXB-182). All devices communicate via Bluetooth with a mobile hub (cell phone), which encrypts the collected data and automatically forwards them to the National Monitoring Center (NMC). In fact, the NMC serves as a link between the physician and the patient. Physicians and nurses have access to the measured values through a password-protected web portal provided by the NMC in real-time from any device with an Internet connection (see also Figure 1). The biggest advantage is that the values registered outside the set-up range trigger the alarms which are immediately sent by SMS (short message service) to the responsible staff member who evaluates the situation and takes action within 30 minutes, if needed. Should the physician need to contact a patient, he/she can send an SMS directly through the portal or call him/her.
 |
Figure 1 Schematic depiction of methodology. |
There are two types of alarms: warning and critical. Critical alarms for blood pressure and pulse are represented by values of systolic blood pressure (SBP) <90mmHg, diastolic blood pressure (DBP) <50mmHg or >90mmHg and pulse <40/min or >130/min. A body temperature >37.0°C sets off a warning alarm and >37.9°C sets off a critical alarm. The values of blood pressure and pulse are considered auxiliary in the process of decision-making.
Should a patient forget to perform a measurement, they are automatically reminded by SMS, which is followed by a phone call from an NMC staff member. Patients are also able to ring an emergency telephone number to directly report both software and hardware-related technical issues to the NMC.
Results
Between March 1, 2018 and August 1, 2020, 50 hemato-oncological patients were recruited with a median of 52 years (19–80). The patient cohort included 22 patients (44%) treated for acute leukemia (acute myeloid leukemia, acute lymphoblastic leukemia and acute promyelocytic leukemia), 12 (24%) for malignant lymphoma (Hodgkin’s lymphoma and non-Hodgkin’s lymphoma), 8 (16%) for multiple myeloma, 6 (12%) for chronic lymphocytic leukemia, 1 (2%) for myelodysplastic syndrome and 1 (2%) for very severe aplastic anemia (see also Table 1).
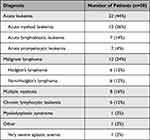 |
Table 1 Characteristics of Patient Cohort (by Diagnosis) |
The median duration of monitoring was 123.5 days (1–720). As of August 1, 2020, from a total of 50 patients, 46% (23/50) were still being actively monitored, 26% (13/50) had discontinued monitoring due to achieving hematologic remission, 14% (7/50) had withdrawn consent after various lengths of monitoring periods (1 day to 207 days), 10% (5/50) had died (none of them of febrile neutropenia) and 4% (2/50) had undergone the initial interview and education, but did not start the measurements – one due to death, the other one changed her mind and withdrew consent. Two patients (one with multiple myeloma and one with acute myeloid leukemia) experienced two separate measuring periods due to relapse.
We registered a total of 168 warning and 129 critical alarms of body temperature in 74% patients (37/50) and 599 critical alarms of blood pressure/pulse in 82% patients (41/50). Among these alerts, six were attributed to incipient febrile neutropenia and one to severe respiratory infection in an immunocompromised patient. The patients concerned (four treated for acute myeloid leukemia and two for multiple myeloma) were contacted by the physician and urgent hospitalization and rapid administration of broad-spectrum intravenous antibiotics were recommended. None of these patients developed septic shock. Six cases of fever were managed in the outpatient unit – 4 infectious complications required anti-infective therapy (genital herpes, upper respiratory tract infection) and two patients had already been taking antibiotics.
The designated physicians checked all 297 alarms of body temperature through the NMC portal and patients needed to be contacted in 18.5% of the cases (55/297). Among these 55 events, 13 needed medical assistance, as mentioned above, which although amounting to only 4.4% of all the alarms, constituted approximately one-quarter of all conducted telephone interventions (23.4%). Remaining alarms were associated with measurement errors, broken measuring devices or incorrect measurement techniques.
Discussion
The blending of information communication technologies and medicine is definitely nothing new. The concept of telemedicine is ever-changing and expanding. Remote monitoring is not the only practical application. eHealth covers everyday matters such as electronic prescriptions and the very useful ePACS (electronic Picture Archiving and Communication System), which enables sharing patient imaging and visual documentation among all the hospitals and about 25% of the outpatient clinics in Czech Republic.9 With regards to cancer, teleoncology is experiencing a kind of a boom, especially in the current COVID-19 era, when in-person visit to the oncology centre can be challenging in terms of potential spread of the virus among the patients and health care professionals as well.10,11 Telehealth solutions are also making its way into cancer care guidelines.12 Communication technologies are helpful in cancer diagnostics (telepathology, telegenetics, teleradiology, etc.), treatment, survivorship care and palliative care.2,13–15 Pharmaceutical clinical trials might also benefit from telehealth (wearables, mobile technology, sensors) by obtaining more timely data, which should decrease trial length and lessen drug development costs.16
There are various ongoing and completed clinical studies and projects aiming to determine the benefit of telemedicine interventions during active treatment in both solid oncology and hemato-oncology. The most important is the electronic Symptom Management using the Advanced Symptom Management System (ASyMS) Remote Technology (eSMART; NCT02356081), an international, randomized controlled trial that evaluated the use of mobile phone technology to manage chemotherapy-related toxicities in people with breast cancer, colorectal cancer, Hodgkin’s lymphoma and non-Hodgkin’s lymphoma from 12 centres in five European countries (Austria, Greece, Norway, Ireland, Great Britain).17–19 The patients in the intervention arm used a mobile phone application where not only objective values of physiological functions were registered (e.g. body temperature) but their subjective feelings as well (e.g. fatigue, nausea, anxiety). eSMART proved favourable in terms of improving anxiety, health-related quality of life, self-efficacy and supportive care needs. The incidence of neutropenic events was higher in the intervention group, but the rates of hospital admissions and deaths were comparable between both groups.19 Similar studies are underway also in Canada and Australia.20,21 Another randomized controlled trial investigated patient-reported symptom monitoring during cancer treatment and proved the telemedicine feasible in this setting. Health-related quality of life improved in the intervention group, monitored patients were less frequently admitted to the hospital and remained on chemotherapy longer. However, targeted adverse symptoms did not include fever or infectious complications.22
As seen above, telemedicine clinical trials often recruit patients suffering from hematologic and solid malignancies together.17,18,23 Therefore, valid and applicable results specific to hemato-oncology are quite difficult to find. With regard to the behaviour of diseases, our patients are specific and more prone to acquiring life-threatening infections due to longer periods of neutropenia.5 That is precisely why we chose to monitor just one, but in our opinion, the most important, parameter which is potentially severe infection at its beginning. Our goal is to shorten the time-to-antibiotics, thus maximizing patient safety while making the process as user-friendly and unburdensome as possible. Access to medical care is not the main problem in the Moravian-Silesian region, but despite thorough patient education of potential treatment-related toxicities and risks, we found that some patients tend to neglect warning signals and look for medical attendance with unnecessary delay resulting in worse outcomes and higher medical expenses.
As expected, several problems arose during the course of the project. There was a rather high proportion of false alarms registered caused mainly by incorrect measuring techniques (e.g. repeated measuring in a very short time period, temperature measuring after taking a shower or sunbathing, etc) and technical problems related to measuring devices (e.g. re-sending previously measured values after restarting the mobile hub, values sent with delay due to switched off mobile hub, etc). We tried to improve the outcomes by performing a more detailed education at the beginning and also in the course of monitoring. However, we were able to address only a part of the problem. Technical errors are, unfortunately, an integral part of remote monitoring technologies and were dealt with by the NMC.24
As mentioned above, the dropout rate was 26% (13/50), which is comparable with other telehealth clinical trials.19,24 Four patients withdrew consent after a very short monitoring period (1–16 days) because of the difficulties they faced related to the use of modern technologies. All of these patients could be considered to be in older age range (66–74 yers). The remaining three patients found requirements of the project too bothersome, thus decided to discontinue.
Two patients involved in our project, both treated for acute leukemia, experienced febrile neutropenia, which was not recorded by the measuring devices – one did not switch on the hub, so the values were not sent to the NMC and the other one presented with the symptoms during regular check-up, after being afebrile in that morning’s measurement. We did not include these in the overall account of detected cases of febrile neutropenia. Nevertheless, neither of these patients needed intensive care unit hospitalization or progressed into septic shock.
Conclusion
Preliminary results of our project seem promising despite the high false alarm rate and low number of included patients and captured events. No septic shock or death associated with treatment-related toxicity has occurred in the monitored group and that is our primary objective. Remote patient monitoring has been useful in improving the safety of high risk hemato-oncological patients in terms of early detection and resolution of treatment-related infectious complications.
The financial cost–benefit analysis is currently being completed. We believe that the final results could help us implement telehealth interventions in the standard of care for hemato-oncological patients, which would be eventually funded by the public health insurance in the Czech Republic.
Ethics Approval and Informed Consent
The ethics committee of University Hospital Ostrava approved this project, and the written informed consent was obtained from all participating patients. The project complies with the Declaration of Helsinki.
Consent for Publication
All authors reviewed the article and gave their consent for publication.
Acknowledgments
The authors thank all the participating patients and their families, Dr Shira Timilsina Godfrey for English editing, Ladislav Vichanek for designing the figure and colleagues from the National Monitoring Center for technical assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research paper was written as a part of a research project of a long-term intersectoral collaboration “Smart technologies for the improvement of quality of life in cities and regions”, identification number CZ.02.1.01/0.0/0.0/17_049/0008452. This project is funded by European Union Social Fund, Operational Programme “Research, Development and Education” led by the Ministry of Education, Youth and Sports of the Czech Republic. The leader of the project consortium is the Faculty of Science, University of Ostrava.
Disclosure
Prof. Dr Roman Hájek reports grants and/or personal fees for advisory board from Janssen, Celgene, BMS, Takeda, Novartis, Amgen, Pharma Mar, AbbVie, GSK, Oncopeptides, and Sanofi, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Harris J, Cheevers K, Armes J. The emerging role of digital health in monitoring and supporting people living with cancer and the consequences of its treatments. Curr Opin Support Palliat Care. 2018;12(3):268–275. doi:10.1097/SPC.0000000000000362
2. Sirintrapun SJ, Lopez AM. Telemedicine in cancer care. Am Soc Clin Oncol Educ Book. 2018;38:540–545. doi:10.1200/EDBK_200141
3. Hanlon P, Daines L, Campbell C, McKinstry B, Weller D, Pinnock H. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res. 2017;19(5):e172. doi:10.2196/jmir.6688
4. Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27:v111–v118. doi:10.1093/annonc/mdw325
5. White L, Ybarra M. Neutropenic fever. Emerg Med Clin North Am. 2014;32(3):549–561. doi:10.1016/j.emc.2014.04.002
6. Ko BS, Ahn S, Lee YS, Kim WY, Lim KS, Lee JL. Impact of time to antibiotics on outcomes of chemotherapy-induced febrile neutropenia. Support Care Cancer. 2015;23(9):2799–2804. doi:10.1007/s00520-015-2645-5
7. Rosa RG, Goldani LZ. Cohort study of the impact of time to antibiotic administration on mortality in patients with febrile neutropenia. Antimicrob Agents Chemother. 2014;58(7):3799–3803. doi:10.1128/AAC.02561-14
8. Ko HF, Tsui SS, Tse JWK, Kwong WY, Chan OY, Wong GCK. Improving the emergency department management of post-chemotherapy sepsis in haematological malignancy patients. Hong Kong Med J. 2015;21(1):10–15. doi:10.12809/hkmj144280
9. Bruthans J. ePACS - (neprávem) “ten druhý vzadu.”. Med Tribune. 2019;15(5):A6–A6.
10. Battineni G, Nittari G, Sirignano A, Amenta F. Are telemedicine systems effective healthcare solutions during the COVID-19 pandemic? J Taibah Univ Med Sci. 2021;16(3):305–306. doi:10.1016/j.jtumed.2021.02.009
11. Battineni G, Pallotta G, Nittari G, Amenta F. Telemedicine framework to mitigate the impact of the COVID-19 pandemic. J Taibah Univ Med Sci. 2021;16(2):300–302. doi:10.1016/j.jtumed.2020.12.010
12. Pareek P, Vishnoi JR, Kombathula SH, Vyas RK, Misra S. Teleoncology: the youngest pillar of oncology. JCO Glob Oncol. 2020;6:1455–1460. doi:10.1200/GO.20.00295
13. Cox A, Lucas G, Marcu A, et al. Cancer survivors’ experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19(1):e11. doi:10.2196/jmir.6575
14. Ho A, Quick O. Leaving patients to their own devices? Smart technology, safety and therapeutic relationships. BMC Med Ethics. 2018;19. doi:10.1186/s12910-018-0255-8
15. Yildiz F, Oksuzoglu B. Teleoncology or telemedicine for oncology patients during the COVID-19 pandemic: the new normal for breast cancer survivors? Future Oncol.2020. 16(28):2191–2195. doi:10.2217/fon-2020-0714
16. Cox SM, Lane A, Volchenboum SL. Use of wearable, mobile, and sensor technology in cancer clinical trials. JCO Clin Cancer Inf. 2018;(2):1–11. doi:10.1200/CCI.17.00147
17. Fox P, Darley A, Furlong E, et al. The assessment and management of chemotherapy-related toxicities in patients with breast cancer, colorectal cancer, and Hodgkin’s and non-Hodgkin’s lymphomas: a scoping review. Eur J Oncol Nurs. 2017;26:63–82. doi:10.1016/j.ejon.2016.12.008
18. Maguire R, Fox PA, McCann L, et al. The eSMART study protocol: a randomised controlled trial to evaluate electronic symptom management using the advanced symptom management system (ASyMS) remote technology for patients with cancer. BMJ Open. 2017;7(5):e015016. doi:10.1136/bmjopen-2016-015016
19. Maguire R, McCann L, Kotronoulas G, et al. Real time remote symptom monitoring during chemotherapy for cancer: European multicentre randomised controlled trial (eSMART). BMJ. 2021;374:n1647. doi:10.1136/bmj.n1647
20. Breen S, Ritchie D, Schofield P, et al. The Patient Remote Intervention and Symptom Management System (PRISMS) - a Telehealth- mediated intervention enabling real-time monitoring of chemotherapy side-effects in patients with haematological malignancies: study protocol for a randomised controlled trial. Trials. 2015;16:472. doi:10.1186/s13063-015-0970-0
21. Moradian S, Krzyzanowska M, Maguire R, et al. Feasibility randomised controlled trial of remote symptom chemotherapy toxicity monitoring using the Canadian adapted Advanced Symptom Management System (ASyMS-Can): a study protocol. BMJ Open. 2020;10(6):e035648. doi:10.1136/bmjopen-2019-035648
22. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. doi:10.1200/JCO.2015.63.0830
23. Rico TM, Dos Santos Machado K, Fernandes VP, et al. Use of text messaging (SMS) for the management of side effects in cancer patients undergoing chemotherapy treatment: a randomized controlled trial. J Med Syst. 2020;44(11). doi:10.1007/s10916-020-01663-x
24. Simblett S, Greer B, Matcham F, et al. Barriers to and facilitators of engagement with remote measurement technology for managing health: systematic review and content analysis of findings. J Med Internet Res. 2018;20(7):e10480. doi:10.2196/10480
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
