Back to Journals » Journal of Pain Research » Volume 12
Management of acute pain in dementia: a feasibility study of a robot-assisted intervention
Authors Demange M , Pino M, Kerhervé H , Rigaud AS, Cantegreil-Kallen I
Received 11 October 2018
Accepted for publication 18 February 2019
Published 7 June 2019 Volume 2019:12 Pages 1833—1846
DOI https://doi.org/10.2147/JPR.S179640
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor E Alfonso Romero-Sandoval

Manon Demange,1,2 Maribel Pino,1,2 Hélène Kerhervé,1,2 Anne-Sophie Rigaud,1,2 Inge Cantegreil-Kallen1,2
1Department of Geriatrics, Broca Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France; 2Research Team 4468, Paris Descartes University, Paris, France
Background: The management of pain is particularly challenging in patients with moderate to severe dementia owing to the loss of communication ability or underlying causes such as behavioral symptoms. It is often associated with health care professionals’ frustration and feeling of helplessness. The present study determined a framework and examined the feasibility of an innovative intervention using the PARO® ,robot for the management of acute pain in dementia.
Method: A mixed-methods research design combining qualitative (five focus groups) and quantitative (questionnaire survey) approaches was used to define the intervention framework. We recruited 57 health care professionals from various medical and paramedical specialties (eg, nursing auxiliaries, nurses, physicians, psychologists) and with expertise in gerontology. The feasibility of the intervention was subsequently assessed with 12 patients suffering from dementia in painful situations to validate the procedure.
Results: Four main issues have been addressed: 1) the identification of a core group of painful situations associated with care (washing, dressing/change, transfer/mobilization), currently considered as inefficiently managed; 2) the selection of an appropriate assessment methodology including criteria and tools for pain evaluation; 3) the definition of health professionals’ training needs and organizational requirements for their implementation; and 4) the perceived usefulness of a robot-assisted intervention for the management of pain in dementia in daily practice. The feasibility study showed that the predefined intervention framework was applicable and acceptable for the majority of professionals and patients.
Conclusion: A consistent and feasible intervention framework for the management of painful situations associated with care in dementia using the PARO robot was defined. Understanding of professionals’ needs, opinions and perceived obstacles regarding the intervention was a useful step in the preparation of the forthcoming clinical trial.
Keywords: pain, pain management, dementia, nonpharmacological intervention, PARO®, robot, feasibility study
Introduction
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience”.1 The IASP’s definition highlights the subjective experience of pain, which results from a complex interplay of influences (somatic, psychological and sociocultural factors). Around 60–80% of elderly people living in nursing homes regularly experience pain.2,3 Specific painful situations have been described in elderly patients without cognitive impairment during daily nursing care (bathing, preparation, transfers, dressing changes) and rehabilitation sessions.4 However, prevalence data of painful situations in dementia are not commonly found in the literature and appropriate management strategies are needed for this population.
Pharmacological intervention is considered a first-line treatment for pain in dementia, in spite of the known side effects (eg, constipation, confusion, behavioral disorders, psychomotor retardation).5 According to a French retrospective and observational study of 10,818 people living in 99 nursing homes, elderly people are high, and often chronic, consumers of analgesics.6 Thus, 62% consume at least one analgesic medication, 51% are chronic consumers, 11% have an acute analgesic consumption and 25% consume analgesics during both chronic and acute periods. Difficulties in the detection of pain in dementia probably lead to a misuse of analgesic medications and incorrect doses in this population.2,7
Other studies also indicated that persons with dementia (PwD) are a susceptible patient group in which pain is frequently underrecognized and undertreated.8 The inability to successfully communicate pain in advanced dementia is a major barrier to effective treatment.9 Identification of pain is further challenged by the overlapping symptomatology of pain with behavioral and psychological symptoms of dementia (BPSD),10,11 which commonly affect PwD. This close relationship between BPSD and pain is of particular importance since BPSD are usually treated with neuroleptics and benzodiazepines, which can cause adverse effects that may even be life-threatening (eg, stroke, death).9 According to Corbett et al,7 differentiation between pain and behavioral symptoms is highly context dependent and pain tools have to assess facial expressions of pain or vocalizations instead of “typical BPSD” such as agitation.
A key recommendation of the International Psycho-geriatric Association12 is the use of nonpharmacological approaches as the first-choice treatment for behavioral problems, and pharmacotherapy as a second line in PwD. Indeed, a wide range of nonpharmacological interventions for PwD exists and some have been studied for the management of pain in older adults, such as music therapy, therapeutic touch, audiovisual stimuli and relaxation.13,14 These interventions are based on the distraction method, which can induce an analgesic effect through competition between the cognitive treatment of the distraction stimulus and that of the painful situation.15 Thus, this competition may be explained by the limited resources of the attentional system to treat several stimuli simultaneously. Research on populations of adults and children often supports the hypothesis that distraction methods have a positive impact on pain modulation. Most of these interventions have also shown positive results on health status, quality of life, socialization and functional capacity in PwD.13 However, to date, little is known about the effect of psychosocial interventions on acute pain in patients with advanced dementia. Therefore, there is still a need for the development of innovative interventions.
At a time when modern technologies are assuming a central role in our society, we are witnessing an important evolution in the use of social robots in health care interventions. In this research area, numerous studies have identified social robots as appropriate therapeutic tools in advanced dementia. Such is the case for PARO® (Intelligent System Co., Kyoto, Japan) (Figure 1), an animal-like robot modeled on a baby harp seal and specifically designed for psychogeriatric care. PARO weighs approximately 2.7 kg and is covered with white artificial fur. It is equipped with five types of sensor: tactile, light, sound-recognition, temperature and posture, with which it can track human motion, pay attention to someone and perceive its environment.16,17 For instance, it can recognize the direction of a voice and some words (eg, its name). PARO responds to its environmental stimuli by making a noise, and moving its head, flippers or eyes.18,19 Most PARO-mediated interventions have been successful in reducing behavioral disorders,20–23 loneliness or stress levels,16 and also encouraging communication and/or social behaviors in dementia.17 Some researchers have hypothesized that PARO may facilitate the transfer of emotions, reassuring and calming the patients.21
 | Figure 1 PARO® robot seal. |
Nevertheless, to date there are limited scientific data on the effects of robot-assisted therapies on pain management in PwD. One of the few studies to investigate this issue was conducted by Petersen et al24 and consisted of a randomized controlled trial aimed at evaluating the effectiveness of a group therapeutic intervention using PARO in treating dementia-related symptoms (eg, anxiety, depression). The 3-month intervention consisted of three weekly 20-minute sessions in which participants were seated at a table on which PARO was placed and were invited to interact with the robot. Participants in the control group received standard activity programs (eg, music, physical activity). The results showed, among other things, a significant reduction in the use of pain medication in the PARO intervention group, suggesting an area for further research. However, although these results indicate a potential application of robot-assisted therapies for pain management in PwD, the use of PARO as a distracting and pleasant stimulus during painful situations remains to be investigated. This would entail the design and development of an intervention program, a process that requires careful examination for several reasons.
First, the development of innovative psychosocial interventions for dementia care is complex because these programs usually involve several interacting components that should be considered for their implementation and assessment (eg, the need to tailor the intervention to individual and contextual characteristics).25 Second, developing an intervention requires identifying important parameters that will be required to assess its impact (eg, inclusion criteria for the target population, willingness and availability of professionals to participate in the program, procedures, assessment strategies).26 These elements will provide the framework of the intervention. For this reason, a key success factor for these interventions is the inclusion of relevant stakeholders in the conception of the intervention,27 especially members of care teams. Finally, aspects related to the acceptability of the intervention and the willingness to integrate new care strategies within daily professional routines should be explored and dealt with, to reduce the risk of failure.
The general purpose of this study is two-fold. First, to determine a general framework for an intervention using the PARO robot as a mediation tool for the management of pain in PwD. Second, to examine the feasibility of this intervention, and to validate the procedure for the future crossover and randomized controlled trial.
Method
Study design
The study was conducted in two successive phases (Figure 2):
- Phase 1. Intervention framework definition: a co-design approach was adopted to integrate the perspectives of members of care teams working in a daily practice with PwD. A mixed-method research design combining qualitative and quantitative methodologies (focus groups [FGs] and questionnaire survey, respectively) was used to collect their views. This mixed method allows the same phenomenon to be analyzed using two different methodologies,28 and enables potential similarities and differences between particular aspects of this phenomenon to be pointed out.29 It also helps researchers to explore the same questions at the micro- and macro-levels.28 The mixed-method research design is known to have good results when studying complex health-related themes.30
- Phase 2. Feasibility assessment: examination of the procedure established in the previous phase for the use of the robot in the management of pain in real-life situations.
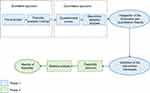 | Figure 2 The sequential design of the study. |
Participants
We enrolled 57 dementia care professionals in Phase 1. Potential participants were given information about the purpose and nature of the study during formal meetings and through posters. Phase 1 participants belonged to various medical and paramedical specialties (nursing auxiliary, nurse, medico-psychological assistant, psychologist and physician) and were recruited from four nursing homes in the St Etienne region (France) and the long-term unit of Broca geriatric hospital (Paris, France). Stratified random sampling was used to allocate these participants to either an FG or the questionnaire survey. Five strata were established based on the center involved.
Phase 2 included feasibility sessions with 12 persons over 65 years old, suffering from acute pain (observation of pain symptoms or prescription of analgesics) and diagnosed with major neurocognitive disorders according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).31 The DSM-V provides the following criteria for the diagnosis of major neurocognitive disorders (also known as dementia): 1) evidence of significant cognitive decline from a previous level of performance in one or more cognitive domains (complex attention, executive function, learning and memory, language, perceptual–motor or social cognition), 2) which interferes with independence in daily activities, and 3) which is not explained by a context of delirium or another mental disorder (eg, depressive disorder). Patients who suffered from psychotic symptoms and decompensated psychiatric conditions were excluded. Phase 2 of the study was conducted at Broca geriatric hospital.
All the participants (and, for patients, their legal representatives when necessary) received written and oral information about the research in accordance with the Declaration of Helsinki. Only those who provided informed consent prior to participation were included. This study was approved by the Broca Hospital clinical research committee.
Procedure and measures
Phase 1: intervention framework definition
The FGs allowed the collection of data regarding the experiences, professional routines and pain management practices in PwD by nursing-home care professionals. A facilitation guide, detailing the FG procedures, was developed to reproduce the same outline in all groups. This guide included 1) the presentation phase, 2) an introduction to the objectives of the FG and explanation of confidentiality principles that applied, 3) the specification of the mediator's and participants’ roles, and 4) a set of questions used to guide the discussions, grouped into three dimensions:
- Painful situations in dementia: participants were asked to discuss which daily situations are painful for patients, including pain indicators. The FG participants were also asked to share their current pain management techniques and their perceptions about them.
- Pain assessment: the second part of the exchanges concerned pain-rating tools known and used in routine clinical practice, and assessment of the difficulties encountered.
- Intervention tool, the PARO robot: participants were invited to share their experiences (for those who had already used the robot) and/or expectations concerning the use of the PARO robot.
FGs were videotaped and led by a trained psychologist mediator (MD), who encouraged all group members to participate equally. The total duration of an FG was approximately 1 hour. FGs were conducted using an academic research approach,32 which involves conducting the FG in the location of the target population. Thus, one FG was conducted in each of the centers involved in the study.
Based on feedback from the FGs, a self-administered questionnaire was developed by the research team to allow the possibility of quantifying opinions and practices from a maximum number of dementia care professionals. Thus, we selected the most relevant topics from FG analysis to design the content of the questionnaire (eg, the questions asked, the multiple-choice answers submitted) and added some new items about the PARO robot. The final 18-item questionnaire was structured in three sections:
- Painful situations and pain indicators in dementia: participants were asked to indicate daily painful situations in PwD among seven preselected items based on FG analysis and results from the literature,4,33,34 and to choose the most prevalent and most important indicators for the detection of pain in dementia.
- Feasibility of assessment tools: two pain measurement instruments were selected according to their psychometric proprieties, the ALGOPLUS® scale35 and the PAINAD® scale,36 and then submitted to participants, who were requested to indicate their level of agreement with four feasibility dimensions (ease of administration, understanding of the statements, availability of a validated French translation, time taken to administer the instrument) with a four-point Likert-type scale. These feasibility aspects were chosen according to the recommendations of a European consensus on outcome measures for psychosocial research in dementia care.37
- Perceived usefulness of the PARO robot: professionals were first asked about their knowledge and practice of the PARO robot with yes/no questions. Then, participants had to indicate the perceived clinical utility of PARO for the management of pain.
A summary of the questionnaire is presented in Table 1.
 | Table 1 Summary of dimensions and questions of the questionnaire survey |
Phase 2: feasibility assessment
After defining the intervention framework, health professionals had the opportunity to put it into practice in several feasibility assessment sessions (Figure 2), which corresponded to the acute pain situations selected by Phase 1 analysis. Each patient was randomly allocated to one of the four main painful situations selected. Feasibility sessions were conducted by the health professional who usually took care of the patient, and were supervised by one of the researchers (MD). Sessions consisted of using the PARO robot as a distracting stimulus during the painful situation while assessing the patient’s pain (before and during the situation) with the pain-rating scale identified by Phase 1 analysis.
Areas of focus for the feasibility study were selected according to recommendations from the literature.38,39 Thus, feasibility assessments included the three main following criteria:
- Data collection assessments: do health professionals understand the standardized pain scale and the data collection method? Do they respond with unusable data? How long does it take to fill in the scale? Does the data assessment involve a reasonable amount of time, or does it create a burden for the health professional?
- Implementation of the robot-based intervention: to what extent can the PARO-based mediation be successfully implemented in painful situations? Does this intervention create an additional burden? What is the perceived usefulness of this intervention?
- Patients’ eligibility criteria: are the established criteria clear and sufficient (too inclusive or restrictive)?
Assessments were performed by the researcher (MD), who observed the course of each feasibility session and conducted informal interviews at the end of sessions.
Data analysis
Data analysis was performed while preserving the anonymity of participants. Participants’ characteristics were described by means, SDs and percentages for categorical variables.
Qualitative analysis was used with contributions from the FG. Each session was videotaped and transcribed. Then, transcripts were examined according to a six-step inductive thematic analysis40 by a member of the research team, as follows:
- Step 1. Familiarization with the data: read and reread transcripts.
- Step 2. Defining relevant segments: segment the FG speech so that an excerpt (a word, a sentence or a paragraph) represents one idea.
- Step 3. Generating initial coding system: identify, analyze and categorize excerpts (ie, main relevant segments of FG speech) into parent codes (ie, key themes).
- Step 4. Reviewing potential subthemes: define subcodes, referring to secondary themes within key themes; and if necessary into sub-subcodes, referring to other topics in secondary themes.
- Step 5. Defining and naming themes: be able to clearly state what key themes or subthemes refer to, and provide definitions of all key themes and subthemes.
- Step 6. Mapping themes: design a concept map from existing links between key themes and subthemes.
An additional round of coding was performed by another member of the research team using the same six-step thematic analysis.
Descriptive statistics were applied to the answers from the questionnaires and graphic analyses were performed. The chi-squared test was used for comparison between percentages. A p-value <0.05 was considered significant. Data analysis was conducted using SPSS software (version 24.0; IBM Corp., Armonk, NY, USA).
Results
Phase 1: intervention framework definition
FG discussions were conducted with 18 health professionals: seven nurses (38.9%), six nursing auxiliaries (33.3%), four medico-psychological assistants (22.2%) and one physician (5.6%). On the 50 self-administered questionnaires distributed to the multidisciplinary care team, 39 (78%) were returned. Respondents to the questionnaire survey were mostly nurses (35.9%), nursing auxiliaries (35.9%) and medico-psychological assistants (20.5%). Characteristics of study participants are summarized in Table 2.
 | Table 2 Sociodemographic characteristics of Phase 1 participants |
Acute pain situations
Analyses of FG and questionnaire data were consistent and showed a core group of frequent acute pain situations in PwD (Figures 3 and 4). In particular, the sample of health professionals participating in the questionnaire survey identified acute five pain situations: 1) bathing and skin care (93.1%), 2) dressing/change (82.8%), 3) transfer/mobilization (82.8%), 4) wound care and bedsores (55.2%), and 5) blood tests (37.9%).
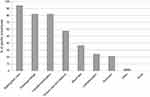 | Figure 3 Percentage of reported painful procedures in persons with dementia according to health professionals. |
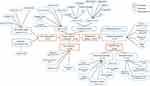 | Figure 4 Concept map from FG of key themes and subthemes related to painful situation in dementia.Abbreviations: FG, focus group; n, number of excerpts from FG participants. |
Eighteen FG excerpts relating to the subtheme “type” of painful situations in dementia were transcribed. The most cited situations were: transfer/mobilization (n=6), nursing care (n=5), hygiene care (n=5) and feeding (n=1). One participant reported no painful care situations in PwD.
Participants’ opinions in both groups coincided on the fact that a patient’s mood and behavior are useful indicators of pain in this context, especially aggressive behaviors (eg, physical violence, insults), opposition, facial expressions (eg, sad face, grimace), vocalizations and body language (eg, tense, rigid, pulling or pushing away). All FG professionals also agreed that behavioral manifestations of pain were more frequent in the morning, because of the amount of care received by the patient, and in the evening, for professionals in three out of four FGs, because of the anxiety caused by sundown syndrome, which tends to exacerbate pain. Results of the FGs revealed that 74% of health professionals used negative adjectives (eg, difficult, hostile, long) to describe the process and course of painful care (Figure 4). These negative perceptions were related to the average execution time of care, conflict management and the need to negotiate with or distract the patient during the care: “We try to draw patients’ attention to something else […] but it’s very difficult with these patients. The moment you lose their attention, it’s over. We cannot do anything” (Nursing auxiliary, 25 years old).
Concerning pain management in dementia, the results showed that management strategies were varied (Figure 4). Among the 20 FG excerpts transcribed, one belonged to pharmacotherapeutic and 19 to nonpharmacological options. All FGs admitted that these strategies had not been effective to date:
We have to execute care procedures very quickly and relieving the pain of patients is not always easy. Sometimes, we try several strategies in vain. It’s discouraging and we often have the impression that we are not doing a good job. (Nurse, 32 years old)
Six participants (31.6%) reported not using any type of nonpharmacological treatment with their patients (Figure 4).
Assessment methodology
The ALGOPLUS scale35 was found to be one of the most frequently used pain instruments in clinical practice with patients suffering from dementia by FG participants (Figure 4), followed by the PAINAD scale36 and the Doloplus® scale for geriatric chronic pain (Figure 4).41 Requiring a brief rating time was considered a key aspect to make the assessment feasible, especially if repetitive pain monitoring is required: “If we have to assess patients’ pain several times, the rating time is very important” (Nurse, 32 years old). These results are in line with analysis of the questionnaire survey. Thus, most of the respondents (93.1%) reported using a pain scale in routine clinical practice with patients. These respondents used the ALGOPLUS scale and fill out items without difficulty.
Regarding the feasibility dimensions of both pain-rating scales, 94.9% (n=37) of health professionals considered understanding the ALGOPLUS statements “rather easy” or “very easy”, versus only 53.8% (n=27) for the PAINAD scale. The same applied to the method of administration: 92.3% (n=36) of respondents considered administration of the ALGOPLUS scale “rather simple” or “very simple”, versus 69.2% (n=27) for the PAINAD scale. Finally, the time required for the ALGOPLUS assessment was judged “rather appropriate” or “very appropriate” by 97.4% (n=37) of professionals, versus 63.1% (n=24) for the PAINAD scale.
A chi-squared analysis demonstrated statistically significant differences in the understanding of the scales’ statements (χ2=9.89, p<0.01), the time necessary for assessment (χ2=14.04, p<0.0001) and the method of administration (χ2=7.52, p<0.001) between the two scales.
Finally, the effective possibility of being able to accurately detect the presence and frequency of painful behaviors was a matter of overall concern in the FGs, this being the main criticism made of the rating scales currently used.
Acceptability, adherence and barriers to PARO-based intervention
FG participants expressed their concern about the introduction of the PARO robot in painful care situations. The lack of time for the introduction of the robot to the patient was one of the most frequent barriers mentioned, as well as the limited human resources in their health care institutions: “I think that it would be necessary to provide the assistance of an additional person in order to introduce and manage the robot […]. We are currently alone to perform care” (Nursing auxiliary, 25 years old). Overall, 35.7% of the transcribed excerpts (n=5) related to these barriers and involved three out of four FGs. Training needs regarding the use of the robot were also mentioned (n=4) in all four FGs: “The lack of training is a problem because we do not know how to use this robot” (Nurse, 22 years old). “Training is important to know how to introduce the robot, use it and anticipate patients’ reactions” (Nurse, 27 years old). Only one professional pointed out concerns in terms of hygiene: “If a patient has a soiled or infected wound, I do not really think that we could use PARO” (Physician, 46 years old).
The PARO robot was also associated with positive opinions and strong expectations of professionals. Nineteen excerpts from the FGs illustrated the usefulness of the PARO robot for the management of pain. Analysis indicated two main perceived interests of the robot-mediated intervention: to create a distraction (n=6) and calm down patients during painful care situations (n=5) (Figure 4). Almost half of the participants in the FGs perceived potential positive repercussions on the patient–carer relationship:
When we become familiar with the use of the robot in painful situations, it is possible that the perception of patients towards nursing staff changes. […] PARO could contribute to ease tensions and establish other relationships with our patients. (Nurse, 34 years old)
Concerning the intention to use the robot, the results revealed that half of the FGs stated that they were ready to use PARO in the future despite the organizational barriers described, if positive impacts were demonstrated by the forthcoming crossover and randomized controlled trial. Analysis of the questionnaire survey showed that among professionals who used the PARO robot, 78.6% considered the possibility of working with it during painful situations. In the same way, 73.3% of PARO users would be ready to adopt the robot in pain management of PwD. These percentages only related to the answers of four types of professionals: nurse, nursing auxiliary, medico-psychological assistant and psychologist. The chi-squared test showed that there was no significant difference in the distribution of intentions to use the robot between the two groups of PARO users (χ2=7.89, p>0.05).
Phase 2: feasibility assessment
Twelve feasibility sessions were organized in four different categories of painful situations: 1) bathing and skin care, 2) dressing/change, 3) transfer/mobilization, and 4) wound care. Each category was tested with three patients with dementia. Feasibility sessions consisted of performing the painful care as usual while assessing the patient’s pain with the ALGOPLUS scale (before and during the care), and using the PARO robot as a distracting stimulus.
Data collection assessments
Results from informal interviews confirmed that health professionals were used to assessing pain with the ALGOPLUS scale. Most filled out the items without difficulty and reported no additional burden. Cases of missing or unusable data were minor (35%), mostly found in the scales completed by professionals with little experience in geriatrics. The chi-squared analysis demonstrated a significant difference in the distribution of missing data between professionals with more than 5 years of experience in geriatric care and those with less than 5 years of experience (χ2=6.28, p<0.05). We estimated that the average time required to complete the scale was 2.3 minutes.
Implementation of the robot-mediated intervention
The implementation of the robot-based intervention was successful in 10 out of 12 painful situations. In one case, the implementation did not succeed during wound care because of a lack of training in the use of the robot. The nurse used PARO only at the beginning of the wound care and then set the robot aside for the rest of the procedure. She explained that she did not feel experienced enough with the robot and that she did not know how to answer the patient’s questions regarding PARO. Another case of implementation failure was observed during a transfer process and was caused by a refusal of the PARO robot by the patient. The reason for refusal given by the patient was a lack of interest in “animals”.
Overall, five professionals (42%) requested the assistance of the researcher during the care. A feeling of clumsiness was reported by health professionals in six out of 12 feasibility situations. This feeling was mainly associated with the newness and innovative nature of the project. However, it is worth noting that professionals had a more positive perception of the usefulness of the PARO robot after feasibility sessions. Good usefulness of PARO was reported by professionals for three situations: 1) facilitating nursing care, 2) distracting the patient during a painful situation, and 3) reducing patients’ aggressive behaviors. Only one nursing auxiliary perceived no usefulness of the robot.
According to the professionals, patients’ adherence to the robot was considered a key aspect in successfully implementing the intervention in daily practice. Observations during feasibility tests showed positive attitudes toward the PARO robot for 92% of patients, regardless of the type of painful situation. Only one refusal was observed. Most of the patients displayed verbal and/or non-verbal behaviors towards the PARO robot (eg, caresses, smiles, kisses, hugs) and also communicated and shared their feelings with the robot.
Patients’ eligibility criteria
Concerning the eligibility criteria of patients, our feasibility study protocol stipulated eligibility for elderly people over 65 years old: 1) those suffering from acute pain, and 2) those diagnosed with dementia according to the criteria of the DSM-V. Eligibility criteria were considered too inclusive and too vague for more than half of the professionals (58.3%). They suggested also restricting recruitment to patients with severe neurocognitive disorders, considered as the most suitable target for such robot-based interventions. Most of the participants also recommended widening inclusion to as many painful situations as possible in order to avoid missed opportunities. Nevertheless, in the present study, it should be noted that enrollment of patients was lowest in the “wound care” group.
Discussion
This study aimed to develop, refine and test the feasibility of a robot-mediated intervention for the management of acute pain situations in dementia.
From a conceptual framework to a feasible robot-based intervention for the management of acute pain in dementia
Our findings showed that acute pain situations in dementia are mostly related to care situations. Thus, our conceptual framework codified a core group of painful situations associated with care, such as bathing, dressing/change, transfer and mobilization. This result fits with the findings of other authors, who highlight similar painful situations in elderly and hospitalized persons.4,33 These data also demonstrate that the procedures designated as the most painful in this population are routine and harmless care (eg, hygiene care), whereas invasive and less repetitive procedures are rated as less painful by health professionals. This study further supports the idea of a lower pain tolerance when the care situation is repeated than for procedures experienced only once, which has been previously addressed in the literature by Coutaux et al.33
In the present study, acute pain situations were considered to be inefficiently managed in the current care practices. There has been no unique, systematic and efficient solution to date, and health professionals stated an urgent need for a common strategy to improve pain management of PwD. Medication is still considered a first-line choice for the management of pain in this population.5 Despite the preponderance of research on pain in literature, relatively few studies have focused on alternative management of pain in PwD.42,43 The most investigated nonpharmacological options for pain management in elderly care are music therapy,44 cold or heat therapy, therapeutic massage,45,46 supportive verbal communication, supportive touch and relaxation.47 However, none of these interventions has been studied in acute pain specifically relating to care procedures. Moreover, based on opinions gathered in this study, most professionals feel frustrated, helpless and sometimes guilty about care-related pain. Their concern to relieve patients' pain is always present during the course of care and during painful procedures, and provides an important internal motivational factor for participating in the future clinical intervention.
To verify the robustness of our intervention framework and also because it is important that clinicians consider the use of this type of technology in dementia, feasibility sessions were conducted. In these feasibility sessions, successful implementation of the robot-mediated intervention represented 83.3% of cases. This means that the selected painful situations are compatible with the use of the PARO robot as a distracting stimulus. Previous clinical studies on the PARO robot have shown its ability to improve communication and social connections,48,49 mood (eg, decreasing agitation, anxiety–depression symptoms)22 and physiological parameters (eg, stress reduction).24,50 On the one hand, PARO’s effect on anxious and agitated patients is attributed to a reduction of cortisol hormone levels51–53 and to the acute calming effect of the robot's tactile stimulation.22,54 A recent study by Robinson et al55 also pointed out a significant reduction in systolic and diastolic blood pressure, suggesting that a companion robot could induce experiences that buffer stress reactions. On the other hand, studies show that inducing a positive mood or diverting attention away from a painful experience decreases pain perception, whereas focusing attention or negative mood tends to increase the feeling of pain.15,56 This close relationship between emotion, attention and pain acts as an incentive to researchers to investigate its effects in patients.
Finally, appropriate and selective eligibility criteria are essential for the feasibility and design of internally valid randomized controlled trials evaluating the efficacy of an intervention.57 However, studies could have limited use to clinicians if the outcomes have poor external validity and the research is not generalizable to the target population for whom the intervention was developed.58 Selecting suitable eligibility criteria is therefore a great challenge. Tickle-Degnen38 suggested combining published standards criteria for clinical trials in the elderly with clinical experience of target team members. In the present study, slightly more than half of the professionals considered our criteria too wide for eligible patients, but too narrow for available experimental situations. For the future crossover and randomized controlled trial, we plan to eliminate patients with minor neurocognitive disorders as measured by the Mini Mental State Examination (MMSE),59 those with severe motor deficits who could not physically interact with the PARO robot and those with negative past experiences with animals, to avoid adverse events during the interaction with PARO, as recommended by our previous work.60 Nevertheless, experimental painful situations will not be restricted to allow future cluster analysis.
A consistent pain assessment methodology for the intervention
The health professionals agreed that the ALGOPLUS scale is a consensual measure of pain in clinical practice with PwD, thanks to its ease of administration and simple-to-understand statements. This French scale was validated among 349 patients and published with 87% sensitivity and 80% specificity for screening and evaluating acute painful conditions.35 Findings from our feasibility study revealed that participants overall had enough time and capacity to complete data collection procedures, except for inexperienced professionals in geriatrics, suggesting that it will be important to give participants sufficient training and education before the beginning of the clinical study. This is in line with the advice of Achterberg et al,43 who specify that an accurate and validated pain assessment tool, supported by better training and support for care staff, is needed to address the current inadequate management of pain in dementia.
Finally, we also outlined similarities between the feasibility study outcomes and opinions of professionals in the framework definition phase: informal indicators of pain used in routine clinical practice match the five items (observational areas) of the ALGOPLUS scale. This result is another factor that could contribute to our population completing the pain assessments with little difficulty, as planned.
Barriers to and facilitators for implementing the intervention
One of our main objectives was to conceive a feasible robot-based intervention for the management of pain so that health professionals could consider the benefits of the use of this type of technology, as well as the potential limitations involved in using a robot.
First, we identified organizational barriers, such as lack of time and human resources, to facilitate the implementation of the PARO-based intervention during painful care situations. This finding fits with other studies which investigated barriers to research implementation in the care of older people. “Lack of time” to implement research in practice or “insufficient time on the job to implement new ideas” was mentioned as an obstacle.61–63 Nursing staff are overworked, “time is limited”, and they are too busy to implement evidence-based procedures into practice.64 This implies that research utilization in clinical practice should address multiple social factors simultaneously. The solution emphasized by Parahoo62 is to promote a context in which health care practitioners recognize the need to improve their care, “seek the knowledge and skills to do so, and feel supported, encouraged and valued”. Also, time spent on introducing the robot must be taken into account in relation to the benefits of the intervention.
Second, professionals showed difficulties in using the robot owing to a lack of sufficient training at all levels (eg, introducing the robot to patients, proposing physical contact with the robot and anticipating patients’ questions). Guidelines regarding the introduction of the PARO robot in this particular context will be a priority for future research. As Bowen et al39 pointed out, guidelines are required in social science interventions to produce a set of relevant and valid outcomes, but also to guarantee the suitability of researchers’ ideas. To address the current lack of training, an implementation guide will be created to allow practitioners to be trained to undertake the robot-based intervention following a predetermined protocol. An implementation guide will also provide a standardized intervention protocol which will enable replication of the methodological procedure.
However, facilitators for adoption of the intervention were also identified in the present study. There was a high participation rate in all phases of the study, which reflects professionals' motivation and interest regarding the intervention. Moreover, most of the professionals reported a diversity of potential useful applications of PARO (eg, distracting the patient from the painful situation, reducing aggressive behaviors) and half of them perceived positive repercussions on the patient–carer relationship. Parahoo62 established a top 10 of research facilitators in the nursing field, among which he mentioned in particular “motivated staff” and “research seen as beneficial to patient care”. Perceived usefulness also influences intention to use a technology, and subsequently predicts its use, as postulated by different technology acceptance models.65
Between 73% and 78% of health professionals in this study reported an intention to use the PARO robot in the future. This trend was motivated by the perceived potential positive impact of PARO on patients’ pain. Professionals who perceived a need for PARO support in the management of pain, specifically nurses, nursing auxiliaries and medico-psychological assistants, seemed more disposed to project themselves using it, as previously observed by Pino et al.66 These authors explain that a pronounced intention to use socially assistive robots in the present or at a future time is related to the need for support services. Also, individuals who believe in the benefits of the technology will tend to accept it.66,67 Thus, the dimension of PARO acceptance will be evaluated in the forthcoming clinical trial, since this factor is known to significantly influence nonpharmacological outcomes.68,69
Limitations
Phase 1
First, our study showed that health professionals were used to assessing pain with the ALGOPLUS scale in routine clinical practice. This result could constitute a bias favoring positive evaluations regarding the feasibility of the assessment tool. However, one of the purposes of Phase 1 of the study was to select a standardized and validated pain scale which was easy to administer and understand by the health care professionals, in order to use it in a future crossover and randomized controlled trial. The fact that professionals used the ALGOPLUS scale in their everyday practice is, rather, a strength. Second, despite its strengths as a research methodology, mixed-methods design presents several limitations. A mixed method allows analysis of a question at both a micro- and a macro-level (ie, the individual and the group, respectively). This means that the researcher uses FGs to collect information regarding a topic and then conducts a survey with a larger group to point out potential similarities or differences in the responses. The present study includes five “mini-FGs”70,71 consisting of three to six participants. Individual interviews could have been conducted to provide more data at a micro-level, since some authors have found that FG participants take the side of the majority or say “what they supposed to say” about a particular topic.72 Fern73 also showed that FG participants produce only 60–70% as many ideas as they would have done in individual interviews, and with a lower quality of ideas.
Phase 2
First, the present feasibility study evaluated a conceptual framework in four categories of painful situations. This limits the generalization of our conclusions about the implementation of the robot in all painful care situations in dementia. Second, our feasibility tests included a small number of patients (n=12). However, the patients were few because Phase 2 of the study was designed to examine and validate the feasibility of an intervention, rather than its clinical impact on patients’ pain. Third, some factors that may affect the course of feasibility tests were not controlled. For instance, the level of professional experience in geriatric care and the level of practice with the PARO robot of health professionals involved in the study should be taken into account. Finally, our study used a small set of measures. Future clinical studies should broaden their scope beyond acute pain measures to look at other clinical outcomes related to pain (eg, anxiety) and health care professionals’ perspectives.
Conclusion
This study finalized a conceptual framework for an intervention using the PARO robot for the management of acute pain in PwD, using a mixed-methods research design, and successfully tested its feasibility. We identified experimental situations as well as important parameters (patients’ eligibility criteria, assessment methodology, professionals’ needs, opinions, perceived obstacles and facilitators) needed to ensure the successful implementation of a future clinical trial.
Acknowledgments
The research reported in this publication was supported by the Paul Bennetot Fondation under the aegis of the Fondation de l’Avenir, and by the Fondation de France.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Merskey H, Bogduk N. Classification of Chronic Pain. Seattle: International Association for the Study of Pain Press; 1994:210.
2. Corbett A, Husebo B, Malcangio M, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. 2012;8(5):264. doi:10.1038/nrneurol.2012.53
3. Pickering G, Jourdan D, Dubray C. Acute versus chronic pain treatment in Alzheimer’s disease. Eur J Pain. 2006;10(4):379–384. doi:10.1016/j.ejpain.2005.06.010
4. Lhuillery D, Cosquéric G. [Douleurs induites par les soins: analyse de l’évolution des données d’une enquête annuelle de prévalence de la douleur]. Douleurs. 2008;9(3):113–117.
5. Belfihadj K. [Prise en soins de la douleur en EHPAD]. Npg. 2018;18(106):218–225. French. doi:10.1016/j.npg.2018.02.003
6. Clot-Faybesse P, Bertin-Hugault F, Blochet C, et al. Analgesic consumption in nursing homes: observational study about 99 nursing homes. Geriatr Psychol Neuropsychiatr Vieil. 2017;15(1):25–34.
7. Corbett A, Husebo BS, Achterberg WP, Aarsland D, Erdal A, Flo E. The importance of pain management in older people with dementia. Br Med Bull. 2014;111(1):139–148.
8. Horgas AL, Tsai P-F. Analgesic drug prescription and use in cognitively impaired nursing home residents. Nurs Res. 1998;47(4):235–242.
9. Guerriero F, Sgarlata C, Maurizi N, et al. Pain management in dementia: so far, not so good. Vascular. 2016;50:75.
10. Caligiuri MP, Peavy G, Galasko DR. Extrapyramidal signs and cognitive abilities in Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16(9):907–911. doi:10.1002/gps.443
11. Van der Steen JT, Sampson EL, Van den Block L, et al. Tools to assess pain or lack of comfort in dementia: a content analysis. J Pain Symptom Manage. 2015;50(5):659–675. doi:10.1016/j.jpainsymman.2015.05.015
12.
13. Quinlan-Colwell A, D‘Arcy YM. Compact Clinical Guide to Geriatric Pain Management: An Evidence-Based Approach for Nurses. New York: Springer Publishing Company; 2011.
14. Park J, Hughes AK. Nonpharmacological approaches to the management of chronic pain in community-dwelling older adults: A review of empirical evidence. J Am Geriatr Soc. 2012;60(3):555–568. doi:10.1111/j.1532-5415.2011.03846.x
15. Villemure C, Bushnell CM. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95(3):195–199. doi:10.1016/S0304-3959(02)00007-6
16. Broekens J, Heerink M, Rosendal H. Assistive social robots in elderly care: a review. Gerontechnology. 2009;8(2):94–103. doi:10.4017/gt.2009.08.02.002.00
17. Shibata T, Wada K. Robot therapy: a new approach for mental healthcare of the elderly–a mini-review. Gerontology. 2011;57(4):378–386. doi:10.1159/000319015
18. Wada K, Shibata T, Asada T, Musha T. Robot therapy for prevention of dementia at home. J Rob Mechatron. 2007;19(6):691. doi:10.20965/jrm.2007.p0691
19. Wada K, Shibata T, Musha T, Kimura S. Robot therapy for elders affected by dementia. IEEE Eng Med Biol Mag. 2008;27(4):53–60. doi:10.1109/MEMB.2008.919496
20. Roger K, Guse L, Mordoch E, Osterreicher A. Social commitment robots and dementia. Can J Aging. 2012;31(1):87–94. doi:10.1017/S0714980811000663
21. De Sant’ Anna M, Morat B, Rigaud AS. [Adaptabilité du robot Paro dans la prise en charge de la maladie d’Alzheimer sévère de patients institutionnalisés]. Npg. 2012;12(67):43–48. French. doi:10.1016/j.npg.2011.10.002
22. Jøranson N, Pedersen I, Rokstad AMM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16(10):867–873. doi:10.1016/j.jamda.2015.05.002
23. Valentí Soler M, Agüera-Ortiz L, Olazarán Rodríguez J, et al. Social robots in advanced dementia. Front Aging Neurosci. 2015;7:133. doi:10.3389/fnagi.2015.00133
24. Petersen S, Houston S, Qin H, Tague C, Studley J. The utilization of robotic pets in dementia care. J Alzheimers Dis. 2017;55(2):569–574. doi:10.3233/JAD-160703
25. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new medical research council guidance. BMJ. 2008;337:a1655. doi:10.1136/bmj.a1655
26. Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10(1):67. doi:10.1186/1471-2288-10-67
27. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi:10.1186/1748-5908-4-50
28. Morse JM. Mixed Method Design: Principles and Procedures. London: Routledge; 2016.
29. Bernardi L, Keim S, Von Der Lippe H. Social influences on fertility: A comparative mixed methods study in Eastern and Western Germany. J Mix Methods Res. 2007;1(1):23–47.
30. Östlund U, Kidd L, Wengström Y, Rowa-Dewar N. Combining qualitative and quantitative research within mixed method research designs: a methodological review. Int J Nurs Stud. 2011;48(3):369–383. doi:10.1016/j.ijnurstu.2011.04.013
31.
32. Hennink MM. International Focus Group Research: A Handbook for the Health and Social Sciences. Cambridge: Cambridge University Press; 2007.
33. Coutaux A, Salomon L, Rosenheim M, et al. Care related pain in hospitalized patients: a cross-sectional study. Eur J Pain. 2008;12(1):3–8. doi:10.1016/j.ejpain.2007.05.002
34. Ambrogi V, Tezenas Du Montcel S, Collin E, Coutaux A, Bourgeois P, Bourdillon F. Care-related pain in hospitalized patients: severity and patient perception of management. Eur J Pain. 2015;19(3):313–321. doi:10.1002/ejp.549
35. Rat P, Jouve E, Pickering G, et al. Validation of an acute pain-behavior scale for older persons with inability to communicate verbally: algoplus. Eur J Pain. 2011;15(2):198.
36. Warden V, Hurley AC, Volicer L. Development and psychometric evaluation of the Pain Assessment in Advanced Dementia (PAINAD) scale. J Am Med Dir Assoc. 2003;4(1):9–15. doi:10.1097/01.JAM.0000043422.31640.F7
37. Moniz-Cook E, Vernooij-Dassen M, Woods R, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health. 2008;12(1):14–29. doi:10.1080/13607860801919850
38. Tickle-Degnen L. Nuts and bolts of conducting feasibility studies. Am J Occup Ther. 2013;67(2):171–176.
39. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–457.
40. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi:10.1191/1478088706qp063oa
41. Lefebvre-Chapiro S. The Doloplus-2 scale: evaluating pain in the elderly. Eur J Palliat Care. 2001;8:191–194.
42. Horgas AL, Elliott AF. Pain assessment and management in persons with dementia. The Nurs Clin North Am. 2004;39(3):593–606.
43. Achterberg WP, Pieper MJ, van Dalen-Kok AH, et al. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471.
44. Gutgsell KJ, Schluchter M, Margevicius S, et al. Music therapy reduces pain in palliative care patients: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):822–831. doi:10.1016/j.jpainsymman.2012.05.008
45. Mok E, Woo CP. The effects of slow-stroke back massage on anxiety and shoulder pain in elderly stroke patients. Complement Ther Nurs Midwifery. 2004;10(4):209–216. doi:10.1016/j.ctnm.2004.05.006
46. Sansone P, Schmitt L. Providing tender touch massage to elderly nursing home residents: a demonstration project. Geriatr Nurs. 2000;21(6):303–308. doi:10.1067/mgn.2000.108261
47. Frampton M. Experience assessment and management of pain in people with dementia. Age Ageing. 2003;32(3):248–251.
48. Pfadenhauer M, Dukat C. Robot caregiver or robot-supported caregiving? Int J Soc Rob. 2015;7(3):393–406.
49. Robinson H, MacDonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14(9):661–667. doi:10.1016/j.jamda.2013.02.007
50. Bemelmans R, Gelderblom GJ, Jonker P, De Witte L. Socially assistive robots in elderly care: a systematic review into effects and effectiveness. J Am Med Dir Assoc. 2012;13(2):114–120. doi:10.1016/j.jamda.2010.10.002
51. Chau D, Osborne TF. Using Technology to Improve Care of Older Adults. New York: Springer Publishing Company; 2017.
52. Wada K, Shibata T. Living with seal robots—its sociopsychological and physiological influences on the elderly at a care house. IEEE Trans Rob. 2007;23(5):972–980. doi:10.1109/TRO.2007.906261
53. Saito T, Shibata T, Wada K, Tanie K. Examination of change of stress reaction by urinary tests of elderly before and after introduction of mental commit robot to an elderly institution.
54. Mordoch E, Osterreicher A, Guse L, Roger K, Thompson G. Use of social commitment robots in the care of elderly people with dementia: a literature review. Maturitas. 2013;74(1):14–20. doi:10.1016/j.maturitas.2012.10.015
55. Robinson H, MacDonald B, Broadbent E. Physiological effects of a companion robot on blood pressure of older people in residential care facility: a pilot study. Australas J Ageing. 2015;34(1):27–32. doi:10.1111/ajag.12099
56. Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J Neurosci. 2009;29(3):705–715. doi:10.1523/JNEUROSCI.3822-08.2009
57. Van Spall HGC, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–1240. doi:10.1001/jama.297.11.1233
58. Guyatt GH, Sinclair J, Cook DJ, Glasziou P. Users’ guides to the medical literature: XVI. How to use a treatment recommendation. Evidence-based medicine working group and the cochrane applicability methods working group. JAMA. 1999;281(19):1836–1843.
59. Folstein MF, Folstein SE, McHugh PR. Mini-mental state ». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
60. Demange M, Lenoir H, Pino M, Cantegreil-Kallen I, Rigaud AS, Cristancho-Lacroix V. Improving well-being in patients with major neurodegenerative disorders: differential efficacy of brief social robot-based intervention for 3 neuropsychiatric profiles. Clin Interv Aging. 2018;13:1303–1311. doi:10.2147/CIA.S152561
61. Fink R, Thompson CJ, Bonnes D. Overcoming barriers and promoting the use of research in practice. J Nurs Adm. 2005;35(3):121–129.
62. Parahoo K. Barriers to, and facilitators of, research utilization among nurses in Northern Ireland. J Adv Nurs. 2000;31(1):89–98.
63. Pettengill MM, Gillies DA, Clark CC. Factors encouraging and discouraging the use of nursing research findings. Image J Nurs Sch. 1994;26(2):143–147.
64. Brown CE, Wickline MA, Ecoff L, Glaser D. Nursing practice, knowledge, attitudes and perceived barriers to evidence-based practice at an academic medical center. J Adv Nurs. 2009;65(2):371–381. doi:10.1111/j.1365-2648.2008.04878.x
65. Venkatesh V, Morris MG, Davis GB, Davis FD. User acceptance of information technology: toward a unified view. MIS Q. 2003;27:425–478. doi:10.2307/30036540
66. Pino M, Boulay M, Jouen F, Rigaud A-S. Are we ready for robots that care for us? » Attitudes and opinions of older adults toward socially assistive robots. Front Aging Neurosci. 2015;7:141. doi:10.3389/fnagi.2015.00141
67. Young JE, Hawkins R, Sharlin E, Igarashi T. Toward acceptable domestic robots: applying insights from social psychology. Int J Soc Rob. 2009;1(1):95. doi:10.1007/s12369-008-0006-y
68. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003.
69. Jin J, Sklar GE, Min Sen Oh V, Chuen Li S. Factors affecting therapeutic compliance: A review from the patient’s perspective. Ther Clin Risk Manag. 2008;4(1):269–286.
70. Kitzinger J. Qualitative research: introducing focus groups. BMJ. 1995;311(7000):299–302.
71. Litosseliti L. Using Focus Groups in Research. London: A&C Black; 2003.
72. Morgan DL. Focus groups. Annu Rev Sociol. 1996;22(1):129–152.
73. Fern EF. The use of focus groups for idea generation: the effects of group size, acquaintanceship, and moderator on response quantity and quality. J Mark Res. 1982;1–13.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
