Back to Journals » International Journal of Women's Health » Volume 15
Management of a Nulliparous IVF Patient with a Declined Ovarian Reserve After Discovery of an Atypical Ovarian Endometriotic Cyst
Authors Ke X, Liang XF, Wang F
Received 21 July 2023
Accepted for publication 26 September 2023
Published 2 October 2023 Volume 2023:15 Pages 1475—1480
DOI https://doi.org/10.2147/IJWH.S431837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Xue Ke, Xue-Fei Liang, Fang Wang
Department of Reproductive Medicine, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
Correspondence: Fang Wang, Chief Department of Reproductive Medicine, Chengdu Women’s and Children’s Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China, Email [email protected]
Purpose: Endometriosis (EM) is a common cause of infertility, and an ovarian endometriotic cyst may affect the ovarian reserve, ovulation, and endometrial receptivity. The majority of EM cases are benign; however, EM may also be prone to malignant transformation, associated with infiltrative growth, and recurrent or distant metastasis. In this study, we report the management of an atypical cyst discovered through ovarian endometriotic cyst puncture prior to controlled ovarian stimulation (COS).
Case Presentation: The patient required in vitro fertilization treatment due to EM and bilateral fallopian tube obstruction. Prior to initiating gonadotropin (Gn) treatment, the right ovarian EM cyst was punctured; cytological pathology of the obtained fluid revealed an atypical morphology. Subsequently, the case was discussed with the patient and ethically reviewed. Gn was initiated according to the patient’s wishes, and six day-3 embryos were finally obtained and cryopreserved. Afterwards, laparoscopic cystectomy of the ovarian endometrioma revealed no malignant transformation. The patient achieved clinical pregnancy after resuscitation and transplantation of the embryo.
Conclusion: In summary, patients with EM-associated infertility are at risk of ovarian cancer formation when undergoing assisted reproduction treatment; therefore, this risk should be evaluated and minimized before initiating such treatment.
Keywords: ovarian cyst, endometriosis, atypical cyst, in vitro fertilization, cyst puncture
Introduction
Endometriosis (EM) is the most common reason female patients request assisted reproduction, as it results in an infertility rate of as high as 30%–50%.1 Ectopic endometrial tissue on the ovary is referred to as an ovarian endometriotic cyst, which is a common type of ovarian cyst. It may affect the ovarian reserve, ovulation, and the endometrial receptivity, resulting in infertility. Before assisted reproduction and surgical treatment for endometriosis are implemented, comprehensive assessment of ovarian cyst size and ovarian reserve should be conducted to determine the appropriate order in which these should be performed.
Cyst puncture is a surgical procedure commonly performed during the assisted reproduction process, whereas cystectomy should be avoided before assisted reproduction, especially for patients with a decreased ovarian reserve.2,3 Cyst puncture preserves ovarian function and facilitates subsequent controlled ovarian stimulation (COS). Ultrasound-guided, transvaginal ovarian cyst puncture is associated with benefits such as little bleeding, low cost, and high patient acceptance; in addition, it reduces the risk of injury to normal ovarian tissues posed by cyst stripping surgery.4,5 At the same time, it is a convenient technique for the ultrasonic counting of antral follicles and monitoring of follicle size, and has little influence on subsequent COS. Moreover, for infertile patients receiving assisted reproduction due to EM, particularly those who reject laparoscopic surgery, it reduces the risk of malignant transformation of EM and improves the clinical pregnancy rate.
After ovarian cyst puncture, fluid from the cyst should be subjected to cytological examination. To our knowledge, the management of a patient in whom an atypical ovarian endometriotic cyst was discovered prior to Gn administration, has not been reported. After approximately 20 years of performing assisted reproduction at our reproductive center, we report our first case of such a discovery upon cyst puncture before initiating COS for in vitro fertilization (IVF) treatment.
Case Presentation
A 33-year-old female (G0P0) had attempted pregnancy for more than two years of regular, unprotected sexual intercourse. Her menstruation was regular, with normal ovulation and an anti-Müllerian hormone concentration of 1.03 ng/mL. Her basic endocrine parameters were as follows: follicle-stimulating hormone (FSH), 10.3 IU/L; luteinizing hormone (LH), 6.5 IU/L; and estradiol (E2), 127 pmol/mL. She had 5–6 antral follicles, experienced moderate dysmenorrhea, and had a CA125 concentration of 23.6 IU/mL. B-mode ultrasound revealed two dense, dot-like echoes of 4.4 × 3.5 and 1.8 × 1.5 cm, respectively, in the right ovary, consistent with chocolate cysts; hysterosalpingography revealed a normal uterus, distal obstruction of the right fallopian tube, and proximal obstruction of the left fallopian tube. The patient had no history of surgery and no known family history of malignancy. Gynecological examination revealed the following: a vulva typical of a nulliparous, sexually active female; smooth vaginal mucosa; no haphalgesic nodules in the posterior fornix; a smooth cervix; an anteverted, normal-sized uterus with fair activity; and in the right adnexa, a cyst the size of a hen’s egg, exhibiting no tenderness. Semen examination of the husband revealed no abnormalities. Karyotype analysis was normal for both the patient and her husband. We diagnosed the patient with primary infertility; an ovarian endometriotic cyst; a pelvic inflammatory disease sequela (bilateral fallopian tube obstruction); and a declined ovarian reserve. Considering her declined ovarian reserve, surgery might have further reduced ovarian function.
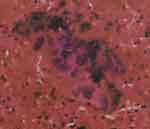
We carefully explained the findings to the patient and her husband, and recommended IVF, to accumulate embryos, followed by surgical treatment, as necessary. As a result, the patient received luteal phase support via long-acting agonist treatment, according to our center’s long protocol for ovarian stimulation. To be specific, we injected the patient with 1.5 mg triptorelin acetate. After 20 days, the down-regulation standard was achieved (E2, 98.2 pmol/mL; FSH, 3.23 IU/L; LH, 0.34 IU/L; progesterone, 0.48 pg/mL), and we proposed a change of regimen to Gn. We conducted a re-examination via ultrasound, which revealed that the cyst in the right ovary was approximately the same size as before. As the cyst interfered with antral follicle counting and would interfere with subsequent follicle monitoring, we recommended that the patient receives transvaginal ovarian cyst puncture. We used a 17G ovum aspiration needle to puncture the right ovarian cyst under transvaginal ultrasound guidance, and approximately 12 mL of a dark-brown, viscous liquid was extracted for cytological examination. We observed a small amount of epithelial cells against a background of abundant red blood cells (Figure 1). Its morphology was atypical (Figure 2).
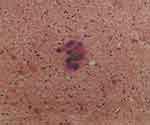 |
Figure 1 Abnormal epithelial cells discovered in the fluid obtained via ovarian endometriotic cyst puncture, against a background of abundant red blood cells. |
After receiving ethical approval and repeatedly communicating with the patient and her husband, they requested us to initiate IVF-assisted reproduction, to accumulate embryos, before surgical treatment of the cyst. Therefore, we initiated the 10-day COS process by injecting the patient with 300 IU human menopausal Gn. We retrieved eight metaphase-II oocytes, eight two-pronuclear oocytes, and six day-3 embryos, the latter of which were immediately cryopreserved. In the following month, we conducted laparoscopic cystectomy of the right ovarian endometriotic cyst. During surgery, we observed that the right ovary had increased in size to approximately 7 × 6 × 4 cm and contained multiple separated cysts, filled with a chocolate-like liquid. No abnormality was observed in the left adnexa. Postoperative histopathological results suggested a right ovarian endometriotic cyst. Three months after the surgery, frozen embryo transplantation (FET) was performed using two embryos, and an artificial FET cycle with down-regulation was adopted to successfully induce clinical pregnancy.
Discussion
The majority of EM cases are benign; however, EM may also be prone to malignant transformation, associated with infiltrative growth, and recurrent or distant metastasis. New recent findings show that the glandular and stromal components of endometriosis originate from different sources. The epithelial components of endometriosis contain cancer-related mutations.6 In a retrospective, observational cohort study conducted in the USA, recruiting 12,193 females evaluated for infertility, 13 infertile patients with EM were also diagnosed with ovarian cancer. Infertile patients with EM had a 2.72-fold increased risk of ovarian cancer in comparison with those without EM.7 Benoit et al8 reviewed 20,686 EM patients that were followed up for 11 years; the relative risk ratio of malignant transformation was 1:18 for EM patients compared to controls. Consequently, when a cyst is diagnosed as atypical through cytological diagnosis after ovarian EM cyst puncture, further histological examination should be performed to determine whether malignant transformation has occurred.
In the present case, cytopathological analysis of the fluid in the ovarian endometriotic cyst revealed a small amount of endothelial cells against a background of abundant red blood cells. Therefore, the possibility of a borderline ovarian tumor or a malignant tumor could not be excluded, and laparoscopic cystectomy was necessary for confirmation. However, this could not immediately be performed, as the patient was nulliparous and had a declined ovarian reserve. Embryo freezing should be proposed for fertility preservation, as a well-established option, because it is recommendable for married women with low ovarian reserve and advanced age.9 For this patient, embryo cryopreservation was the optimal method to yield the highest probability of a successful clinical pregnancy. However, embryo cryopreservation poses several risks for patients with an ovarian EM cyst:
Therefore, along with respecting the patient’s wishes and reproductive counselling in the multidisciplinary management,11 it is of particular importance to assess the possibility of malignancy before embryo cryopreservation. Multiple studies suggest that, for ovarian tumor patients requiring fertility treatment, ovarian tumor size, ultrasonic features of the tumor, and patient symptoms play a vital role in distinguishing benign and malignant tumors. Heidemann et al12 discovered that the risk of malignant transformation of a cyst increased over time. Particularly, EM patients followed up over >10 years had the highest risk of malignant transformation. Kadan et al13 and Thomsen et al14 identified a larger cyst diameter as an independent risk factor of malignant transformation. With regard to our case, the patient had a lower risk of malignant transformation, in terms of cyst age and features. As far as cyst fluid analysis was concerned, the atypical nature of the cyst was not a specific indication of malignancy.15 Normal cells are transformed to tumor cells through progressively acquiring epigenetic alterations and driver mutations, leading to alterations of tissue structure and cell morphology. Atypical EM (aEM) consists of cells that may be benign or malignant, exhibiting pale or hyperchromatic nuclei, and moderate to pronounced pleomorphism; in addition, an elevated ratio of nucleus to cytoplasm, together with crowding and stratification of tall epithelial cells, is observed.16 Nonetheless, the concept of aEM is contentious and has been propounded by numerous authors for various diseases. Atypia is common in endometriotic cysts and is usually accompanied by pigment-rich macrophages, inflammation, increased fibrosis, and a loss of endometrioid stroma; such cysts may be caused by repetitive episodes of hemorrhaging.17 In a review, Varma et al18 stated that aEM was present in 12–35% of ovarian EM cases. Pathologists frequently depict nuclear atypia when they report such cases, although the term “aEM” may not be used. Czernobilsky and Morris15 noted the possibility that aEM is reactive in nature, not necessarily associated with malignancy. Follow-up visits on patients with aEM have revealed no increase in the development of cancer.19–21
Nonetheless, when cytopathology reveals the atypical ovarian endometriotic cyst, patients are at risk of developing endometriosis-associated ovarian cancer (EAOC). Since Sampson first reported a case of EAOC in 1925,22 a large number of studies have revealed that EM consists of similar genetic changes to that of ovarian cancer. Lu et al23 revealed a genetic correlation coefficient of 0.4 for EM and ovarian cancer; EM was most closely correlated with ovarian clear-cell carcinoma. Wang et al24 conducted a cohort study on 5945 EM patients and 23,780 non-EM cases in Taiwan and discovered that the former had an increased risk of ovarian cancer compared with the latter. Therefore, EM-associated malignant transformation has been widely acknowledged, and atypical cysts should not be disregarded.
Before conducting the procedure to confirm EAOC, it is necessary to communicate the risks of COS with patients. Numerous scholars have investigated the mechanisms of EM malignant transformation in terms of tissue pathology, metabolic disturbance, hormone and receptor malfunction, and molecular biology. However, no consensus has been reached, to date. For patients with suspected aEM, or a borderline or malignant ovarian tumor, it remains controversial whether COS is associated with the development of ovarian cancer. Modesitt et al10 reported in 2002 that, among 21 EM malignant transformation patients, 13 had received hormone replacement therapy (primarily with estrogen). However, more recent large-scale studies indicate that medication for infertility is unrelated to ovarian tumor occurrence. Bjørnholt et al25 retrospectively analyzed 96,545 infertile women in Denmark referred to fertility clinics from 1963 to 2006 and discovered that the risk of ovarian cancer was not related to the use of clomiphene citrate or Gn. Diergaarde et al26 reviewed two large case–control studies and also revealed that ovarian stimulation therapy did not apparently increase the risk of ovarian cancer; however, they commented that it may have been too early to evaluate such a relationship, as most of the subjects had just reached the typical age of ovarian cancer development. Consequently, more large-scale studies with long follow-up times are required to accurately evaluate the relationship of assisted reproductive technology with ovarian cancer.
Conclusion
In summary, patients with EM-associated infertility are at risk of ovarian cancer formation when undergoing assisted reproduction treatment; therefore, this risk should be evaluated and minimized before initiating such treatment. In some cases, patients may have a reduced ovarian reserve, increasing their urgency to achieve pregnancy. Where analysis of such a patient reveals a low probability of malignant transformation, the procedure should be:
Abbreviations
EP, ectopic pregnancy; IVF, in vitro fertilization; FET, frozen embryo transfers; REP, repeated ectopic pregnancy.
Ethical Approval
Ethical approval was provided by the medical ethics committee of Chengdu Women’s and Children’s Central Hospital. The patient involved in this case report has been anonymized, and she acknowledges that she cannot be identified via the paper.
Declaration of Patient Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
We would like to express our deep appreciation for the support offered by the pathology department.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflict of interest for this work.
References
1. Macer ML, Taylor HS. Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am. 2012;39(4):535–549. doi:10.1016/j.ogc.2012.10.002
2. Popovic J, Stefanovic M, Trajkovic Dinic SP, et al. Recurrent endometriosis and IVF: is it still an enigma? Clin Exp Obstet Gynecol. 2018;45(3):410–413. doi:10.12891/ceog4173.2018
3. Legendre G, Catala L, Morinière C. Relationship between ovarian cysts and infertility: what surgery and when? Fertil Steril. 2014;101(3):608–614. doi:10.1016/j.fertnstert.2014.01.021
4. Zolnierczyk P, Cendrowski K, Sawicki W. Transfundal puncture of a large ovarian cyst with hysteroscopic and ultrasonographic guidance. Int J Womens Health. 2015;7:527–529. doi:10.2147/IJWH.S82339
5. Kim YJ, Cha SW, Kim HO. Serum anti-Müllerian hormone levels decrease after endometriosis surgery. J Obstet Gynaecol. 2017;37(3):342–346. doi:10.1080/01443615.2016.1239071
6. Habib N, Buzzaccarini G, Centini G, et al. Impact of lifestyle and diet on endometriosis: a fresh look to a busy corner. Prz Menopauzalny. 2022;21(2):124–132. doi:10.5114/pm.2022.116437
7. Brinton LA, Lamb EJ, Moghissi KS, et al. Ovarian cancer risk associated with varying causes of infertility. Fertil Steril. 2004;82(2):405–414. doi:10.1016/j.fertnstert.2004.02.109
8. Benoit L, Arnould L, Cheynel N, et al. Malignant extraovarian endometriosis: a review. Eur J Surg Oncol. 2006;32(1):6–11. doi:10.1016/j.ejso.2005.08.011
9. Zaami S, Stark M, Signore F, et al. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur J Contracept Reprod Health Care. 2022;27(4):335–340. doi:10.1080/13625187.2022.2045936
10. Modesitt SC, Tortolero-Luna G, Robinson JB, et al. Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol. 2002;100(4):788–795. doi:10.1016/s0029-7844(02)02149-x
11. Falzone L, Scandurra G, Lombardo V, et al. A multidisciplinary approach remains the best strategy to improve and strengthen the management of ovarian cancer (Review). Int J Oncol. 2021;59(1):53. doi:10.3892/ijo.2021.5233
12. Heidemann LN, Hartwell D, Heidemann CH, et al. The relation between endometriosis and ovarian cancer—a review. Acta Obstet Gynecol Scand. 2014;93(1):20–31. doi:10.1111/aogs.12255
13. Kadan Y, Fiascone S, McCourt C, et al. Predictive factors for the presence of malignant transformation of pelvic endometriosis. Gynecol Oncol. 2015;185:23–27.
14. Thomsen LH, Schnack TH, Buchardi K, et al. Risk factors of epithelial ovarian carcinomas among women with endometriosis: a systematic review. Acta Obstet Gynecol Scand. 2017;96(6):761–778. doi:10.1111/aogs.13010
15. Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
16. LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19(9):1080–1084. doi:10.1016/S0046-8177(88)80090-X
17. McCluggage WG. Endometriosis-related pathology: a discussion of selected uncommon benign, premalignant and malignant lesions. Histopathology. 2020;76(1):76–92. doi:10.1111/his.13970
18. Varma R, Rollason T, Gupta JK, et al. Endometriosis and the neoplastic process. Reproduction. 2004;127(3):293–304. doi:10.1530/rep.1.00020
19. Seidman JD. Prognostic importance of hyperplasia and atypia in endometriosis. Int J Gynecol Pathol. 1996;15(1):1–9. doi:10.1097/00004347-199601000-00001
20. Fukunaga M, Nomura K, Ishikawa E, et al. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30(3):249–255. doi:10.1046/j.1365-2559.1997.d01-592.x
21. Sainz de la Cuesta R, Eichhorn JH, Rice LW, et al. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol. 1996;60(2):238–244. doi:10.1006/gyno.1996.0032
22. Sampson JA. Endometrial carcinoma of the ovary, arising in the endometrial tissue in that organ. Arch Surg. 1925;10(1):1–72. doi:10.1001/archsurg.1925.01120100007001
23. Lu Y, Cuellar-Partida G, Painter JN, et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum Mol Genet. 2015;24(10):5955–5964. doi:10.1093/hmg/ddv306
24. Wang KC, Chang WH, Lee WL, et al. An increased risk of epithelial ovarian cancer in Taiwanese women with a new surgico-pathological diagnosis of endometriosis. BMC Cancer. 2014;14(1):831. doi:10.1186/1471-2407-14-831
25. Bjørnholt SM, Kjaer SK, Nielsen TSS, et al. Risk for borderline ovarian tumors after exposure to fertility drugs: results of a population-based cohort study. Hum Reprod. 2015;30(1):222–231. doi:10.1093/humrep/deu297
26. Diergaarde B, Kurta ML. Use of fertility drugs and risk of ovarian cancer. Curr Opin Obstet Gynecol. 2014;26(3):125–129. doi:10.1097/GCO.0000000000000060
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.