Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Management and Characteristics of Embolism and Thrombosis After COVID-19 Vaccination: Scoping Review
Authors Phianhasin L , Ruksakulpiwat S , Kruahong S, Kuntajak P, Kelman GB, Benjasirisan C
Received 15 May 2023
Accepted for publication 25 August 2023
Published 20 September 2023 Volume 2023:16 Pages 2745—2772
DOI https://doi.org/10.2147/JMDH.S421291
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lalipat Phianhasin,1,* Suebsarn Ruksakulpiwat,1 Suratsawadee Kruahong,1 Premgamon Kuntajak,1 Glenda B Kelman,2 Chitchanok Benjasirisan1,*
1Faculty of Nursing, Mahidol University, Bangkok, Thailand; 2Department of Nursing, Russell Sage College, Troy, NY, USA
*These authors contributed equally to this work
Correspondence: Chitchanok Benjasirisan, Department of Medical Nursing, Faculty of Nursing, Mahidol University, 2 Prannok Road, Siriraj, Wanglang, Bangkoknoi, Bangkok, 10700, Thailand, Tel +66 98-272-4330, Email [email protected]
Abstract: This scoping review aims to 1) identify characteristics of participants who developed embolism and/or thrombotic event(s) after COVID-19 vaccination and 2) review the management during the new vaccine development of the unexpected event(s). This review was conducted following PRISMA for scoping review guidelines. Peer-reviewed articles were searched for studies involving participants with embolism and/or thrombotic event(s) after COVID-19 vaccination with the management described during the early phase after the approval of vaccines. The 12 studies involving 63 participants were included in this review. The majority of participants’ ages ranged from 22 to 49 years. The embolism and/or thrombotic event(s) often occur within 30 days post-vaccination. Five of the included studies reported the event after receiving viral vector vaccines and suggested a vaccine-induced immune thrombotic thrombocytopenia as a plausible mechanism. Cerebral venous sinus thrombosis was the most frequently reported post-vaccination thrombosis complication. In summary, the most frequently reported characteristics and management from this review were consistent with international guidelines. Future studies are recommended to further investigate the incidence and additional potential complications to warrant the benefit and safety after receiving COVID-19 vaccine and other newly developed vaccines.
Keywords: COVID-19 vaccine, thromboembolism, cerebral venous sinus thrombosis, vaccine-induced immune thrombotic thrombocytopenia
Introduction
COVID-19 is an infectious disease caused by a newly discovered coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The vaccine breakthrough has influenced controlling the pandemic by decreasing the rate of new infections among individuals who have been vaccinated.2 Generally, the development of a safe vaccine takes years of research prior to products used in clinical trials.3 For the COVID-19 vaccine. Several different types of COVID-19 vaccines have been developed with unprecedented speed and with the use of diverse technologies, including inactivated, protein-based, viral vector, and RNA and DNA vaccines.4
Minor side effects, like fever, fatigue, headache, and muscle pain, are normal body responses after COVID-19 vaccination, yet some side effects can cause adverse outcomes.5 Embolism and/or thrombotic event(s) have been reported as possible adverse events associated with some type of COVID-19 vaccination.5 Although the incidence is rare, it can result in life-threatening complications. Correspondingly, because several other vaccines have also reported thrombocytopenia incidents, such as active diphtheria, measles–mumps–rubella, and influenza vaccination, embolism and/or thrombotic event(s) are likely to be adverse effects of future vaccines.6 Consequently, this scoping review aims to identify characteristics of participants who developed embolism and/or thrombotic event(s) after COVID-19 vaccination and review the management of unexpected event(s) during the new vaccine development in order to guide future management and prevent from life-threatening complications.
Methods
The PRISMA for scoping reviews was utilized as a review protocol to guide the review.7 Four electronic databases, including PubMed, MEDLINE, Web of Science, and CINAHL Plus Full-Text databases, were systematically searched from June to July 2021, representing the time of the extensive utilization of COVID-19 vaccines and right before the European Medicines Agency (EMA) announced a possible link to embolism and/or thrombotic event(s).8 Reference lists of the included studies were manually searched to obtain relevant studies. All references identified were stored in EndNote. The detailed search strategies are available in Supplementary Data 1.
Inclusion/Exclusion Criteria
Peer-reviewed articles were included on the incident of embolism and thrombosis after COVID-19 vaccination and its management. In this scoping review, embolism and thrombosis are defined as
A collective term for pathological conditions which are caused by the formation of a blood clot (thrombus) in a blood vessel, or by blocking of a blood vessel with an embolus, undissolved materials in the bloodstream.9
The terms embolism and thrombosis also include thromboembolism, which is an obstruction of a blood vessel by thrombus in the bloodstream. The details of the inclusion and exclusion criteria of the review are provided in Table 1.
 |
Table 1 Study Inclusion and Exclusion Criteria |
First, titles and abstracts were screened independently by the first two authors. Then, the two authors assessed the full text to consider the relevance of the study following the inclusion and exclusion criteria. Finally, any disagreements were resolved using a third author or consensus among the authors.
Assessment of Methodological Quality
The Joanna Briggs Institute (JBI) critical appraisal tools were used as the assessment tools. The tools can assess various types of studies.10,11 Two independent authors performed quality control for the eligible studies. After the eligible studies were finalized, two authors evaluated the methodological quality of each eligible study independently. Any disagreements were solved by consensus or by the decision of a third author.
Data Charting
The summary data (see Table 2) developed for the review included the following data: references, country, study type, COVID-19 vaccine information, sample size, characteristics of the patients, medical history, prescription medication, clinical presentation, embolism and/or thrombotic event(s), coexisting conditions, the potential mechanism of the incidents, and clinical management. The first two authors completed data extraction, and the data was verified by the other authors.
 |
Table 2 Summary Table of Embolism and/or Thrombotic Event(s) After the COVID-19 Vaccination and Its Management |
Data Synthesis
After the eligible studies were assessed by the JBI critical appraisal tools, the data was analyzed using a narrative approach, specifically thematic synthesis. The analysis revealed six themes; 1) Characteristics of participants; 2) Vaccine information; 3) Potential mechanism; 4) Clinical presentation, embolism and/or thrombotic event(s), and coexisting conditions and; 5) Clinical management.
The first theme includes a synthesis of gender, age, medical history, and prescription medication. Since there is no validated or standardized measure for the age range that is suitable to classify the population of COVID-19 vaccine administration’s age, the preferred age range was used. In addition, the ICD10 version 2016 was utilized to describe the diagnosis, signs, and symptoms in the clinical presentation, embolism and/or thrombotic event(s), and coexisting conditions.24
Results
Search Results
Among 191 initially identified articles, 12 studies fulfilled the inclusion criteria and were included in the scoping review (see Figure 1). The description of the included studies is summarized in Table 2.
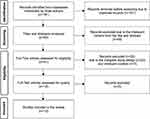 |
Figure 1 The figure was adapted from Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Reprint–Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151(4):264–269.25 The figure displays the selection method of qualified studies and shows the four phases of conducting a scoping review: identification, screening, eligibility, and inclusion. In each phase, the number of records or articles is reported along with the reasons for exclusion. The final phase results in a set of included studies were 12 studies that are relevant to the research question and scope of the review. |
Quality Assessment
The assessment of the risk of bias is presented in Supplementary Data 2.
Findings of the Review
Characteristics of Participants (See Table 3)
 |
Table 3 Characteristics of Participants in the Included Studies |
Gender and Age
Twelve studies identified a total of 63 participants; 46 females (73.02%)12–16,18–20,22 and 17 males (26.98%)15–19,21,23 who experienced embolism and/or thrombotic event(s). The majority of the studies reported participants’ age ranges from 22 to 49 years (n = 9 studies),15–23 followed by 50–65 years (n = 6 studies),12–14,18–20 12–21 years (n = 2 studies),19,20 and older than 65 years (n = 2 studies).19,20
Medical History and Prescription Medication
Medical history and prescription medication were classified as known and unknown thrombosis risk factors based on previous literature.26,27
There were participants with known thrombosis risk factors shown in five studies (10 participants),12,18–20,23 comprising type 2 diabetes mellitus (n = 1 study; 1 participant),12 hypertension (n = 1 study; 1 participant),18 single-vessel coronary artery disease (SVD) (n = 1 study; 1 participant),23 previous deep venous thrombosis (DVT) (n = 1 study; 1 participant),19 and obesity (n = 1 study; 6 participants).20 Other medical histories were cited in seven studies (14 participants).12,14–18,20
Prescription medications known to cause thrombosis were reported as the following; contraceptive tablets or vaginal rings (n = 4 studies; 5 participants)12,18–20 and hormone-replacement treatment (n = 1 study; 1 participant).18 Other prescription medications are recorded in Table 3 (n = 4 studies; 14 participants).12,16–18
Vaccine Information
Among the 12 studies included, two types and three different brands of COVID-19 vaccines were reported being used; BNT162b2, EP2163 mRNA COVID-19 vaccine, or Pfizer (n = 2 studies; 2 participants),12,23 Ad26.COV2.S COVID-19 viral vector vaccine, or Janssen/Johnson & Johnson (J&J) (n = 1 study; 10 participants),20 and Oxford-AstraZeneca ChAdOx1 nCoV-19, Covishield, adenoviral vector vaccine, Vaxzevria, or AstraZeneca (AZ) (n = 9 studies; 49 participants).13–19,21,22 Forty-seven participants of those included in the literature had embolism and/or thrombotic adverse reaction(s) after the first dose of vaccination (n = 9 studies; 47 participants)12,13,15,17–21,23 and four studies (16 participants)14–16,22 did not provide data about the sequence of dosing of the vaccination. Time duration from vaccination to the admission of embolism and/or thrombotic adverse reaction(s) ranged across studies from 0 to 28 days; 0–7 days (n = 7 studies; 11 participants),12,15,17–19,21,22 8–14 days (n = 8 studies; 35 participants),13–20,22 15 to 21 days (n=5 studies; 12 participants),15,19,20,22,23 22–28 days (n = 3 studies; 5 participants),16,19,20 and 29–35 days (n = 1 study; 1 participant).16
Potential Mechanism
Potential mechanisms of embolism and/or thrombotic event(s) after the COVID-19 vaccination are reported in Table 2. The review found that vaccine-induced thrombotic thrombocytopenia (VITT) was reported as the potential mechanism of embolism and/or thrombotic event(s) after the COVID-19 vaccination most frequently (n = 5 studies).12,15,18,19,21 Amidst these studies, one study reported that VITT occurs due to two possible mechanisms.15 First, the adenovirus binds to platelets and causes platelet activation, and free DNA in the vaccine could be a potential trigger of these Platelet Factor 4 (PF4) reactive antibodies. Another study demonstrated that exposure to AZ might trigger the expression of antiplatelet antibodies, resulting in VITT.22 Three studies reported that embolism and/or thrombotic event(s) after the COVID-19 vaccination were conceivably due to the immunological mechanism, autoimmune heparin-induced thrombocytopenia (HIT)-like mechanism in which platelet-activating antibodies develop without heparin exposure is suspected.13,17,20
Other potential mechanisms were demonstrated in the included studies. One study showed that COVID-19 vaccine recipients who have a history of allergy to the immunological response to the mRNA vaccine and the dysregulation of the surface receptor may have triggered or activated thrombosis formation.23 Another study showed that disseminated intravascular coagulation (DIC) is the possible mechanism of embolism and/or thrombotic event(s) that occur with COVID-19 recipients after getting vaccinated.14 One study reported as unclear mechanism.16
Clinical Presentation, Embolism and/or Thrombotic Event(s), Coexisting Conditions
The ICD-10 WHO Version 2016 was utilized to classify as shown in Table 4.
 |
Table 4 Clinical Presentations, Embolism and/or Thrombotic Events, and Coexisting Conditions Classified by ICD10 (Version 2016) |
Clinical Presentation
Not all included studies provided data regarding presenting symptoms of embolism and/or thrombotic event(s). Of the 12 studies with data available, conditions related to diseases of the musculoskeletal system and connective tissue were reported in five studies (8 events),12,16–18,20 and conditions related to diseases of the circulatory system were reported in three studies (4 events).12,16,20 Other findings included conditions related to diseases of eye and adnexa (n = 6 studies; 10 events),13,17,18,20,22 diseases of nervous system (n = 4 studies, 7 events),14,17,18,22 general symptoms and signs (n = 7 studies; 26 events),15,17,18,20–23 and symptoms and signs involving the digestive system and abdomen (n = 4 studies; 9 events).15,18,20,21
Embolism and/or Thrombotic Event(s)
Ten studies reported 49 incidents of cerebrovascular diseases.13–15,17–23 Other embolism and/or thrombotic events were intracardiac thrombosis (n = 2 studies; 2 events),15,19 ischemic heart diseases (n = 2 studies; 2 events),14,19 pulmonary heart disease and diseases of pulmonary circulation (n = 5 studies; 13 events),12,14,15,19,20 and diseases of arteries, arterioles and capillaries (n = 2 studies; 2 events).15,19 Also, diseases of veins, lymphatic vessels and lymph nodes were reported as embolism and/or thrombotic events, and this category included phlebitis and thrombophlebitis (n = 5 studies; 9 events),12,15,16,19,20 portal vein thrombosis (n = 4 studies; 7 events),14,18–20 and other venous embolism and thrombosis (n = 6 studies; 21 events).13–15,18–20
Coexisting Conditions
Among coexisting conditions based on data available, diseases of the circulatory system were most frequently cited (n = 7 studies; 22 events).14,15,17–21 Subsequently, diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism were reported in one study (5 events).19
Clinical Management
Details are summarized in Figure 2.
Laboratory and Imaging Findings
COVID-19 and coagulation tests, including platelet, D-dimer, fibrinogen, INR, aPTT, and heparin-PF4 ELISA test, were assessed in 12 studies,12–23 as demonstrated in Table 5. The data showed that nine studies (59 participants)13–15,17–22 reported thrombocytopenia. Another three studies had a normal level of platelet count.12,16,23 Additionally, ten studies (71.43%)12,14–16,18–23 assessed D-dimer levels, which showed elevated D-dimer levels in eight studies (51 participants)12,14,15,18–22 and normal level in two studies (3 participants).16,23 However, eight studies12,14,15,17–21 that assessed fibrinogen levels reported a variety of ranges from low (n = 6 studies, 31 participants)15,17–21 to normal (n= 5 studies; 16 participants),14,15,18–20 and high (n = 3 studies; 4 participants).12,15,19
 |
Table 5 Laboratory Findings of Included Studies |
INR and aPTT were assessed in six studies.12,15,18–20,23 Seven participants had a normal INR level (3 studies),15,18,23 and one study (5 participants) had a high INR level.15 Among these five studies, the majority of those participants had a normal aPTT (n = 23 participants)12,15,18,19,23 followed by high (n = 2 studies, 11 participants)15,19 and low level of aPTT (n = 1 study; 3 participants).19
To determine the cause of bleeding disorder and embolism and/or thrombotic event(s), the heparin-PF4 ELISA test was tested in seven studies (26 participants), and the results were positive.12,15,17,19–21 For other bleeding disorder tests, IgG and IgM antiplatelet antibodies, platelet suspension immunofluorescence test, monoclonal antibody-specific immobilization of platelet antigens assay, and serotonin release assay (SRA) were positive in the 4 patients (n = 2 studies).13,20 Also, in some studies, the participants were further tested (n = 3 studies; 15 participants)13,20,22 in thrombophilia, immunologic and functional HIT assays, and IgG antibodies against PF4, which showed negative.
In addition, embolism and/or thrombotic event(s) were evaluated with imaging studies, such as positive duplex ultrasonography (n = 1 study; 1 participant)12 and ultrasound (n = 1 study; 2 participants)16 for evidence of DVT. For those who were diagnosed with CVST after vaccination with COVID-19 vaccine, they were tested with magnetic resonance imaging (MRI) (n = 3 studies; 5 participants),14,20,22 computed tomography (CT) (n = 5 studies; 12 participants),14,18,20,21,23 and digital subtraction angiography (DSA) (n = 1 study; 3 participants).22
Medical Treatment
Most participants were clinically managed by anticoagulation treatment and other treatments, including intravenous steroids, blood products, intravenous immunoglobulin (IVIG), thrombolysis procedures, and symptom management. Participants in eight studies (21 participants) received heparin therapy and low molecular weight heparin (LMWH).12,13,15,17,18,20,22,23 Of eight studies, six studies (11 participants) received heparinization therapy and LMWH as an initial treatment. Then, the participants’ treatment was subsequently switched to non-heparin treatment, such as rivaroxaban, phenprocoumon, apixaban, and dabigatran.12,13,15,20,22,23 Some studies (n = 4 studies; 10 participants) were continued on heparin and LMWH from the beginning of treatment.15,17,18,22 Also, some studies (n = 4 studies; 8 participants) reported that participants were initially treated with non-heparinization treatment, such as rivaroxaban, apixaban, dabigatran, and clopidogrel after diagnosis of embolism and/or thrombosis was made.16,20,21,23
In addition to anticoagulation, other treatments were used to manage the patient with embolism and/or thrombotic event(s) after COVID-19 vaccination, indicating in some studies that additional treated with systemic corticosteroids. Moreover, immunoglobulin therapy was used in some participants (n = 4 studies; 13 participants).17,18,20,21
Discussion
VITT or TTS may result in embolism and/or thrombotic event(s) after vaccine administration.5 Several types of vaccines were documented to develop a rare adverse effect of acute thrombocytopenia after vaccinations, such as live-attenuated (MMR and varicella-zoster), recombinant DNA (hepatitis B virus), and inactivated vaccines (influenza).6 In terms of COVID-19 vaccination, it is thought to be due to autoantibodies directed against PF4 that activate platelets and cause venous and arterial thromboembolism in the absence of heparin exposure, similar to other types of spontaneous HIT.28 Despite unclear mechanisms of embolism and/or thrombotic event(s) following COVID-19 vaccination, thrombocytopenia is a condition reported in the majority of included studies.12,13,15,17–22
Approach to the Clients
A previous study regarding the management of thrombocytopenia suggested performing a detailed history taking, including a family history of thrombocytopenia, medical history (recent viral and bacterial infections, vaccinations, malignancies, recent travels, and recent transfusions), and concomitant medications (heparin), in order to find causes and treat appropriately.29 In particular COVID-19 vaccination, there are several aspects to consider VITT.
Age, Gender, and Prescription Medications
Individuals who had thromboembolic events reported following COVID-19 vaccination occurred most frequently in participants aged 22–49, followed by 50–65 years, and least frequently in participants aged 12–21 and 65 years and older.12,13,15,17–22 The scoping review’s findings, similar to current statistics from other studies, indicated that younger individuals (18–55 years old) are more likely to develop thrombosis with thrombocytopenia syndrome (TTS) following COVID-19 adenovirus vector-based (AZ and J&J) vaccinations than older adults (56 years and older).5 Additionally, there are gender-specific risk factors among females, such as oral contraceptive pill usage and pregnancy/postpartum period.30 To our knowledge, estrogen-containing oral contraceptive pills (EOCP) are well known for increasing the risk of VTE. The risk is greater with early usage, particularly within the first 6–12 months.12
Types of Vaccines
Ten of the 12 studies reviewed13–22 reported embolism and/or thrombotic event(s) after viral vector vaccines (AZ and J&J), while the other two included studies on mRNA vaccines (Pfizer).12,23 Vaccine technologies and manufacturing among these potential COVID-19 vaccines are different, suggesting that clot adverse reactions are more prevalent following one type of vaccine than any other type. Among the included studies, viral vector vaccines are the most predominant type of vaccine causing embolism and/or thrombotic event(s).13–22
Sequence of Dose
In all 12 studies reviewed, embolism and/or thrombotic event(s) occurred after the first dose of the COVID-19 vaccination, consistent with other research findings. In contrast, one case report described a worsening symptom of CVST after the second dose of mRNA Pfizer vaccine.23 In this case, the participant presented with mild to moderate headache and giddiness 16 days after the first dose but refused medical treatment due to the participant’s consideration from the exertion of working. The second dose was administered, and the participant reported worsening symptoms in the following two days. The symptoms may either progress or are induced by the second dose.
In addition, as COVID-19 has constantly been evolving, the CDC encouraged to receive a booster dose.31 After the first, second, and booster doses of COVID-19 vaccination, individuals and health-care providers should be aware of and closely observe any adverse reactions. A further investigation comparing an individual who received the first, second, additional, and booster doses should be done.
Suggested Interval for VITT Surveillance
VITT is suspected in individuals who develop thrombocytopenia and/or thrombosis following vaccine administration.32 The suggested interval for VITT surveillance following COVID-19 vaccination recommended by Warkentin and Cuker is 5–30 days post-vaccination.32 As mentioned in previous studies reviewed of the 12, the time interval 5–30 days post-vaccination may be an appropriate surveillance period for VITT for individuals and health-care providers.22,33 In the studies reviewed, the most commonly reported time frame after viral vector vaccination events occurred during 8–14 days. The longest time frame found after viral vector vaccination to admission was 29 days after the first dose of the AZ.16
Among the 12 studies reviewed, individuals with VITT were likely to seek medical assistance when more aggressive symptoms developed, such as sudden onset leg pain, orbital pain, severe headache, visual disturbance, and hemiparesis. For these reasons, recent vaccination status should be assessed.
In summary, monitoring for any adverse reactions up to 30 days post-vaccination may be appropriate. Specifically, embolism and/or thrombotic event(s) may need close observation during initially 8–14 days post-vaccination. Any adverse signs and symptoms need to be reported immediately to health-care providers.
Physical Examination
General physical examination, including inspection, palpation, percussion, and auscultation, was indicated in previous management of thrombocytopenia.29 Moreover, special attention for the physical examination can be performed based on different embolisms and/or thrombotic event(s) resulting from different clinical presentations and coexisting conditions. Although not all the included studies specify clinical presentations and coexisting conditions, some specific incidents can be discussed based on the data available.
CVST was reported in 9 studies (46 events),14,15,17–23 and the reported data shared similar clinical presentations. Headache was the most frequent symptom stated among the reported cases.17–23 In addition, a review of practical guidelines for CVST reported that red flags for headache from CVST included new-onset, persistent, worse with the Valsalva maneuver, and not improved with regular analgesia.34
The time interval from vaccination to the presentation of CVST is also relevant to CVST which is possibly associated with the COVID-19 vaccine. A study about clinical characteristics of CVST with VITT showed that symptoms onset began 5–24 days after the first dose of COVID-19 vaccination and congruent with the review’s findings that the time interval was 2–25 days after vaccination.14,17,18,20–23,35 Coexisting condition also consistent with a previous study demonstrated that approximately one-third of CVST patients experienced parenchymal hemorrhage along with more severe symptoms onset.36
DVT was found in four studies with 8 events reported,12,16,19,20 but this review also notes clinical presentation and coexisting conditions in two included studies (3 events).12,16 General presentations indicated in included studies are leg pain and swelling as revealed in other DVT cases.12,16,37 Furthermore, participants developed signs and symptoms of DVT at day 7, 27, and 29 after the first dose of Pfizer and two unknown sequences of doses of AZ, respectively.12,16 In contrast, an article revealed a participant who experienced DVT shortly after the second dose of the mRNA vaccine.38 For coexisting conditions of DVT, one study reported that a participant developed PE.12
Clinical Management
The previous management of thrombocytopenia indicated that one treatment approach is removing the potential cause of thrombocytopenia, such as discontinuing medication and treating infection.29 This is consistent with the review’s findings that management from included studies did not continue the second dose of COVID-19 vaccination.13,15–19,21,22 In addition, there was evidence of positive COVID-19 cases following 14–27 days after the first dose of the COVID-19 vaccine, which is difficult to determine if they were infected prior to vaccination.39 Therefore, COVID-19 RT-PCR or serology testing should be considered to evaluate the potential exposure from COVID-19 virus that may cause the thrombosis complication.
Laboratory testing is essential for differential diagnosis between isolated thrombocytopenia and pancytopenia, and CBC must be taken. Apart from platelet count that can get from CBC, the review’s findings reported that D-dimer concentration, fibrinogen level, and clotting time are the initial blood tests that have been used to detect abnormal clotting activity.13–22 Additionally, the coagulation blood tests found in the included studies were consistent with the guideline for clinical management of thrombosis with TTS following vaccination to prevent coronavirus disease from WHO.5,12,15,19–21
According to the guidelines established by WHO, the recommendation has been suggested that the individual presenting with the symptoms of TTS occurred within 30 days after the COVID-19 vaccination to be referred to a tertiary-care hospital and be managed by a multidisciplinary team.5 Initial assessment should include a CBC before starting empiric administration of IVIG and anticoagulation. If the patient has thrombocytopenia, such patients are considered a suspect case of VITT; the patients should be further evaluated through D-dimer, fibrinogen, heparin-PF4 ELISA, and imaging for thrombosis. When the proper diagnosis has been made, or while waiting for heparin-PF4 ELISA results, non-heparin therapy and IVIG are recommended. Although there is no evidence to confirm that using heparin in the suspected VITT case will result in an aggravated condition, five included studies have been switched the treatments from heparin to non-heparin therapy after positive results of heparin-PF4 ELISA were detected.12,13,15,20,22 Another critical recommendation from the WHO guideline is to oppose platelet infusion for those who have VITT in all cases except that the patients have severe thrombocytopenia and were required to proceed with emergency surgery.
For patients who have incompatible initial laboratory results with VITT, the American Society of Hematology suggests continuing assessment for VITT and using non-heparin treatment since the patients might be in an early stage of VITT.40 Once VITT has been ruled out, or a plausible alternative diagnosis has been made, the standard treatment of embolism and/or thrombotic event(s) could be consequently given.
The findings from this review are also consistent with other guidelines published during the early phase, such as the guidelines from National Institute for Health Care Excellence and the International Society on Thrombosis and Haemostasis.41,42 As more information became available about VITT, diagnostic guidelines would also improve. However, due to the varied and often subtle presentations of VITT, diagnostic accuracy remain limited. The help from health-care professionals undertaking the research on this topic would be crucial to ensure that the vaccine works safely to increase vaccine confidence and trustworthiness of the healthcare system.
Scoping Review Limitations
Several limitations were identified in this scoping review. This scoping review included only English-language studies, so studies reported in other languages were not included in this scoping review, which may exclude some types of vaccines. For example, Sinopharm and Sinovac (inactivated virus vaccines) were developed by China, and Sputnik V (a viral vector vaccine) was developed by Russia.43 In terms of the reliability of result findings, the majority of included studies are case reports and case series due to limited information in the early stage of the COVID-19 vaccine, so the results of the review should be interpreted with caution. Additionally, the majority of included studies gathered data in Europe, so the results may not be generalizable to other countries in aspects of management and variety of vaccines. Another limitation is the limited time frame only during the early development of the vaccine, which excludes a protein-based COVID-19 vaccine type (Novavax) that develops later.44
Conclusion
The review results suggested monitoring up to 30 days post COVID-19 vaccination, especially the first dose, would be critical to observe any adverse reactions; however, embolism and/or thrombotic event(s) can also occur after that period and requires close monitoring. These suggestions were consistent with WHO guidelines for diagnosis and management of TTS following the COVID-19 vaccination developed and other guidelines at that time.
The timely development of guidelines to manage VITT and other serious side effects from newly developed guidelines is a crucial part for vaccine safety surveillance to ensure that the health-care profession will be able to identify and adequately manage the unexpected events. Meanwhile, the health-care profession is the key person to identify the incidence of side effects and the population at risk to help refine the guideline. Then, vaccine safety surveillance should be continued to ensure that the benefit still outweighs the risk for people receiving the vaccine and help build public trust and protect lives from serious infectious diseases.
Acknowledgments
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Mahidol University provided funding for an open-access publication fee for this review.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213–218. doi:10.1016/j.ajem.2020.09.065
2. Dyer O. Covid-19: US reports low rate of new infections in people already vaccinated. BMJ. 2021;373:n1000. doi:10.1136/bmj.n1000
3. Shahcheraghi SH, Ayatollahi J, Aljabali AA, et al. An overview of vaccine development for COVID-19. Ther Deliv. 2021;12(3):235–244. doi:10.4155/tde-2020-0129
4. World Health Organization. Listings of WHO’s response to COVID-19; March 26, 2023. Available from: https://www.who.int/news/item/29-06-2020-covidtimeline.
5. World Health Organization. Guidance for clinical case management of thrombosis with thrombocytopenia syndrome (TTS) following vaccination to prevent coronavirus disease (COVID-19); March 26, 2023. Available from: https://apps.who.int/iris/bitstream/handle/10665/342999/WHO-2019-nCoV-TTS-2021.1-eng.pdf.
6. Olivieri B, Betterle C, Zanoni G. Vaccinations and autoimmune diseases. Vaccines. 2021;9(8):815. doi:10.3390/vaccines9080815
7. Tricco AC,et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
8. European Medicines Agency. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets; March 26, 2023. Available from: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood.
9. National Center for Biotechnology Information. Embolism and thrombosis; March 26, 2023. Available from: https://www.ncbi.nlm.nih.gov/mesh/68016769.
10. Munn Z, Barker T Hugh, Moola S, et al. Methodological quality of case series studies. JBI Database of Systematic Reviews and Implementation Reports. 2019;18(10):2127–2133. doi:10.11124/JBISRIR-D-19-00099
11. Joanna Briggs Institute. Critical appraisal tools; March 26, 2023. Available from: https://jbi.global/critical-appraisal-tools.
12. Al-Maqbali JS, Al Rasbi S, Kashoub MS, et al. A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Am J Case Rep. 2021;22:e932946. doi:10.12659/AJCR.932946
13. Bayas A, Menacher M, Christ M, Behrens L, Rank A, Naumann M. Bilateral superior ophthalmic vein thrombosis, ischaemic stroke, and immune thrombocytopenia after ChAdOx1 nCoV-19 vaccination. Lancet. 2021;397(10285):e11. doi:10.1016/S0140-6736(21)00872-2
14. D’Agostino V, Caranci F, Negro A, et al. A rare case of cerebral venous thrombosis and disseminated intravascular coagulation temporally associated to the COVID-19 vaccine administration. J Pers Med. 2021;11(4):285. doi:10.3390/jpm11040285
15. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi:10.1056/NEJMoa2104840
16. Haakonsen HB, Nystedt A. Deep vein thrombosis more than two weeks after vaccination against COVID-19. Tidsskr nor Laegeforen. 2021. 141. doi:10.4045/tidsskr.21.0274
17. Mehta PR, Apap Mangion S, Benger M, et al. Cerebral venous sinus thrombosis and thrombocytopenia after COVID-19 vaccination - a report of two UK cases. Brain Behav Immun. 2021;95:514–517. doi:10.1016/j.bbi.2021.04.006
18. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi:10.1056/NEJMoa2104882
19. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi:10.1056/NEJMoa2105385
20. See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. J Am Med Assoc. 2021;325(24):2448–2456. doi:10.1001/jama.2021.7517
21. Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep. 2021;14(6):e243931. doi:10.1136/bcr-2021-243931
22. Wolf ME, Luz B, Niehaus L, Bhogal P, Bazner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. doi:10.3390/jcm10081599
23. Zakaria Z, Sapiai NA, Ghani ARI. Cerebral venous sinus thrombosis 2 weeks after the first dose of mRNA SARS-CoV-2 vaccine. Acta Neurochi. 2021;163(8):2359–2362. doi:10.1007/s00701-021-04860-w
24. World Health Organization. ICD-10 Version: 2016; March 26, 2023. Available from: https://icd.who.int/browse10/2016/en.
25. Moher D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264. doi:10.7326/0003-4819-151-4-200908180-00135
26. Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral contraceptives and HRT Risk of thrombosis. Clin Appl Thromb Hemost. 2018;24(2):217–225. doi:10.1177/1076029616683802
27. Johns Hopkins Medicines. Thrombosis; March 26, 2023. Available from: https://www.hopkinsmedicine.org/health/conditions-and-diseases/thrombosis.
28. Pishko AM, Cuker A. Thrombosis after vaccination with messenger RNA-1273: is this vaccine-induced thrombosis and thrombocytopenia or thrombosis with thrombocytopenia syndrome? Ann Intern Med. 2021;174(10):1468–1469. doi:10.7326/m21-2680
29. Izak M, Bussel JB. Management of thrombocytopenia. F1000Prime Rep. 2014;6:45. doi:10.12703/p6-45
30. Martín-Martos F, Trujillo-Santos J, Del Toro J, et al. Gender differences in patients with venous thromboembolism and five common sites of cancer. Thromb Res. 2017;151(Suppl 1):S16–s20. doi:10.1016/s0049-3848(17)30061-0
31. Centers for Disease Control and Prevention. CDC endorses ACIP’s updated COVID-19 vaccine recommendations; March 26, 2023. Available from: https://www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html.
32. Cuker TEWA COVID-19: vaccine-induced immune thrombotic thrombocytopenia (VITT). Wolters Kluwer; March 26, 2023. Available from: https://www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt.
33. Lee EJ, Cines DB, Gernsheimer T, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi:10.1002/ajh.26132
34. Ulivi L, Squitieri M, Cohen H, Cowley P, Werring DJ. Cerebral venous thrombosis: a practical guide. Pract Neurol. 2020;20(5):356–367. doi:10.1136/practneurol-2019-002415
35. Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke. 2021;52(7):2478–2482. doi:10.1161/strokeaha.121.035564
36. Krajíčková D, Klzo L, Krajina A, Vyšata O, Herzig R, Vališ M. Cerebral venous sinus thrombosis: clinical characteristics and factors influencing clinical outcome. Clin Appl Thromb Hemost. 2016;22(7):665–672. doi:10.1177/1076029615576739
37. Mayo Clinic. Deep vein thrombosis (DVT); March 26, 2023. Available from: https://www.mayoclinic.org/diseases-conditions/deep-vein-thrombosis/symptoms-causes/syc-20352557.
38. Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16(3):803–804. doi:10.1007/s11739-021-02685-0
39. Public Health Ontario. Severe outcomes among confirmed cases of COVID-19 following vaccination in Ontario: December 14, 2020 to February 26, 2023; March 26, 2023. Available from: https://www.publichealthontario.ca/-/media/documents/ncov/epi/covid-19-epi-confirmed-cases-post-vaccination.pdf?la=en.
40. Bussel JB, Connors JM, Cines DB, et al. Vaccine-induced immune thrombotic thrombocytopenia. American Society of Hematology; March 26, 2023. Available from: https://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia.
41. NIf H, Excellence C. COVID-19 rapid guideline: vaccine-induced immune thrombocytopenia and thrombosis (VITT); 2021.
42. Thrombosis ISo, Haemostasis. ISTH interim guidance for the diagnosis and treatment on vaccine‐induced immune thrombotic thrombocytopenia; 2021.
43. World Health Organization. Interim statement of the COVID-19 subcommittee of the WHO Global Advisory Committee on vaccine safety on AstraZeneca COVID-19 vaccine. Saudi Med J. 2021;42(5):581–582.
44. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes emergency use of Novavax COVID-19 vaccine, adjuvanted; March 26, 2023. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-emergency-use-novavax-covid-19-vaccine-adjuvanted.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

