Back to Journals » Infection and Drug Resistance » Volume 15
MALDI-TOF MS-Based Clustering and Antifungal Susceptibility Tests of Talaromyces marneffei Isolates from Fujian and Guangxi (China)
Authors Fang L , Liu M, Huang C, Ma X, Zheng Y, Wu W, Guo J, Huang J, Xu H
Received 28 February 2022
Accepted for publication 16 June 2022
Published 1 July 2022 Volume 2022:15 Pages 3449—3457
DOI https://doi.org/10.2147/IDR.S364439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lili Fang,1– 3,* Minxue Liu,4,* Chunlan Huang,5,* Xiaobo Ma,1– 3 Yanqing Zheng,1– 3 Wenjuan Wu,6 Jian Guo,6,* Jiangshan Huang,1– 3 Heping Xu1– 3
1Department of Laboratory Medicine, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China; 2Xiamen Key Laboratory of Genetic Testing, Xiamen, Fujian, People’s Republic of China; 3School of Public Health, Xiamen University, Xiamen, Fujian, People’s Republic of China; 4The Maternal & Child Health Hospital, The Children’s Hospital, The Obstetrics & Gynecology Hospital of Guangxi Zhuang Autonomous Region, Nanning, Guangxi, People’s Republic of China; 5Department of Clinical Laboratory, Liuzhou People’s Hospital, Liuzhou, Guangxi, People’s Republic of China; 6Department of Laboratory Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Heping Xu; Jiangshan, Hung Department of Laboratory Medicine, The First Affiliated Hospital of Xiamen University, Xiamen, Fujian, People’s Republic of China, Email [email protected]; [email protected]
Introduction: Talaromyces marneffei is a life-threatening pathogen that causes systemic talaromycosis in immunocompromised and acquired immunodeficiency syndrome (AIDS) patients. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) as a tool to cluster T. marneffei isolates is rarely reported and the data on antifungal susceptibility of T. marneffei isolated in the southern region of China, especially in Fujian, is hardly found.
Methods: MALDI-TOF MS was used to cluster 135 T. marneffei isolates, and the minimum inhibitory concentration (MIC) values of amphotericin B, itraconazole, posaconazole, voriconazole, fluconazole, anidulafungin, micafungin, caspofungin and 5-fluorocytosine with Sensititre YeastOne™ YO10 assay were measured during January 2017 to October 2020 in Fujian and Guangxi.
Results: MALDI-TOF MS correctly identified 100% of the T. marneffei isolates. Hierarchical clustering of MALDI-TOF peak profiles identified four different clusters. MICs for itraconazole, posaconazole, voriconazole and amphotericin B were as follows: ≤ 0.015– 0.03 μg/mL, ≤ 0.008– 0.03 μg/mL, ≤ 0.008– 0.06 μg/mL, ≤ 0.12– 1 μg/mL, respectively. MICs for echinocandins and fluconazole were comparatively high.
Conclusion: Since only simple sample preparation is required and since results are available in a short period of time, MALDI-TOF MS can be considered as a method for identification and clustering of T. marneffei. Itraconazole, posaconazole, voriconazole and amphotericin B can be used to treat T. marneffei infected patients due to the low MICs.
Keywords: Talaromyces marneffei, MALDI-TOF, antifungal susceptibility, HIV-infected, non-HIV-infected
Introduction
Talaromyces marneffei, a temperature dimorphic fungus, belongs to division Ascomycota, class Eurotiomycetes, order Eurotiales, family Trichocomaceae, genus Talaromyces. T. marneffei is considered an emerging life-threatening pathogenic fungus that causes systemic talaromycosis in Southeast Asia and the southern region of China.1 T. marneffei infection is not only the third most common secondary disease of AIDS but also an emerging opportunistic mycosis in non-HIV-infected and immunocompromised patients.2,3 T. marneffei infections can cause different clinical symptoms in HIV-infected and non-HIV-infected patients,4 requiring distinct treatments.5,6 Since the disease has a high mortality rate and is associated with relapse, early identification of the fungus and quick diagnosis of disease are essential. Further, improvements in treatment options are especially important for non-HIV-infected patients.7
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) can be used to successfully identify the most clinically relevant fungi8,9 and is an effective method by which to cluster microorganism.10–14
T. marneffei strains may develop resistance during prolonged therapy with the antifungal drugs, as a result of relapse and/or treatment failure. However, as an important guideline for the clinical antifungal drugs use, antifungal susceptibility results of T. marneffei are rarely reported in the southern regions of China as far as we have searched. Such results would be very useful for clinical antifungal treatment of patients. The Sensititre YeastOne™ YO10 assay has been shown to be suitable for antifungal drug susceptibility testing of the yeast phase of T. marneffei by Lei et al.15
Herein, we report results of a T. marneffei study in the southern regions of China with the following objectives: (1) evaluate the application performance of MALDI-TOF MS for identification of T. marneffei isolates and (2) determine T. marneffei minimal inhibitory concentrations (MICs) for amphotericin B, itraconazole, posaconazole, voriconazole, fluconazole, anidulafungin, micafungin, caspofungin, and 5-fluorocytosine.
Materials and Methods
Ethics Statement
This study was approved by the local Ethics Committee of The First Affiliated Hospital of Xiamen University and complied with the Declaration of Helsinki (2008). Written and informed consent was obtained from all participants.
Isolation of T. marneffei Strains
A total of 135 T. marneffei isolates were collected from the infected patients between January 2017 and October 2021 in Fujian and Guangxi, China. Among them, 78 strains were from Guangxi, and 57 strains were from Fujian. All the 135 T.marneffe strains were stored at −80°C, thawed for 24 hours at 20°C, transferred to Sabouraud dextrose agar (SDA) medium, and incubated for 96 hours at 25°C. Multiple colonies were transferred to potato dextrose agar(PDA) medium for cultivation at 35°C and enrichment for later use. All T. marneffei strains were identified by the following criteria: (1) demonstration of thermal dimorphism by showing a conversion from the yeast form at 37°C to the mold form at 25°C, (2) production of a diffusible red pigment from the mold form when cultured at 25°C on SDA, (3) microscopic morphology of mycelia, including the presence of conidiophore-bearing biverticillate penicillin and each penicillus being composed of four or five metulae with smooth walled conidia,16 as well as (4) sequencing of the internal transcribed spacer (ITS).33
DNA Extraction, PCR Amplification and Sequencing
DNA extraction was from 1- to 2-week-old colonies grown on malt extract agar using an Ultraclean™ Microbial DNA isolation Kit (MoBio, Solana Beach, USA) with extracts stored at −20°C. For PCR, a standard thermal cycle was used, which ran 35 cycles and had a 55°C annealing temperature. The ITS region was amplified using the primers pair V9G and LS266. For the DNA-dependent RNA polymerase II, the second largest subunit (RPB2), the primer-pair RPB2-5F and RPB2-7Cr, was used with a step-up PCR that started with 5 cycles and an annealing temperature of 48°C, followed by 5 cycles at 50°C, and 25 cycles at 52°C. β-tubulin (BenA) was amplified with primer pair Bt2a and Bt2b. Primer pair, T10 and Bt2b, was used with a 52°C annealing temperature for Islandici species. Sequencing reactions used the BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, CA) with the same primer sets used for PCR amplification. Sequences were determined with an ABI PRISM 3730xl genetic analyser (Applied Biosystems, California, USA). Sequence contigs were assembled using Seqman Pro v. 9.0.4 (DNAstar Inc.) and newly generated sequences deposited into GenBank. Accession numbers are included in the phylogenetic trees, with accession numbers of ex-type sequences provided in the accepted species list.
Sample Preparation for Matrix-Assisted Laser Desorption/Ionization Time-of-Flight MS
Autof (Autof KIT): One or two isolated colonies were picked with a moistened swab and the fungal material placed in a 1.5 mL micro-centrifuge tube containing 300 μL deionized water, and thoroughly mixed. Next, 900 μL of anhydrous ethanol was added and mixed. The supernatant was completely removed after centrifugation (12,000 rpm for 2 min), tubes were dried 2–5 min until there was no residual ethanol, 10 μL of 70% formic acid was added, and mixed. Next, 10 μL acetonitrile was added, mixed, and the supernatant containing extracted protein was obtained after centrifugation (12,000 rpm for 2 min). One microliter of the protein extract was placed onto a plate, dried at room temperature, and 1 μL α-cyano-4-hydroxycinnamic acid (CHCA; Zybio Inc., Chongqing, China) matrix solution was added. The plate was dried at room temperature and then placed into the instrument for analysis.
MALDI-TOF MS
Mass spectrometry analysis was performed using MALDI-TOF MS EXS3000 (Zybio Inc., Chongqing, China). MS spectra were obtained in linear mode within a range of 2000–20,000 Da. E. coli ATCC 25922 was used for mass calibration and instrument parameter optimization, with an average deviation of molecular weight less than 300 ppm after correction. MS data were analyzed using MDT Master (version 1.1). As specified by the manufacturer’s instructions, log scores ≥2.0 were accepted for the identification at the species level, and log scores <2.0 and ≥1.7 were used for identification at the genus level or the presumptive species level. Log scores below 1.7 were considered unreliable.
Each sample was coated with two targets. High-quality spectra with stable baseline, abundant protein peaks, and even distribution were selected. The iDBac (version 1.1.10) software was used to create the dendrogram based on the main spectrum projection (MSP), using the algorithm of the unweighted pair-group method with arithmetic means (UPGMA).
Cluster analysis was performed by spectra comparison within the database according to the manufacturer’s instructions (Zybio Inc., Chongqing, China). Mass spectrometry analysis was performed with MDT Master to calculate the height and area of spectrum peaks. Welch’s t test was used to determine peaks with statistical differences. Finally, an output file was generated. The spectra of peaks from different types of strains were collected together. Hierarchical clustering of the spectra was performed by applying the ward’s method and cosine distance between all pairs of peak profiles.
Antifungal Susceptibility Testing
The Sensititre YeastOne™ YO10 assay has been shown to be suitable for antifungal testing of the yeast phase of T. marneffei by Lei et al.15 In order to investigate the antifungal susceptibility of T. marneffei strains, the Sensititre YeastOne™ YO10 assay (ThermoFisher Scientific, Cleveland, OH, USA) was used according to manufacturer’s instructions. The Disposable Sensititre panels, which are 96-well plates, contain serial twofold dilutions of dried antifungal agents including amphotericin B, itraconazole, posaconazole, voriconazole, fluconazole, anidulafungin, micafungin, caspofungin and 5-fluorocytosine. MICs were in the range of 0.12–8 μg/mL, 0.015–16 μg/mL, 0.008–8 μg/mL, 0.008–8 μg/mL, 0.12–256 μg/mL, 0.015–8 μg/mL, 0.008–8 μg/mL, 0.008–8 μg/mL, 0.06–64 μg/mL, respectively.
A 0.5 McFarland sterile saline suspension of the sporangiospores was prepared using the spectrophotometric method. Then, 20 µL of the suspension was added into 11 mL YeastOne micro-inoculum broth, with a final concentration of 1.5–8 × 103 CFU/mL. 100 µL of the micro-inoculum broth was dispensed into each well of the dried panels for rehydration. Finally, the panels were enclosed with adhesive seals and incubated for 72 hours at 37°C in a non-CO2 atmosphere (the yeast form of T. marneffei grows better at 37°C than at 35°C). Quality control strains of Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included with each assessment.
MICs for amphotericin B were read as having the lowest drug concentration with no visible growth. But for the other antifungal drugs, the MICs were defined as the lowest concentrations necessary to inhibit 80% of growth compared to the positive control. Antifungal susceptibility testing was performed on three different days, with MIC values reported as the median of the results for the 3 days.
Results
Clinical Data and Specimen Distribution
Of the 135 T. marneffei isolates, 82.22% (111/135) of the strains were isolated from HIV-infected patients and 17.78% (24/135) were from non-HIV-infected patients. Of the 24 T. marneffei isolates from HIV negative patients, 54.2% (13/24) were from Fujian, and the other 45.8% (11/24) were from Guangxi.
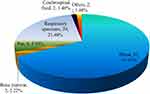
Most T. marneffei strains were isolated from blood, accounting for 67.41% (91/135). Respiratory specimens were the second most prevalent, accounting for 21.48% (29/135), followed by pus, which accounted for 5.93% (8/135) (Figure 1).
MALDI-TOF MS
MALDI-TOF MS correctly identified 100% of the T. marneffei isolates. Hierarchical clustering of MALDI-TOF peak profiles identified four different clusters. Interestingly, all T. marneffei strains, except for strain 5, from HIV-negative patients belonged to cluster II in Fujian, which was different in Guangxi. The strains of non-HIV-infected patients belonged to cluster III and IV from Guangxi (Figure 2).
Antifungal Susceptibility Results
Results for the in vitro antifungal susceptibility of the 135 T. marneffei isolates are listed in Table 1. The MIC range for amphotericin B, itraconazole, posaconazole, voriconazole, fluconazole, anidulafungin, micafungin, caspofungin and 5-fluorocytosine were ≤0.12–1 μg/mL, ≤0.015–0.03 μg/mL, ≤0.008–0.03μg/mL, ≤0.008–0.06 μg/mL, 0.25–16 μg/mL, ≤0.015- ≥8 μg/mL, ≤0.008- ≥8 μg/mL, ≤0.008- ≥8 μg/mL, ≤0.06–4 μg/mL, respectively. No difference in antifungal susceptibility between Fujian and Guangxi was found. There was no difference between the HIV group and non-HIV group (Supplementary Tables 1 and 2).
 |
Table 1 In vitro MIC (μg/ml) of the 135 T. marneffei Strains Against 9 Antifungals as Determined by YeastOne Method |
Amphotericin B was found to have low MIC for all of the isolates with a MIC ≤ 1 μg/mL. MIC50 and MIC90 were 0.25 μg/mL and 0.5 μg/mL, respectively. MICs for triazoles, except fluconazole, were generally in congruence and low. More than 90% of the T. marneffei isolates had MICs ≤ 0.015 μg/mL for itraconazole, posaconazole, and voriconazole. MIC50 and MIC90 for itraconazole were the lowest of the three evaluated azoles (≤0.015 μg/mL). Fluconazole had a reduced antifungal effect for the T. marneffei yeast form (MIC range, 0.25–≥16 µg/mL), with MIC50 and MIC90 of 2 μg/mL and 4 μg/mL, respectively. The susceptibilities results indicated that echinocandins might have lower activity against T. marneffei yeasts, 68.89% (93/135) isolates against anidulafungin with MIC ≥ 4 μg/mL and 70.37% (95/135) strains to micafungin with MIC ≥8 μg/mL. The activity of caspofungin against T. marneffei yeasts was comparatively effective with an MIC50 of 2 μg/mL. 5-fluorocytosine may be active against some T. marneffei yeasts with an MIC50 of 0.25 μg/mL and an MIC90 of 0.5 μg/mL.
Discussion
Bacterial typing is an important method to identify the route of pathogen transmission. For fungal identification, MALDI-TOF MS provides an incomparable advantage.8 Dhieb et al11 reported that MALDI-TOF geographical clustering of C. glabrata was congruent with microsatellite length polymorphism (MLP) genotyping. Usbeck et al12 reported that mass fingerprints of 33 Saccharomyces strains, which are commonly used in wine fermentations, were generated by MALDI-TOF MS once sample preparation and instrument settings were optimized. As a reference method, delta-PCR was chosen to study the genetic diversity of the employed strains. Finally, MALDI-TOF MS, acting at the level of the proteome, provides valuable information about the relationship between yeast strain and biological applications. Theresa Bartosch et al obtained distance dendrograms for MALDI-TOF MS analysis of T.verrucosum and other keratinophilic fungi strains. Results demonstrated the dermatophyte, T. benhamiae, to have the highest similarity to T. verrucosum. Two clusters of T. verrucosum isolates were identified.13
In this study, MALDI-TOF MS correctly identified 100% of the T. marneffei isolates. Hierarchical clustering of MALDI-TOF peak profiles identified four distinct clusters. However, due to small sample size, further studies are needed to confirm this observation.
T. marneffei, was originally identified as Penicillium marneffei. It was renamed as Talaromyces marneffei by R.A. Samson based on gene sequencing analysis in 2011.17 Today, it is an important thermal dimorphic fungus causing systemic mycosis in south-east Asia.1,18,19 Furthermore, T. marneffei infections often have a high mortality rate and are associated with relapse, even though antifungal susceptibility results for T. marneffei are rarely reported.
The Sensititre YeastOne method is usually used to study the antifungal AST in Candida species and has been used for the analysis of other fungi. Hsuan-Chen Wang20,21 reported that the YeastOne method could be used as an alternative for azole susceptibility testing of Aspergillus species and for detection of A. fumigatus TR34/L98H isolates. Jesu’s Guinea22 reported that Sensititre YeastOne was suitable for the analysis of A. fumigatus itraconazole and voriconazole MICs when results were assessed at 48 h of incubation. Fatima Zohra Delma23 reported essential agreement between Sensititre YeastOne and EUCAST/CLSI for amphotericin B, 5-flucytosine, fluconazole, and voriconazole with >89%/>93% for MTS and for EUCAST/CLSI 57%/>75%. Major error rates are low for amphotericin B and fluconazole (<3%) and a bit higher for the other drugs (<8%). The YeastOne™ YO10 assay was shown to be suitable for susceptibility testing of the yeast phase of T. marneffei by Lei et al.15 For dimorphic fungi, antifungal drug susceptibility can differ between mycelial and yeast forms.24,25 The activity of antifungal drugs against the parasitic form of this dimorphic fungus (yeast) was evaluated in this study.26,27 There is no standard method by which to evaluate dimorphic fungi. The Sensititre method performed in the study was modified from M27-A guidelines approved by the National Committee for Clinical Laboratory Standards (NCCLS).28
The data in Table 1 show MIC values without susceptibility categories, whether intermediate or resistant, in that there are no established T. marneffei breakpoints for antifungal agents. Herein, outcomes for antifungal drug sensitivity are in accordance with previous reports.15,29 Among the tested azoles, posaconazole had the lowest MIC50 and MIC90 for T. marneffei yeasts. Results are consistent with previous reports demonstrating the activity of posaconazole against the yeast phase of T. marneffei.30,31 The yeast forms of the T. marneffei isolates were also inhibited by itraconazole and voriconazole with MIC50 and MIC90 ≤ 0.015 μg/mL. These results suggest that amphotericin B had good antifungal activity against the T. marneffei isolates, which is consistent with the report of Liu et al.32 However, toxicity should be considered when using amphotericin B for clinical management of talaromycosis. MICs for fluconazole, 5-fluorocytosine, echinocandins, anidulafungin, micafungin, and caspofungin were high, which suggests that these antifungal agents have little or no activity against the T. marneffei at yeast phase. Overall, an in vitro analysis of antifungal susceptibilities found the antifungal activity of triazoles to be superior to echinocandins and 5-fluorocytosine for the yeast phase of T. marneffei.
There are limitations to this study. First, this is a study with a relatively small study population. Second, a broader range of clinical isolates from multiple settings would be useful. Third, clinical information should be included in future studies. Fourth, there is no standard cluster method by which to compare the results derived herein by MALDI-TOF MS. Each of these limitations should be addressed by future investigations.
Conclusions
Since only simple sample preparation is required and since results are available in a short period of time, MALDI-TOF MS can be considered as a method for identification and clustering of T. marneffei. Itraconazole, posaconazole, voriconazole, and amphotericin B can be used to treat T. marneffei infected patients based on their relatively low MICs in vitro.
Abbreviations
AIDs, acquired immune deficiency syndrome; MALDI-TOF MS, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; T. marneffei, Talaromyces marneffei; MIC, the minimum inhibitory concentration; HIV, human immunodeficiency virus; SDA, Sabouraud dextrose agar; PDA, potato dextrose agar; ITS, the internal transcribed spacer; CHCA, α-cyano-4-hydroxycinnamic acid; MLP, microsatellite length polymorphism; EUCAST, European Committee on Antimicrobial Susceptibility Testing; CLSI, Clinical and Laboratory Standards Institute; NCCLS, the National Committee for Clinical Laboratory Standards.
Ethics Statement
This study was approved by the local Ethics Committee of The First Affiliated Hospital of Xiamen University and complied with the Declaration of Helsinki (2008). Written and informed consent was obtained from all participants.
Acknowledgments
We would like to acknowledge Zybio Inc., Chongqing, China, for technical Support. Lili Fang, Minxue Liu, Chunlan Huang and Jian Guo are co-first authors for this study.
Funding
This work was supported by (1) the Fujian Health Education Joint Research Project [2019-WJ-42], (2) the Fujian Province Natural Science Foundation of China [2020J011233], (3) Guangxi Health Commission Key Lab of Fungi and Mycosis Research and Prevention [ZZH2020004], and (4) the First Affiliated Hospital of Guangxi Medical University Provincial and Ministerial Key Laboratory Cultivation Project: Guangxi Key Laboratory of Tropical Fungi and Mycosis Research [YYZS2020006].
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Vanittanakom N, Cooper CR
2. Peng J, Chen Z, Cai R, et al. Recovery from Talaromyces marneffei involving the kidney in a renal transplant recipient: a case report and literature review. Transpl Infect Dis. 2017;19(4):e12710. doi:10.1111/tid.12710
3. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5:e19. doi:10.1038/emi.2016.18
4. Kawila R, Chaiwarith R, Supparatpinyo K. Clinical and laboratory characteristics of penicilliosis marneffei among patients with and without HIV infection in Northern Thailand: a retrospective study. BMC Infect Dis. 2013;13(1):464. doi:10.1186/1471-2334-13-464
5. Lang Q, Pasheed Chughtai A, Kong WF, Yan HY. Case report: successful treatment of pulmonary Talaromyces marneffei infection with posaconazole in a renal transplant recipient. Am J Trop Med Hyg. 2020;104(2):744–747. doi:10.4269/ajtmh.20-0909
6. Chi XH, Xue YM, Wang QS, Li GP, Zhou HS, Qi YS. Diagnosis and treatment of diffusible Penicillium marneffei in human immunodeficiency virus-negative patients: a challenge for the physician. Indian J Med Microbiol. 2017;35(4):617–619. doi:10.4103/ijmm.IJMM_15_418
7. Lau SK, Lam CS, Ngan AH, et al. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry for rapid identification of mold and yeast cultures of Penicillium marneffei. BMC Microbiol. 2016;16:36. doi:10.1186/s12866-016-0656-0
8. Sun Y, Guo J, Chen R, et al. Multicenter evaluation of three different MALDI-TOF MS systems for identification of clinically relevant filamentous fungi. Med Mycol. 2021;59(1):81–86. doi:10.1093/mmy/myaa037
9. Seyfarth F, Wiegand C, Erhard M, Graser Y, Elsner P, Hipler UC. Identification of yeast isolated from dermatological patients by MALDI-TOF mass spectrometry. Mycoses. 2012;55(3):276–280. doi:10.1111/j.1439-0507.2011.02086.x
10. Fang L, Xu H, Ren X, et al. Epidemiology and risk factors for carbapenem-resistant Klebsiella pneumoniae and subsequent MALDI-TOF MS as a tool to cluster KPC-2-producing Klebsiella pneumoniae, a retrospective study. Front Cell Infect Microbiol. 2020;10:462. doi:10.3389/fcimb.2020.00462
11. Dhieb C, Normand AC, Al-Yasiri M, et al. MALDI-TOF typing highlights geographical and fluconazole resistance clusters in Candida glabrata. Med Mycol. 2015;53(5):462–469. doi:10.1093/mmy/myv013
12. Usbeck JC, Wilde C, Bertrand D, Behr J, Vogel RF. Wine yeast typing by MALDI-TOF MS. Appl Microbiol Biotechnol. 2014;98(8):3737–3752. doi:10.1007/s00253-014-5586-x
13. Bartosch T, Heydel T, Uhrlass S, et al. MALDI-TOF MS analysis of bovine and zoonotic Trichophyton verrucosum isolates reveals a distinct peak and cluster formation of a subgroup with Trichophyton benhamiae. Med Mycol. 2018;56(5):602–609. doi:10.1093/mmy/myx084
14. Yu K, Huang Z, Li Y, et al. Establishment and application of matrix-assisted laser desorption/Ionization time-of-flight mass spectrometry for detection of Shewanella genus. Front Microbiol. 2021;12:625821. doi:10.3389/fmicb.2021.625821
15. Lei HL, Li LH, Chen WS, et al. Susceptibility profile of echinocandins, azoles and amphotericin B against yeast phase of Talaromyces marneffei isolated from HIV-infected patients in Guangdong, China. Eur J Clin Microbiol Infect Dis. 2018;37(6):1099–1102. doi:10.1007/s10096-018-3222-x
16. Wong SS, Ho TY, Ngan AH, Woo PC, Que TL, Yuen KY. Biotyping of Penicillium marneffei reveals concentration-dependent growth inhibition by galactose. J Clin Microbiol. 2001;39(4):1416–1421. doi:10.1128/JCM.39.4.1416-1421.2001
17. Samson RA, Yilmaz N, Houbraken J, et al. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud Mycol. 2011;70(1):159–183. doi:10.3114/sim.2011.70.04
18. Li HR, Cai SX, Chen YS, et al. Comparison of Talaromyces marneffei infection in human immunodeficiency virus-positive and human immunodeficiency virus-negative patients from Fujian, China. Chin Med J. 2016;129(9):1059–1065. doi:10.4103/0366-6999.180520
19. Wang YG, Cheng JM, Ding HB, et al. Study on the clinical features and prognosis of Penicilliosis marneffei without human immunodeficiency virus infection. Mycopathologia. 2018;183(3):551–558. doi:10.1007/s11046-017-0236-3
20. Wang HC, Hsieh MI, Choi PC, Wu CJ. Comparison of the sensititre YeastOne and CLSI M38-A2 microdilution methods in determining the activity of Amphotericin B, Itraconazole, Voriconazole, and Posaconazole against Aspergillus Species. J Clin Microbiol. 2018;56(10). doi:10.1128/JCM.00780-18
21. Wang H-C, Hsieh M-I, Choi P-C, Wu C-J. Comparison of the Sensititre YeastOne and CLSI M38-A2 Microdilution Methods in Determining the Activity of Amphotericin B, Itraconazole, Voriconazole, and Posaconazole against Aspergillus Species. Mycology. 2018;56(10):e00780–18.
22. Guinea J, Pelaez T, Alcala L, Bouza E. Comparison of Sensititre YeastOne with the NCCLS M38-A microdilution method to determine the activity of amphotericin B, voriconazole, and itraconazole against clinical isolates of Aspergillus fumigatus. Diagn Microbiol Infect Dis. 2006;56(1):53–55. doi:10.1016/j.diagmicrobio.2006.03.004
23. Delma FZ, Al-Hatmi AMS, Buil JB, et al. Comparison of MIC test strip and sensititre YeastOne with the CLSI and EUCAST broth microdilution reference methods for in vitro antifungal susceptibility testing of Cryptococcus neoformans. Antimicrob Agents Chemother. 2020;64(4). doi:10.1128/AAC.02261-19
24. Sar B, Boy S, Keo C, et al. In vitro antifungal-drug susceptibilities of mycelial and yeast forms of Penicillium marneffei isolates in Cambodia. J Clin Microbiol. 2006;44(11):4208–4210. doi:10.1128/JCM.00902-06
25. Nakai T, Uno J, Ikeda F, Tawara S, Nishimura K, Miyaji M. In vitro antifungal activity of Micafungin (FK463) against dimorphic fungi: comparison of yeast-like and mycelial forms. Antimicrob Agents Chemother. 2003;47(4):1376–1381. doi:10.1128/AAC.47.4.1376-1381.2003
26. Hohl TM. Overview of vertebrate animal models of fungal infection. J Immunol Methods. 2014;410:100–112. doi:10.1016/j.jim.2014.03.022
27. Boyce KJ, Andrianopoulos A. Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev. 2015;39(6):797–811. doi:10.1093/femsre/fuv035
28. Alexander BD, Procop GW, Dufresne P, et al. M27 reference method for broth dilution antifungal susceptibility testing of yeasts. Clinical and Laboratory Standards Institute; 2017. (M27, 4th ed).
29. Lau SKP, Xing F, Tsang CC, et al. Clinical characteristics, rapid identification, molecular epidemiology and antifungal susceptibilities of Talaromyces marneffei infections in Shenzhen, China. Mycoses. 2019;62(5):450–457. doi:10.1111/myc.12887
30. Lau SK, Lo GC, Lam CS, et al. In vitro activity of posaconazole against talaromyces marneffei by broth microdilution and Etest methods and comparison to Itraconazole, Voriconazole, and Anidulafungin. Antimicrob Agents Chemother. 2017;61(3). doi:10.1128/AAC.01480-16
31. Filiotou A, Giannaris M, Kaloterakis A, et al. First case of Penicillium marneffei fungemia in Greece and strain susceptibility to five licensed systemic antifungal agents and posaconazole. Am J Med Sci. 2006;332(1):43–45. doi:10.1097/00000441-200607000-00009
32. Liu D, Liang L, Chen J. In vitro antifungal drug susceptibilities of Penicillium marneffei from China. J Infect Chemother. 2013;19(4):776–778. doi:10.1007/s10156-012-0511-7
33. Yilmaz N, Visagie CM, Houbraken J, Frisvad JC, Samson RA. Polyphasic taxonomy of the genus Talaromyces. Stud Mycol. 2014;78:175–341.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.