Back to Journals » International Journal of Nanomedicine » Volume 13
Main trends of immune effects triggered by nanomedicines in preclinical studies
Authors Halamoda-Kenzaoui B , Bremer-Hoffmann S
Received 21 March 2018
Accepted for publication 30 May 2018
Published 17 September 2018 Volume 2018:13 Pages 5419—5431
DOI https://doi.org/10.2147/IJN.S168808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Blanka Halamoda-Kenzaoui, Susanne Bremer-Hoffmann
Directorate F-Health, Consumers and Reference Materials, European Commission Joint Research Centre (JRC), Ispra (VA), Italy
Abstract: The application of nanotechnology to emerging medicinal products is a crucial parameter for the implementation of personalized medicine. For example, sophisticated drug delivery systems can target the diseased tissue by recognizing patient-specific biomarkers while carrying pharmacologically active molecules. However, such nanomedicines can be recognized by the immune system as foreign triggering unexpected biological reactions. The anticipation of the immunogenic potential of emerging nanotechnology-based products in the preclinical phase is challenging due to high interspecies variations between the immune systems of laboratory animals and humans. A close monitoring of the scientific literature is required to better understand the relationship between various immune reactions and the diversity of nanomedicines currently in the development pipeline. We have reviewed the most frequent immune reactions induced by the nanomaterials in vivo and have identified the main effects triggered by lipid-based, polymer-based and inorganic nanoparticles, as the main categories of nanomaterials used in medicine. According to our results, almost 50% of the investigated nanomaterials induced effects related to the activation of the immune system. Among them, complement activation-related hypersensitivity reactions and activation of adaptive immune response were the most frequent effects reported for the lipid-based nanoparticles. However, many of these effects are not or are only partially covered by the current regulatory framework applicable for nanomedicines. In addition, we extracted the most relevant nanospecific properties responsible for the observed biological effects. Our analysis led to identification of the most prevalent measurement endpoints relevant for the assessment of the immunotoxic potential of the nanotechnology-based products and will support the smooth and safe translation of the new formulations to clinical applications.
Keywords: immune reactions, nanomaterials, preclinical testing, in vivo, personalized medicine
Introduction
The application of nanotechnology in health care holds promise for the development of innovative medical products addressing, in particular, unmet medical needs. The possibility to design nanomedicines that are able to transport biologically active molecules and target specifically the diseased tissue makes them a promising tool for the implementation of personalized medicines.1 However, innovative materials as well as the use of foreign proteins used for the design of nanomedicines can be potentially immunogenic. In particular, when intravenously (IV) administered, nanomedicines interact immediately with the blood and immune system. The human immune system comprises innate and adaptive immune mechanisms involving a network of interdependent signaling pathways. The innate immune system generates immediate unspecific defense reactions to encounter microbial invasions, whereas the adaptive immune system engenders delayed but more specific immune response involving the activation of lymphocytes and production of specific antibodies.
Nanoparticles (NPs) were shown to interact with both innate and adaptive immune mechanisms.2 However, the mechanisms and biological consequences of this interaction are not well understood yet. Once in the blood system, NPs interact with plasma proteins which bind to NP surface, forming so-called protein corona (PC).3,4 This process can in a significant way impact the interaction of NPs with the components of the immune system, for example, binding of the immunoglobulins to NP surface facilitates their recognition and uptake by the phagocytic cells in the process called opsonization.5,6
In the area of drug delivery, the combined effect of both nanocarrier and encapsulated drug has to be considered. In several cases, the NP shell protecting the active pharmaceutical ingredient may reduce the toxic effect of the drug on the immune system;7 in other cases, it can act in synergy with it, for example, stimulating the immune response against the tumor.8 However, NP-induced effect can also lead to unwanted immune reactions impacting the safety of nanoformulations. In addition, the immune system is very species specific, and interindividual diversity of the human immune system9 makes preclinical safety assessments of nanomedicines challenging. Particularly, knowledge of which physicochemical properties of NPs can influence a biological effect is needed for the design of safe pharmaceutical products based on nanotechnology.
We have reviewed the most frequent immune reactions induced by the nanomaterials in vivo and identified the main effects triggered by lipid-based, polymer-based and inorganic NPs. In addition, we have extracted the most relevant nanospecific properties responsible for the observed biological effect. Our analysis led to identification of the most prevalent endpoints relevant for the assessment of the immunotoxic potential of the nanotechnology-based products. A detailed knowledge on the interaction of the NPs with the immune system will support the translation of emerging nanomedicines to clinical applications.
Methodological approach
Search management
The present review has to be seen as narrative and not as a systematic review. As the first step, we performed the literature search by using Scopus, Web of Science and PubMed search engines, the most commonly known search engines in the biomedical field. All studies investigating the effects in vivo of all nanomaterials potentially relevant for the nanomedicine field were considered.
The initial search was carried out using the prefix “nano*” AND “immunotoxicity” OR “immune response” OR “hypersensitivity” AND “in vivo” keywords and excluding “vaccine” keyword. The additional search was done using “nano*” AND “complement activation” AND “in vivo” keywords. Other additional searches were done using “liposome” OR “graphene” AND “immunotoxicity” AND “in vivo” keywords.
In the second step, the results were manually curated. Double hits and entries that were out of scope were removed. Reviews, book chapters and conference proceedings were also not considered. Reported effects resulting from the nanoformulations containing active pharmaceutical ingredients were only taken into account when the study indicated the impact of the nanocarrier on the observed biological effect. The results stating absence of significant immune response of the investigated formulations were classified separately. The search was performed from December 2016 till March 2017, and the last update was performed at the end of June 2017.
Quality of research studies
An important aspect to evaluate the quality of the studies reporting an immune effect was the description of the physicochemical characterization of the investigated nanomaterials. Ninety-two percent of the publications provided the physicochemical characterization of the investigated nanomaterial or referred to the previously published physicochemical characterization. Moreover, 59% of them performed additionally the in vitro studies to complete the information obtained from in vivo experiments.
The ability of NPs to interfere with the immune assays, as well as their contamination by the endotoxin should be evaluated before the experiments, since it can highly influence the assay results as well as the reliability of data in independent runs due to batch-to-batch variances. However, reports on the reliability of the measurements as well as information on interference with the test reagents were rarely provided. Therefore, this parameter can be considered as the confounding factor of the performed analysis.
Overview of the results
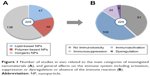
In our search, we considered all categories of nanomaterials potentially used for medical applications. According to the classification of nanomedicines proposed by Wicki et al,10 we grouped the various NPs into lipid-based NPs, polymer-based NPs and inorganic NPs. Among the 229 original research articles investigating the effect on the immune system, 138 (60%) were related to inorganic NPs, 47 (20%) to lipid-based NPs and 46 (20%) to polymer-based NPs (Figure 1A). The majority of these reports (~60%) demonstrated an interaction with the immune system either in terms of the stimulation (47%), suppression (10%) or dysregulation (2.5%) covering the ambiguous effects on the immune system (Figure 1B). In 40% of the studies (91 articles), no significant effect on the immune system was found. Inorganic NPs were responsible for 68% of all described adverse effects on the immune system (not shown).
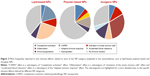
We also extracted the most frequent immune effects related to each category of medical NPs (Figure 2). The majority (61%) of reports related to lipid-based NPs demonstrated an effect on the immune system, including mainly activation of the complement system, complement activation-related pseudoallergy (CARPA) and activation of the adaptive immune response (in particular, poly(ethylene glycol) [PEG]ylated liposomes). Around 65% of tested polymer-based NPs did not show any significant immunotoxicity. The most frequent reaction induced by polymer-based NPs was antigenicity accompanied by the production of specific antibodies and leading to accelerated blood clearance (ABC) of the nanoformulation. Seventy percent of the investigated inorganic NPs induced an adverse effect on the immune system, demonstrating in particular an increased risk of inflammation associated with either innate or adaptive immune response.
Activation of the immune system
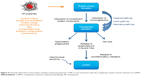
Activation of the immune system was the most frequently observed immune reaction after the administration of nanomaterials in animal studies, independent of the category of the NPs. Stimulation of the immune response can be beneficial to the organism since it supports the recognition and elimination of foreign materials and the defense against bacterial and viral infection. For example, gold nanorods were shown to inhibit respiratory syncytial virus and were able to stimulate antiviral response in mice.11 Currently, a number of studies exploit the immunostimulating properties of NPs used as anticancer therapies12–14 and as vaccine vehicles.15 In this review, though, we focused on unintended immune reactions triggered by NPs as they are not always detected on due time. Uncontrolled and excessive immunostimulation can lead to autoimmune disorders, or alternatively can induce inflammation and damage the tissues even a long time after the exposure. Among the 108 publications reporting on the activation of immune responses, 43 demonstrated the ongoing inflammatory process with the release of proinflammatory cytokines and inflammatory changes in organs and tissues (Figure 3). Oxidative stress generation, interaction with toll-like receptors, responsible for pathogen recognition, and activation of inflammatory pathways such as cellular nuclear factor-κB were pointed out as main mechanisms of NP-induced inflammation.16–19 The inflammatory reactions were almost exclusively induced by inorganic NPs, mainly carbon-based or metal-based NPs, in particular, titanium dioxide NPs, gold NPs,20,21 silica NPs22 and graphene oxide–based nanomaterials.23,24
An important component of the innate immune defense consists of the macrophages covering heterogeneous population of phagocytic cells. Their function is to recognize and engulf foreign bodies including pathogens and cell debris.25 Activation of innate immune cells including the macrophages, NP uptake, generation of oxidative stress and subsequent release of cytokines by the phagocytic cells were among the most reported effects in vitro and in vivo (Figure 3). Upon activation, the macrophages can polarize and acquire proinflammatory M1 phenotype, engendering the response to pathogens or M2 phenotype linked to anti-inflammatory effect and tissue repair mechanisms. The exposure of M2-polarized macrophages to superparamagnetic iron oxide nanoparticles seemed to alter their profile toward M1 phenotype and promote the induction of proinflammatory transcripts.26 Ma et al27 demonstrated that larger graphene oxide displayed a stronger adsorption onto the macrophage plasma membrane and elicited more robust interaction with toll-like receptors and more potent activation of nuclear factor-κB pathway, promoting greater M1 polarization. On the other hand, depending on the surface functionalization, gold nanorods were shown to induce either M1 or M2 macrophage polarization and promote or not inflammatory injury to the liver.28 These findings indicate that by tuning the surface properties, it is possible to reverse the adverse effect of NPs in order to achieve the expected biological profile.
Complement activation
The system of complement is an important part of the innate immunity, designated as first-line defense against pathogenic infections.29 It can be activated via antibodies binding to the antigen (classical pathway), mannose binding lectin pathway or alternative pathway initiated by direct binding of the pathogen to the complement protein. Even if each pathway is triggered differently, they converge at the step where the third complement protein (C3) is cleaved into anaphylatoxin C3a and opsonic component C3b. The cascade of different reactions generates three major effects: 1) proinflammatory process with the release of anaphylatoxins, 2) opsonization of pathogen, which is subsequently eliminated by the phagocytic cells and 3) membrane attack complex leading directly to lysis of the targeted pathogen cell. All three pathways classical, lectin and alternative pathway, can be activated in contact with NPs,30–33 leading to the inflammatory process34 accumulation in the liver and spleen macrophages35 or hypersensitivity reactions (Figure 4). Furthermore, particle recognition by the reactive intravascular macrophages can additionally enhance the release of anaphylatoxin and the proinflammatory reactions as recently confirmed by Wibroe et al.36 The resulting CARPA is a frequent side effect of IV administered liposomal and micelle drugs already on the market or in the development stage (Figure 3).33,37,38 Occasionally, it can lead to life-threatening conditions including pulmonary edema, cardiovascular stress, hypoxia and other hypersensitivity symptoms.
Lipid-based NPs with the functionalized surface were the main category of NPs reported to induce CARPA effect in vivo. However, an appropriate animal model must be used to observe hypersensitivity reactions.39,40 More frequently, the in vitro and in vivo methods for the blood markers of complement activation were employed to assess the effect of investigated NPs. Though it has to be noted that in most cases, a strong complement activation leads to hypersensitivity reactions, interindividual variations in human complement activation responses exist.41 Apart from the lipid-based formulations, polymeric and inorganic NPs also, such as iron oxide NPs and gadolinium conjugates, were reported to activate the complement system.30,31,42,43
However, surface-related properties that can have an influence on the complement system activation are more relevant than the category of a nanomaterial, since they determine the PC composition and its subsequent interaction with other plasma proteins including immunoglobulins and components of the complement system44 (Figure 4). For this reason, the chemical structure of functionalization groups, type and density of the coating, surface charge, hydrophobicity, conditions of cross-linking and even the effect of the encapsulated drug were investigated for their role in the activation of complement system.30,31,43,45,46 Nevertheless, the exact molecular mechanism initiating this interaction still remains to be elucidated.
Activation of the adaptive immune response
The capacity to increase the adaptive immune response via activation of T-cells and B-cells and the subsequent production of specific antibodies are usually associated with biotechnology-derived products. Therapeutic proteins can be recognized as foreign and are presented to T-cells, leading to activation of the cascade of cell-mediated and humoral responses. Upon activation and depending on the type of encountered pathogen, T-cells proliferate and differentiate into T helper 1 cells (Th1), T helper 2 cells (Th2), Th17 or regulatory T lymphocytes. Evaluation of Th1 to Th2 balance following interaction with an investigated nanomaterial can provide insights on the proinflammatory potential and the subsequent immune pathway. Th1 cells play a major role in the cellular immunity and are associated with acute inflammation; Th1 cytokines can induce macrophage polarization toward M1 phenotype. Such Th1-polarized inflammatory response was induced in mice by intratracheal administration of iron oxide NPs, leading to pulmonary inflammation.47,48 Th2 cells promote humoral response, but are also related to allergy disorders, for example, silica NPs were reported to induce allergen-specific Th2-type allergic immune responses in a mouse model.49 In addition, several medical NPs including polymeric micelles and dextran-coated iron oxide NPs were able to stimulate the adaptive immune response inducing production of corresponding immunoglobulins.50–52 A particularly interesting observation is related to PEG coating frequently used in nanomedicine to provide better stabilization and a lower recognition by the immune system in order to increase the drug circulation time.53–55 Interestingly, PEGylated liposomes, iron oxide NPs and gold NPs were reported to lose their long circulating properties when they were administered repeatedly to the same animal. This unexpected phenomenon called “accelerated blood clearance” (ABC) occurred due to the production of specific anti-PEG IgM antibodies labeling the NPs, followed by the increased uptake of NPs by the macrophages and their accumulation in the liver and spleen (Figure 5).56–60 Several attempts were made to find an alternative NP coating which could keep stealth properties of PEG without inducing the ABC phenomenon and compromising the efficacy of drug.61,62
Immunosuppression
Suppressive effect on innate and acquired immune responses can lead to less-effective responses of the organism to pathogen infection. Such effect was observed for 10% of investigated nanomaterials. A decreased production of the specific antibodies in response to antigen, reduced activity of natural killer cells and slower proliferation of lymphocytes have been demonstrated in vitro and in vivo for inorganic NPs, mainly fullerene derivatives63–66 and several metallic NPs.67–71 The diminution of the host immunity against Listeria monocytogenes infection was noted in mice administered with liposome-encapsulated hemoglobin, but this issue has been overcome by the steric stabilization of the formulation and adequate changes in lipid composition.72,73
An important aspect in the assessment of the drug delivery systems is the influence of the encapsulated drug. Depending on the liposome composition and other physicochemical properties of the formulation, the myelosuppressive effect of the encapsulated doxorubicin was shown to be attenuated74 or, on the contrary, amplified, when cumulated with the immunotoxic effect of the carrier.75 Therefore, a simultaneous safety testing of the NP formulation with and without the encapsulated drug could provide more accurate information.
Influence of the physicochemical parameters
A big part of the ongoing research effort concentrates on determining the key physicochemical properties of NPs that can induce or modulate a biological effect. This information has a fundamental importance for drug developers since it allows designing of efficacious and safe therapeutics displaying a reduced risk of immune response, unless such response is needed (immunostimulatory agents, vaccine vehicles etc). Correlation of the physicochemical characteristics with the biological effect is also the first step for determining the so-called critical quality attributes, which are formally designated parameters of manufactured pharmaceutical products enabling evaluation of their quality.76 Among the selected scientific articles, 64% tried to identify such key physicochemical properties of NPs responsible for the observed immune reaction. Surface-related properties such as coating, surface functionalization and charge were among the most critical parameters as reported by the researchers (Figure 6). Indeed, coating of NPs with the hydrophilic polymer not only ensures better steric stabilization, but also has a direct impact on the opsonization process and subsequent interaction with the immune cells. Increased PEG density on the surface of lipid NPs reduced their hemolytic and immunostimulating activity.77 But, on the other hand, the second administration of PEGylated formulations to the same animal resulted in the production of anti-PEG antibodies and the ABC (Figure 5). In addition, the size, the chemical structure and hydrophobicity were also recognized as important properties in many studies. Smaller NPs were, in general, found to have higher immunotoxic potential than the bigger ones,52,78–80 whereas hydrophobicity influenced activation of the complement system81 and cytokine secretion by the splenocytes.82
Finally, it has to be noted that in the biological environment, physical and chemical properties of NPs, such as size, shape or surface properties, can change following NP agglomeration or dissolution. Likewise, the NP PC composition also can undergo dynamic changes influencing subsequent interaction with the immune components.32,83 In addition, some studies seem to indicate that the conformational changes of the proteins involved in the formation of the PC are the key events initiating immunogenic reactions.84,85
Testing of the nanomedicine immunotoxicity
Given the increasing number of nanotechnology-based products in development stage, and the risk of interspecies variations limiting utility of animal studies, cost- and time-effective, humanized in vitro screening methods are needed to detect possible immune-related toxicities of nanomedicines. The evaluation of the state of the macrophages involved in the first-line interaction with NPs and mediating specific and nonspecific immune responses has been suggested as valuable testing approach for predicting the immunotoxic potential of NPs.25,86,87 Macrophage viability, generation of reactive oxygen and nitrogen species, as well as uptake of NPs were among the most tested endpoints studied in vitro (Figure 7B).
  | Figure 7 Most frequently studied endpoints for the in vivo (A) and in vitro/ex vivo (B) assessment of the immunotoxic potential of nanomaterials. |
Another function of phagocytic cells, that is, the release of cytokines/chemokines, leading to inflammation and activation of the immune system, was intensively studied in vivo and in vitro (Figure 7A and B).88 The amount of secreted cytokines analyzed in relevant in vitro models can reflect the in vivo situation and particularly predict severe immune reactions such as cytokine storm.89 However, certain types of NPs can adsorb measured cytokines on their surface, reducing their content in the supernatant and generating false-negative results of the assays.90 Evaluation of the cytokine expression at the protein level or at the gene level (Figure 7B), usually less subject to interferences, can provide a valuable option. The gene expression of the range of cytokines was used by Moyano et al82 to establish a link between NP hydrophobicity and activation of the immune system.
Measurement of the complement activation is particularly important in case of IV administered nanomedicines due to its implications to several other immune effects such as enhanced uptake by the macrophages, hypersensitivity reactions and inflammatory process (Figure 4). Several methods for testing of the complement activation in vivo and in vitro in human blood are available or in the phase of development.91,92 The major issue related to in vivo models is their high interspecies variation and the difficulty to find an appropriate animal model sufficiently predictive for the hypersensitivity reactions in humans.40 Due to their availability and cost-effectiveness, in vitro methods for assessing complement activation are more and more used to design and predict biocompatibility and the circulation time of developed nanoformulations for IV use.31,81,93
The binding of the specific plasma proteins to NP surface (opsonization) is the first reaction of the organism to IV administered NPs and contributes to their recognition and uptake by the immune cells. Detection of the immunogenic potential of NPs at this early step would be optimal to avoid subsequent immunotoxic reactions such as complement activation, activation of macrophages and lymphocytes, proinflammatory reactions etc. Some attempts have been already made to correlate the PC “fingerprint” of gold NPs with the subsequent internalization by the cells.94 However, much more information needs to be gathered before we are able to properly link the composition of NP PC with the specific reactions of the immune system.
Finally, independent of the investigated endpoint, the reliability and suitability of the method should be evaluated. Due to their specific properties, NPs often interfere with the immune assays. They can adsorb cytokines on their surface, reducing their amounts in the supernatant, or interfere with the absorbance and fluorescence measurements.90,95 Another issue is the contamination by the endotoxin, which activates the immune response leading to false-positive result.96,97 A thorough testing of the eventual sample contamination, appropriate controls and the use of several complementary assays should improve the quality of results and help with their interpretation.
Considerations for regulatory evaluations
Currently, nanotechnology-based medicinal products aiming to enter into clinical application have to follow the regulatory path of medicinal products. Relevant guidelines for their evaluation on quality, efficacy and safety have been published by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The potential of these innovative products to interfere with the immune system is assessed with the information based on the preclinical in vivo standard toxicity studies. Immunogenicity assessments are required for biotechnology-derived pharmaceuticals.98 For other nanotechnology-based products not involving biotechnology, changes in relevant parameters including hematology, globulin level in serum, immune organ weight and histology and incidences of infections or tumors can trigger additional immunotoxicity studies.99 However, even though this strategy is functioning for the majority of xenobiotics, it is uncertain whether more subtle NP-induced immune disorders can be detected on time.
The additional studies recommended in the ICH S8 guideline include T-cell–dependent antibody response, immunophenotyping, natural killer cell activity, host resistance studies, macrophage/neutrophil function and cell-mediated immunity performed in vivo, in vitro or ex vivo. However, as such, they cover only partially the immune effects frequently induced by nanomaterials, such as complement activation, inflammation or cytokine release (Table 1). Contrary to medicinal products, complement activation is routinely tested for medical devices.
  | Table 1 Most relevant immune effects reported in the in vivo studies and corresponding regulatory guideline/guidance documents |
Besides the low predictivity of animal models, technical challenges such as the nanospecific optical and chemical properties that may interfere with the results of many conventional assays have to be taken into account. Therefore, many regulators are requesting that validated state-of-the-art in vitro methods containing specific nanosize-related precautions should be developed to assess the immunotoxic profile of NPs for medical use.100,101 The reflection papers issued by the European Medicines Agency in 2013 related to information needs for IV liposomal products,102 iron-based products103 and block copolymer micelle products104 recommend additional immunotoxicity studies including complement activation and macrophage activation, antigenicity and hypersensitivity reactions using validated in vitro and in vivo methods in sensitive models.
Up to now, there is only one standardized method for the evaluation of immunological response to nanoparticulate materials, developed by the American Society for Testing and Materials (ASTM).105 Another ASTM method refers to assessment in vitro of the whole complement activation by solid materials used in medical devices.106 However, it does not address medicinal products or products based on nanotechnology. Complement activation, secretion of proinflammatory cytokines and leukocyte proliferation are part of the assay cascade provided by the European Nanomedicine Characterization Laboratory107 for the preclinical characterization of nanomaterials intended for medical use. More efforts are needed to develop and standardize test methods for assessment of the immunological response to nanotechnology-based pharmaceuticals.
Conclusion
Nanotechnology-based health products are essential for the implementation of personalized medicine. However, many of these products are triggering interaction with the immune system, leading to a compromised safety and efficacy. The activation of the immune system, including the activation of macrophages and the release of cytokines, is the most frequent effect of nanomaterials observed in vivo. Surface-related properties of the investigated nanomaterials were identified as the most critical parameter influencing the observed immune effect. Complement activation and related hypersensitivity reactions as well as antigenicity leading to the production of the specific antibodies and ABC were the main adverse effects described after the administration of lipid-based and polymer-based nanomaterials, the most relevant platforms currently used for the development of nanomedicines. Due to interspecies variations, the relevance of the animal studies used for this purpose remains limited. In consequence, relevant, suitable in vitro testing methods based on humanized cells should be developed for nanotechnology-based medicinal products.
Acknowledgment
The authors would like to acknowledge Dr Marina Dobrovolskaia from Nanotechnology Characterization Laboratory for providing critical comments of the manuscript.
Author contributions
BHK performed the literature search, analyzed the results and drafted the manuscript. SBH conceived the study, helped in data interpretation and revised the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
Any opinions expressed in this publication are those of the authors only, and this paper does not represent an official position of the European Commission. The authors report no conflicts of interest in this work.
References
Herrmann IK, Rösslein M. Personalized medicine: the enabling role of nanotechnology. Nanomedicine. 2016;11(1):1–3. | ||
Kononenko V, Narat M, Drobne D. Nanoparticle interaction with the immune system. Arh Hig Rada Toksikol. 2015;66(2):97–108. | ||
Cedervall T, Lynch I, Foy M, et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl. 2007;46(30):5754–5756. | ||
Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105(38):14265–14270. | ||
Neagu M, Piperigkou Z, Karamanou K, et al. Protein bio-corona: critical issue in immune nanotoxicology. Arch Toxicol. 2017;91(3):1031–1048. | ||
Engin AB, Nikitovic D, Neagu M, et al. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: the cell and immune system. Part Fibre Toxicol. 2017;14(1):22. | ||
Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16(24):6139–6149. | ||
Koshy ST, Mooney DJ. Biomaterials for enhancing anti-cancer immunity. Curr Opin Biotechnol. 2016;40:1–8. | ||
Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. 2017;17(1):21–29. | ||
Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. | ||
Bawage SS, Tiwari PM, Singh A, et al. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomedicine. 2016;12(8):2299–2310. | ||
Zheng H, Wen S, Zhang Y, Sun Z. Organosilane and polyethylene glycol functionalized magnetic mesoporous silica nanoparticles as carriers for CpG immunotherapy in vitro and in vivo. PLoS One. 2015;10(10):e0140265. | ||
Campbell DF, Saenz R, Bharati IS, et al. Enhanced anti-tumor immune responses and delay of tumor development in human epidermal growth factor receptor 2 mice immunized with an immunostimulatory peptide in poly(D,L-lactic-co-glycolic) acid nanoparticles. Breast Cancer Res. 2015;17(1):48. | ||
Faghfuri E, Yazdi MH, Mahdavi M, et al. Dose–response relationship study of selenium nanoparticles as an immunostimulatory agent in cancer-bearing mice. Arch Med Res. 2015;46(1):31–37. | ||
Zaman M, Good MF, Toth I. Nanovaccines and their mode of action. Methods. 2013;60(3):226–231. | ||
Wang X, Tian J, Yong K-T, et al. Immunotoxicity assessment of CdSe/ZnS quantum dots in macrophages, lymphocytes and BALB/c mice. J Nanobiotechnology. 2016;14(1):10. | ||
Mishra V, Baranwal V, Mishra RK, Sharma S, Paul B, Pandey AC. Titanium dioxide nanoparticles augment allergic airway inflammation and Socs3 expression via NF-κB pathway in murine model of asthma. Biomaterials. 2016;92:90–102. | ||
Hutter E, Boridy S, Labrecque S, et al. Microglial response to gold nanoparticles. ACS Nano. 2010;4(5):2595–2606. | ||
Pinsino A, Russo R, Bonaventura R, Brunelli A, Marcomini A, Matranga V. Titanium dioxide nanoparticles stimulate sea urchin immune cell phagocytic activity involving TLR/p38 MAPK-mediated signalling pathway. Sci Rep. 2015;5(1):14492. | ||
Ng C-T, Li JJ, Balasubramanian SK, You F, Yung L-YL, Bay B-H. Inflammatory changes in lung tissues associated with altered inflammation-related microRNA expression after intravenous administration of gold nanoparticles in vivo. ACS Biomater Sci Eng. 2016;2(11):1959–1967. | ||
Zhang X-D, Wu D, Shen X, Liu P-X, Fan F-Y, Fan S-J. Invivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33(18):4628–4638. | ||
Rabolli V, Badissi AA, Devosse R, et al. The alarmin IL-1α is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 2014;11(1):69. | ||
Cho YC, Pak PJ, Joo YH, Lee H-S, Chung N. In vitro and in vivo comparison of the immunotoxicity of single- and multi-layered graphene oxides with or without pluronic F-127. Sci Rep. 2016;6(1):38884. | ||
Erf GF, Falcon DM, Sullivan KS, Bourdo SE. T lymphocytes dominate local leukocyte infiltration in response to intradermal injection of functionalized graphene-based nanomaterial. J Appl Toxicol. 2017;37(11):1317–1324. | ||
Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today. 2015;10(4):487–510. | ||
Rojas JM, Sanz-Ortega L, Mulens-Arias V, Gutiérrez L, Pérez-Yagüe S, Barber DF. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine. 2016;12(4):1127–1138. | ||
Ma J, Liu R, Wang X, et al. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano. 2015;9(10):10498–10515. | ||
Bartneck M, Ritz T, Keul HA, et al. Peptide-functionalized gold nanorods increase liver injury in hepatitis. ACS Nano. 2012;6(10):8767–8777. | ||
Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33(6):479–492. | ||
Pham CTN, Mitchell LM, Huang JL, et al. Variable antibody-dependent activation of complement by functionalized phospholipid nanoparticle surfaces. J Biol Chem. 2011;286(1):123–130. | ||
Wang G, Griffin JI, Inturi S, et al. In vitro and in vivo differences in murine third complement component (C3) opsonization and macrophage/leukocyte responses to antibody-functionalized iron oxide nanoworms. Front Immunol. 2017;8(2):151. | ||
Chen F, Wang G, Griffin JI, et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12(4):387–393. | ||
van den Hoven JM, Nemes R, Metselaar JM, et al. Complement activation by PEGylated liposomes containing prednisolone. Eur J Pharm Sci. 2013;49(2):265–271. | ||
Meng J, Ji Y, Liu J, et al. Using gold nanorods core/silver shell nanostructures as model material to probe biodistribution and toxic effects of silver nanoparticles in mice. Nanotoxicology. 2014;8(6):686–696. | ||
Hamad I, Christy Hunter A, Rutt KJ, Liu Z, Dai H, Moein Moghimi S. Complement activation by PEGylated single-walled carbon nanotubes is independent of C1q and alternative pathway turnover. Mol Immunol. 2008;45(14):3797–3803. | ||
Wibroe PP, Anselmo AC, Nilsson PH, et al. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat Nanotechnol. 2017;12(6):589–594. | ||
Szebeni J. Hemocompatibility testing for nanomedicines and biologicals: predictive assays for complement mediated infusion reactions. Eur J Nanomed. 2012;1(1):33–53. | ||
Szebeni J, Storm G. Complement activation as a bioequivalence issue relevant to the development of generic liposomes and other nanoparticulate drugs. Biochem Biophys Res Commun. 2015;468(3):490–497. | ||
Jackman JA, Mészáros T, Fülöp T, Urbanics R, Szebeni J, Cho N-J. Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: foundation of a validatable immune toxicity model. Nanomedicine. 2016;12(4):933–943. | ||
Dézsi L, Fülöp T, Mészáros T, et al. Features of complement activation-related pseudoallergy to liposomes with different surface charge and PEGylation: comparison of the porcine and rat responses. J Control Release. 2014;195:2–10. | ||
Benasutti H, Wang G, Vu VP, et al. Variability of complement response toward preclinical and clinical nanocarriers in the general population. Bioconjug Chem. 2017;28(11):2747–2755. | ||
Wang K, Pan D, Schmieder AH, et al. Synergy between surface and core entrapped metals in a mixed manganese–gadolinium nanocolloid affords safer MR imaging of sparse biomarkers. Nanomedicine. 2015;11(3):601–609. | ||
Oommen OP, Duehrkop C, Nilsson B, Hilborn J, Varghese OP. Multifunctional hyaluronic acid and chondroitin sulfate nanoparticles: impact of glycosaminoglycan presentation on receptor mediated cellular uptake and immune activation. ACS Appl Mater Interfaces. 2016;8(32):20614–20624. | ||
Moghimi SM, Simberg D. Complement activation turnover on surfaces of nanoparticles. Nano Today. 2017;15:8–10. | ||
Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63(12):1000–1007. | ||
Coty J-B, Eleamen Oliveira E, Vauthier C. Tuning complement activation and pathway through controlled molecular architecture of dextran chains in nanoparticle corona. Int J Pharm. 2017;532(2):769–778. | ||
Park E-J, Oh SY, Lee SJ, et al. Chronic pulmonary accumulation of iron oxide nanoparticles induced Th1-type immune response stimulating the function of antigen-presenting cells. Environ Res. 2015;143:138–147. | ||
Park E-J, Kim H, Kim Y, Yi J, Choi K, Park K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology. 2010;275(1–3):65–71. | ||
Yoshida T, Yoshioka Y, Fujimura M, et al. Promotion of allergic immune responses by intranasally-administrated nanosilica particles in mice. Nanoscale Res Lett. 2011;6(1):195. | ||
Hara E, Makino A, Kurihara K, Yamamoto F, Ozeki E, Kimura S. Pharmacokinetic change of nanoparticulate formulation “Lactosome” on multiple administrations. Int Immunopharmacol. 2012;14(3):261–266. | ||
Wang G, Inturi S, Serkova NJ, et al. High-relaxivity superparamagnetic iron oxide nanoworms with decreased immune recognition and long-circulating properties. ACS Nano. 2014;8(12):12437–12449. | ||
Liao L, Zhang M, Liu H, et al. Subchronic toxicity and immunotoxicity of MeO-PEG-poly(D,L-lactic-co-glycolic acid)-PEG-OMe triblock copolymer nanoparticles delivered intravenously into rats. Nanotechnology. 2014;25(24):245705. | ||
Gaucher G, Asahina K, Wang J, Leroux J-C. Effect of poly(N-vinyl-pyrrolidone)-block-poly(D, L-lactide) as coating agent on the opsonization, phagocytosis, and pharmacokinetics of biodegradable nanoparticles. Biomacromolecules. 2009;10(2):408–416. | ||
Shan X, Yuan Y, Liu C, Tao X, Sheng Y, Xu F. Influence of PEG chain on the complement activation suppression and longevity in vivo prolongation of the PCL biomedical nanoparticles. Biomed Microdevices. 2009;11(6):1187–1194. | ||
Zhang T, Tang M, Zhang S, et al. Systemic and immunotoxicity of pristine and PEGylated multi-walled carbon nanotubes in an intravenous 28 days repeated dose toxicity study. Int J Nanomedicine. 2017;12:1539–1554. | ||
Yang Q, Lai SK. Anti-PEG immunity: emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–677. | ||
Yang Q, Ma Y, Zhao Y, et al. Accelerated drug release and clearance of PEGylated epirubicin liposomes following repeated injections: a new challenge for sequential low-dose chemotherapy. Int J Nanomedicine. 2013;8:1257–1268. | ||
Ichihara M, Moriyoshi N, Lila ASA, Ishida T, Kiwada H. Anti-PEG IgM Production via a PEGylated Nano-Carrier System for Nucleic Acid Delivery. Methods in Molecular Biology (Clifton, N.J.) 2013;948:35–47. | ||
Judge A, Mcclintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13(2):328–337. | ||
Wang C, Cheng X, Sui Y, et al. A noticeable phenomenon: thiol terminal PEG enhances the immunogenicity of PEGylated emulsions injected intravenously or subcutaneously into rats. Eur J Pharm Biopharm. 2013;85(3):744–751. | ||
Zhang Q, Deng C, Fu Y, Sun X, Gong T, Zhang Z. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol Pharm. 2016;13(6):1800–1808. | ||
Abu Lila AS, Uehara Y, Ishida T, Kiwada H. Application of polyglycerol coating to plasmid DNA lipoplex for the evasion of the accelerated blood clearance phenomenon in nucleic acid delivery. J Pharm Sci. 2014;103(2):557–566. | ||
Hirai T, Yoshioka Y, Udaka A, et al. Potential suppressive effects of two C60 fullerene derivatives on acquired immunity. Nanoscale Res Lett. 2016;11(1):449. | ||
Jovanović B, Anastasova L, Rowe EW, Palić D. Hydroxylated fullerenes inhibit neutrophil function in fathead minnow (Pimephales promelas Rafinesque, 1820). Aquat Toxicol. 2011;101(2):474–482. | ||
Ryan JJ, Bateman HR, Stover A, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–672. | ||
Hamilton RF, Buford MC, Wood MB, Arnone B, Morandi M, Holian A. Engineered carbon nanoparticles alter macrophage immune function and initiate airway hyper-responsiveness in the BALB/c mouse model. Nanotoxicology. 2007;1(2):104–117. | ||
Johnson BM, Fraietta JA, Gracias DT, et al. Acute exposure to ZnO nanoparticles induces autophagic immune cell death. Nanotoxicology. 2015;9(6):737–748. | ||
Kim CS, Nguyen HD, Ignacio RM, et al. Immunotoxicity of zinc oxide nanoparticles with different size and electrostatic charge. Int J Nanomedicine. 2014;9 Suppl 2:195–205. | ||
Moon E-Y, Yi G-H, Kang J-S, Lim J-S, Kim H-M, Pyo S. An increase in mouse tumor growth by an in vivo immunomodulating effect of titanium dioxide nanoparticles. J Immunotoxicol. 2011;8(1):56–67. | ||
de Jong WH, van der Ven LTM, Sleijffers A, et al. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials. 2013;34(33):8333–8343. | ||
Shen CC, Wang CC, Liao MH, Jan TR. A single exposure to iron oxide nanoparticles attenuates antigen-specific antibody production and T-cell reactivity in ovalbumin-sensitized BALB/c mice. Int J Nanomedicine. 2011;6:1229–1235. | ||
Sherwood RL, Mccormick DL, Zheng S, Beissinger RL. Influence of steric stabilization of liposome-encapsulated hemoglobin on Listeria monocytogenes host defense. Artif Cells Blood Substit Immobil Biotechnol. 1995;23(6):665–679. | ||
Zheng S, Beissinger R, Sherwood RL, Mccormick DL, Lasic DD, Martin FJ. Liposome-encapsulated hemoglobin: a red blood cell substitute. J Liposome Res. 1993;3(3):575–588. | ||
Bally MB, Nayar R, Masin D, Cullis PR, Mayer LD. Studies on the myelosuppressive activity of doxorubicin entrapped in liposomes. Cancer Chemother Pharmacol. 1990;27(1):13–19. | ||
Gibaud S, Andreux JP, Weingarten C, Renard M, Couvreur P. Increased bone marrow toxicity of doxorubicin bound to nanoparticles. Eur J Cancer. 1994;30(6):820–826. | ||
Bastogne T. Quality-by-design of nanopharmaceuticals – a state of the art. Nanomedicine. 2017;13(7):2151–2157. | ||
Kumar V, Qin J, Jiang Y, et al. Shielding of lipid nanoparticles for siRNA delivery: impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids. 2014;3:e210. | ||
Kim JH, Kim CS, Ignacio RM, et al. Immunotoxicity of silicon dioxide nanoparticles with different sizes and electrostatic charge. Int J Nanomedicine. 2014;9 Suppl 2:183. | ||
Hu Z, Song B, Xu L, et al. Aqueous synthesized quantum dots interfere with the NF-κB pathway and confer anti-tumor, anti-viral and anti-inflammatory effects. Biomaterials. 2016;108:187–196. | ||
Hirai T, Yoshikawa T, Nabeshi H, et al. Size-dependent immune-modulating effect of amorphous nanosilica particles. Pharmazie. 2011;66(9):727–728. | ||
D’Addio SM, Saad W, Ansell SM, et al. Effects of block copolymer properties on nanocarrier protection from in vivo clearance. J Control Release. 2012;162(1):208–217. | ||
Moyano DF, Goldsmith M, Solfiell DJ, et al. Nanoparticle hydrophobicity dictates immune response. J Am Chem Soc. 2012;134(9):3965–3967. | ||
Lee YK, Choi EJ, Webster TJ, Kim SH, Khang D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int J Nanomedicine. 2015;10:97–113. | ||
Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8(7):543–557. | ||
Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnology. 2013;11(1):26. | ||
Fröhlich E. Value of phagocyte function screening for immunotoxicity of nanoparticles in vivo. Int J Nanomedicine. 2015;10:3761–3778. | ||
Li Y, Italiani P, Casals E, et al. Assessing the immunosafety of engineered nanoparticles with a novel in vitro model based on human primary monocytes. ACS Appl Mater Interfaces. 2016;8(42):28437–28447. | ||
Elsabahy M, Wooley KL. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem Soc Rev. 2013;42(12):5552. | ||
Dobrovolskaia MA, Mcneil SE. Understanding the correlation between in vitro and in vivo immunotoxicity tests for nanomedicines. J Control Release. 2013;172(2):456–466. | ||
Guadagnini R, Halamoda Kenzaoui B, Cartwright L, et al. Toxicity screenings of nanomaterials: challenges due to interference with assay processes and components of classic in vitro tests. Nanotoxicology. 2015;9 Suppl 1:13–24. | ||
Pham CTN, Thomas DG, Beiser J, et al. Application of a hemolysis assay for analysis of complement activation by perfluorocarbon nanoparticles. Nanomedicine. 2014;10(3):651–660. | ||
Meerasa A, G Huang J, Gu FX. CH(50): A revisited hemolytic complement consumption assay for evaluation of nanoparticles and blood plasma protein interaction. Curr Drug Deliv. 2011;8(3):290–298. | ||
Quaglia F, Ostacolo L, de Rosa G, et al. Nanoscopic core–shell drug carriers made of amphiphilic triblock and star-diblock copolymers. Int J Pharm. 2006;324(1):56–66. | ||
Walkey CD, Olsen JB, Song F, et al. Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano. 2014;8(3):2439–2455. | ||
Oostingh GJ, Casals E, Italiani P, et al. Problems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effects. Part Fibre Toxicol. 2011;8(1):8. | ||
Dobrovolskaia MA. Pre-clinical immunotoxicity studies of nanotechnology-formulated drugs: challenges, considerations and strategy. J Control Release. 2015;220:571–583. | ||
Crist RM, Grossman JH, Patri AK, et al. Common pitfalls in nanotechnology: lessons learned from NCI’s Nanotechnology Characterization Laboratory. Integr Biol (Camb). 2013;5(1):66–73. | ||
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Preclinical safety evaluation of biotechnology-derived pharmaceuticals S6(R1). 2011. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S6_R1/Step4/S6_R1_Guideline.pdf. Accessed August 27, 2018. | ||
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. Immunotoxicity studies for human pharmaceuticals S8; 2005. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S8/Step4/S8_Guideline.pdf. Accessed August 27, 2018. | ||
Global summit on regulatory science: nanotechnology standards and applications. Final report. 2016. | ||
Bremer-Hoffmann S, Halamoda-Kenzaoui B, Borgos SE. Identification of regulatory needs for nanomedicines. J Interdiscip Nanomed. 2018;3(1):4–15. | ||
European Medicines Agency. Reflection paper on the data requirements for intravenous liposomal products developed with reference to an innovator liposomal product. London: European Medicines agency; 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/03/WC500140351.pdf. Accessed August 27, 2018. | ||
European Medicines Agency. Reflection paper on the data requirements for intravenous iron-based nano-colloidal products developed with reference to an innovator medicinal product. London: European Medicines agency; 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184922.pdf. Accessed August 27, 2018. | ||
EMA/CHMP. Joint MHLW/EMA reflection paper on the development of block copolymer micelle medicinal products. EMA/CHMP/13099/2013; 2013. | ||
ASTM. Standard Test Method for Evaluation of the Effect of Nanoparticulate Materials on the Formation of Mouse Granulocyte-Macrophage Colonies. ASTM E2525 – 08(2013); 2013. | ||
ASTM. Standard Practice for Testing for Whole Complement Activation in Serum by Solid Materials. ASTM F1984 – 99(2013); 2013. | ||
EUNCL | Nanomedicine Characterisation Laboratory. Available from: http://www.euncl.eu/. Accessed October 28, 2016. | ||
Tao Y, Han J, Dou H. Surface modification of paclitaxel-loaded polymeric nanoparticles: evaluation of invitro cellular behavior and invivo pharmacokinetic. Polymer. 2012;53(22):5078–5086. | ||
Bucharskaya AB, Pakhomy SS, Zlobina OV, et al. Alterations of morphology of lymphoid organs and peripheral blood indicators under the influence of gold nanoparticles in rats. J Innov Opt Health Sci. 2016;09(01):1640004. | ||
Easo SL, Mohanan PV. In vitro hematological and in vivo immunotoxicity assessment of dextran stabilized iron oxide nanoparticles. Colloids Surf B Biointerfaces. 2015;134:122–130. | ||
Ilinskaya AN, Clogston JD, Mcneil SE, Dobrovolskaia MA. Induction of oxidative stress by Taxol® vehicle Cremophor-EL triggers production of interleukin-8 by peripheral blood mononuclear cells through the mechanism not requiring de novo synthesis of mRNA. Nanomedicine. 2015;11(8):1925–1938. | ||
Pondman KM, Tsolaki AG, Paudyal B, et al. Complement deposition on nanoparticles can modulate immune responses by macrophage, B and T cells. J Biomed Nanotechnol. 2016;12(1):197–216. | ||
Herd HL, Bartlett KT, Gustafson JA, Mcgill LD, Ghandehari H. Macrophage silica nanoparticle response is phenotypically dependent. Biomaterials. 2015;53:574–582. | ||
Rudin CM, Marshall JL, Huang CH, et al. Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: a Phase I study. Clin Cancer Res. 2004;10(21):7244–7251. | ||
Liu J, Tian X, Luo N, et al. Sub-10 nm monoclinic Gd2O3:Eu3+ nanoparticles as dual-modal nanoprobes for magnetic resonance and fluorescence imaging. Langmuir. 2014;30(43):13005–13013. | ||
Park EJ, Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett. 2009;184(1):18–25. | ||
Lee S, Kim MS, Lee D, et al. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int J Nanomedicine. 2013;8:147–158. | ||
Beyerle A, Braun A, Merkel O, Koch F, Kissel T, Stoeger T. Comparative in vivo study of poly(ethylene imine)/siRNA complexes for pulmonary delivery in mice. J Control Release. 2011;151(1):51–56. | ||
Murray AR, Kisin E, Leonard SS, et al. Oxidative stress and inflammatory response in dermal toxicity of single-walled carbon nanotubes. Toxicology. 2009;257(3):161–171. | ||
Arancibia S, Barrientos A, Torrejón J, Escobar A, Beltrán CJ. Copper oxide nanoparticles recruit macrophages and modulate nitric oxide, proinflammatory cytokines and PGE2 production through arginase activation. Nanomedicine. 2016;11(10):1237–1251. | ||
Liu H, Yang D, Yang H, et al. Comparative study of respiratory tract immune toxicity induced by three sterilisation nanoparticles: silver, zinc oxide and titanium dioxide. J Hazard Mater. 2013;248–249(1): 478–486. | ||
Huang YJ, Hung KC, Hsieh FY, Hsu SH. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352–20364. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.