Back to Journals » OncoTargets and Therapy » Volume 11
Magnolol exerts anticancer activity in hepatocellular carcinoma cells through regulating endoplasmic reticulum stress-mediated apoptotic signaling
Authors Wang YD, Sun XJ, Yang WJ , Li J, Yin JJ
Received 21 March 2018
Accepted for publication 10 May 2018
Published 28 August 2018 Volume 2018:11 Pages 5219—5226
DOI https://doi.org/10.2147/OTT.S168887
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Ya-Dong Wang,1 Xue-Jun Sun,2 Wei-Jun Yang,3 Jing Li,1 Jia-Jun Yin1
1Department of General Surgery, Affiliated Zhongshan Hospital of Dalian University, Dalian 116001, People’s Republic of China; 2Department of General Surgery, First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, People’s Republic of China; 3Department of General Surgery, The First People’s Hospital of Guiyang, Guiyang 550002, People’s Republic of China
Introduction: Magnolol (Mag), a biologically active compound isolated from the root and stem bark of Magnolia officinalis, has been reported to induce apoptosis in several cancer cell lines in vitro. In the present study, we aimed to determine the anticancer effects of Mag on hepatocellular carcinoma (HCC) cells.
Materials and methods: The HepG2 cells were treated with varying concentrations of Mag (10, 20, and 30 µM) for 48 hours. The effects of Mag on the proliferation, migration, invasion, apoptosis and cell cycle progression of HepG2 cells were respectively detected by MTT assay, transwell assays, and flow cytometric analysis. A HepG2 cell-based tumor-bearing model was established to evaluate the effect of Mag on HCC tumor growth in vivo. The protein expression levels were determined by Western blot analysis.
Results: Our results showed that Mag inhibited the proliferation, migration, and invasion of HepG2 cells in vitro in a dose-dependent manner. In addition, Mag reduced the HCC tumor volume and weight in the mouse xenograft model. Subsequent studies showed that Mag induced apoptosis in HepG2 cells, accompanied by a loss in mitochondrial membrane potential, cytochrome c release, and induction of endoplasmic reticulum stress. Furthermore, inhibition of the endoplasmic reticulum stress by CHOP knockdown restored the effects of Mag in HepG2 cells.
Conclusion: The present study highlighted the possibility of using Mag as a novel therapeutic drug for HCC treatment.
Keywords: hepatocellular carcinoma, magnolol, apoptosis, mitochondrial dysfunction, endoplasmic reticulum stress
Introduction
Cancer is a major public health problem worldwide. Hepatocellular carcinoma (HCC) is one of the most common aggressive cancers and has a relatively high mortality rate.1 Chemotherapy remains the first line of therapeutic methods for HCC, but its effect is still not satisfactory.2 Accordingly, there is an urgent need to identify effective therapeutic agents against HCC with limited side effects.
The endoplasmic reticulum (ER) is the major intracellular organelle for protein folding, calcium storage, and lipid metabolism.3 Under stress conditions, when the protein folding ability is disrupted, misfolded/unfolded proteins are accumulating in the ER lumen, and this phenomenon is called ER stress. ER stress often occurs during apoptosis of tumor cells.4 Accordingly, targeting the inducers of ER stress has been considered as a therapeutic strategy for HCC.
Natural plant products have attracted the interest of researchers as prospective anticancer candidates in recent years.5,6 Magnolol (Mag) is a biological active compound isolated from the root and stem bark of Magnolia officinalis, which has been frequently used in traditional Chinese medicine.7 Accumulating articles have reported that Mag can reduce the malignant traits of several kinds of cancer cells, such as breast cancer,8 non-small-cell lung cancer,9 and gallbladder cancer.10 However, there are scarcely any reports about the effects of Mag in HCC and its mechanisms.
Therefore, in the present study, we aimed to determine the effects of Mag on the proliferation, invasion, and apoptosis of HepG2 cells. Furthermore, the effects of Mag on ER stress-related proteins in HepG2 cells were investigated to account for the molecular mechanisms.
Materials and methods
Cell culture and treatments
The human HCC cells (HepG2 cells) and normal liver cells (LO2 cells), purchased from the American Type Culture Collection (Manassas, VA, USA), were routinely cultured in Dulbecco’s Modified Eagle’s Medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Biowest, Loire, France), 100 U/mL penicillin, and 100 μg/mL streptomycin in an incubator at 37°C with 5% CO2 in a humidified atmosphere.
Mag was purchased from Sigma-Aldrich (St Louis, MO, USA), and Mag stock solution was prepared in DMSO (Sigma-Aldrich) and diluted in culture medium to the final concentration. When 75% confluence was achieved, the cells were treated with the indicated concentration of Mag (10, 20, and 30 μM) for 48 hours.
The siRNA for CHOP (si-CHOP) was designed and synthesized by Shanghai Integrated Biotech Solutions Co., Ltd. (Shanghai, People’s Republic of China). For siRNA transfection, HepG2 cells were seeded on 6-well plates at a density of 2×105 cells/well. When the cells reached 80% confluence, si-CHOP or negative control siRNA (si-NC) was transfected into HepG2 cells using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA). Forty-eight hours after transfection, the cells were prepared for further experiments.
MTT assay
Cell proliferation was quantified by MTT assay. In brief, the cells were plated in 96-well plates at a density of 5×103 cells/well and incubated with various doses of Mag for 48 hours. After that, 20 μL MTT (5 mg/mL; Sigma) was added to each well. The plates were incubated for another 4 hours. The culture medium was then removed and the remaining formazan precipitates were dissolved in 150 μL DMSO. The cell proliferation was determined by measuring the absorbance at 490 nm on a microplate reader (MultiskanEX, Lab Systems, Helsinki, Finland).
Cell cycle analysis
Cell cycle progression was evaluated using the CycleTEST PLUS DNA Reagent Kit (BD Biosciences, San Jose, CA, USA). In brief, after the treatments, the cells were collected, fixed in 70% ethanol at 4°C for 16 hours, and then stained with propidium iodide (PI) for 30 minutes in the dark. Then the stained cells were analyzed by flow cytometry (FACScan®; BD Biosciences).
Cell apoptosis analysis
Cell apoptosis was evaluated using the Annexin-V-FITC Apoptosis Detection Kit (BD Biosciences). Briefly, after the treatments, the cells were collected, washed with PBS, and suspended in 500 μL binding buffer with 5 μL of Annexin-V-FITC and 5 μL PI for 15 minutes in the dark. Then the stained cells were analyzed by flow cytometry.
Transwell migration and invasion assays
Cell migration and invasion were determined using transwell chambers (8 μM pore size; Corning, New York, NY, USA). In brief, a total of 3×105 of HepG2 cells were suspended in serum-free medium and added to the upper chambers. The cells were then treated with different doses of Mag as described above. The lower chambers were filled with 600 μL medium containing 10% fetal bovine serum. After 48 hours, the inside of the inserts was cleaned thoroughly using a cotton swab, and the migrated or invaded cells at the lower membrane surface were fixed with methanol, stained with 1% crystal violet solution for 15 minutes, counted under microscopy, and photographed.
Measurement of mitochondrial membrane potential (MMP)
The MMP was examined by staining the cells with the fluorescent probe JC-1 (Beyotime, Shanghai, People’s Republic of China). Briefly, after treatments, the cells were collected, resuspended in 500 μL of PBS and incubated in 0.5 mL JC-1 working solution in darkness at 37°C for 15 minutes. Then the stained cells were analyzed by flow cytometry.
Western blot analysis
Cells were washed twice with ice-cold PBS and lysed in radioimmunoprecipitation assay lysis buffer (Beyotime) on ice. The mitochondrial and cytosolic fractions were prepared using the Mitochondria/Cytosol Fractionation kit (Abcam, Cambridge, MA, USA). Protein content was determined by the Enhanced BCA Protein Assay kit (Beyotime). Then, equal amounts of proteins were run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% BSA in Tris-buffered saline (TBS) with Tween 20 and then probed overnight at 4°C with primary antibodies, followed by incubation with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 2 hours. The bands were visualized using an enhanced chemiluminescence system (Pierce Biotechnology Inc., Rockford, IL, USA), and the density was quantified using Alphalmager 2200 image software (UVP, Upland, CA, USA). The signal generated by GAPDH or COX IV was used as an internal control.
Xenografts in nude mice
Twenty male nude mice (athymic, Balb/c nu/nu) aged 5–6 weeks (18–20 g/mouse) were purchased from the SLAC Laboratory Animal Company (Shanghai, People’s Republic of China). These mice were randomly divided into 4 groups (n=5/group). HepG2 cells (1×106 cells) were resuspended in 100 μL free serum medium and injected subcutaneously into the right flank of mice. When the tumors reached approximately 100 mm3, the mice in 3 Mag-treated groups were intraperitoneally injected with 10, 20, and 30 mg/kg of Mag every day. Mice in the control group were intraperitoneally injected with corn oil every day. The tumor volume (V) was calculated every 5 days by using the following formula: V = A × B2/2 (A = largest diameter; B = smallest diameter). Tumor growth curves were plotted. On day 30, the mice were sacrificed, and the tumors were removed and weighed. All animal-related procedures were approved by the Ethics Committee of Affiliated Zhongshan Hospital of Dalian University (Dalian, People’s Republic of China) and undertaken in accordance with the Institutional Animal Care and Use guidelines. All efforts were made to minimize suffering.11
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). All of the data are presented as the means ± standard deviation (SD), and subsequently analyzed by one-way analysis of variance, followed by Tukey’s post hoc test. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
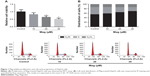
Mag inhibits proliferation and cell cycle progression of HepG2 cells
In order to determine the effects of Mag on HCC cell proliferation, the MTT assay was employed. HepG2 cells were exposed to a range of doses of Mag (10, 20, and 30 μM) for 48 h. We found that Mag exposure suppressed the proliferation of HepG2 cells in a dose-dependent manner (Figure 1A). But Mag exerts little effects on normal LO2 cells (data not shown), indicating the selectivity of Mag in killing cancer cells. To further examine whether the inhibitory effect of Mag on proliferation was associated with cell cycle arrest, the cell cycle progression of HepG2 cells was then determined through flow cytometric analysis. The results showed that administration of Mag increased the number of HepG2 cells in G0/G1 phase of cell cycle in a dose-dependent manner, and this increase was accompanied with a concomitant decrease of cell number in S phase (Figure 1B).
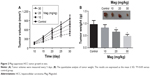
Mag suppresses HCC tumor growth in vivo
On the basis of the in vitro findings, we subsequently aimed to investigate the role of Mag on HCC tumor growth in vivo. A HepG2 cell-based tumor-bearing model was established using athymic nude mice. We observed that treatment of mice with Mag had an inhibitory effect on xenograft tumor growth compared to the control group (Figure 2A), and no obvious adverse effects were found even in the mice treated with the highest dose of Mag. After 30 days, the mice were killed and we found that treatment with Mag dose-dependently suppressed increases in tumor volume and weight (Figure 2B).
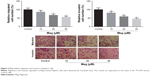
Mag inhibits migration and invasion of HepG2 cells
The migratory and invasive capacities of Mag-treated HepG2 cells were then evaluated through performing transwell assays. After incubation with different doses of Mag (10, 20, and 30μM) for 48 h, we observed that the number of migrated or invaded HepG2 cells was dose-dependently reduced (Figure 3).
Mag promotes apoptosis in HepG2 cells
We further investigated the effects of Mag on HCC cell apoptosis using flow cytometry after Annexin V-FITC/PI staining. As shown in Figure 4A, a dose-dependent increase in the fraction of apoptotic cells was observed in Mag-treated HepG2 cells. We also evaluated the expression of apoptosis-related proteins. The results of Western blot analysis indicated that Mag stimulation resulted in a marked reduction in the Bcl-2 expression and increased the expression of Bax and cleaved PARP-1 in HepG2 cells (Figure 4B). Additionally, Mag treatment induced the release of cytochrome c from mitochondria to cytosol in HepG2 cells (Figure 4C). We then analyzed changes in MMP using JC-1 staining, and the results showed that the MMP in HepG2 cells was disrupted dose-dependently upon Mag exposure, which was proved by the decrease in the ratio of red/green (Figure 4D).
Mag induces ER stress in HepG2 cells
To further investigate the role of ER stress in Mag-induced apoptosis, the expression levels of ER stress-related proteins, including GRP78, p-PERK, p-eIF2α, and CHOP, were detected by Western blot analysis. As shown in Figure 5, Mag treatment significantly activated ER stress, as evidenced by the increased expression levels of ER stress-related proteins, including GRP78, p-PERK, p-eIF2α, and CHOP.
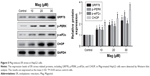
CHOP knockdown abrogated the effects of Mag in HepG2 cells
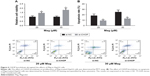
We performed CHOP knockdown experiments using CHOP siRNAs in HepG2 cells. Through Western blot analysis, we found that transfection with si-CHOP significantly reduced the expression levels of CHOP in HepG2 cells (data not shown). Furthermore, as shown in Figure 6A and B, CHOP knockdown obviously restored the decreased cell viability and increased cell apoptosis in Mag-treated HepG2 cells.
Discussion
Mag possesses potential antitumor activities against many types of human cancers. In the present study, the anticancer effects and possible mechanisms of Mag in HCC cells were further investigated.
Excessive proliferation is one of the hallmarks of tumor cells, and mistakes in cell cycle process induce unrestrained cell proliferation.12 A previous study reported the inhibitory effect of Mag on HCC cell proliferation by inhibiting DNA synthesis.13 In the present study, we also confirmed that Mag can inhibit proliferation of human HCC cells in vitro through inducing G0/G1 arrest. Additionally, in the HCC xenograft model, as expected, Mag administration inhibited HCC tumor growth in vivo. We also determined the migration and invasion of Mag-treated HepG2 cells, and found that these cells were less able to migrate and invade. All these data confirmed that Mag has a potential anticancer effect in HCC.
Cancer cells are featured by a reduction in cell apoptosis, and induction of apoptosis is one of the pivotal points in identification of potential anticancer agents.14 The results of the present study demonstrated that Mag led to the imbalance of Bcl-2 family expression, thus enhancing the apoptosis of HepG2 cells. The mitochondrion-dependent pathway is the most common apoptotic pathway in vertebrate animal cells.15 As an early step involved in mitochondria-mediated apoptosis, MMP disruption is induced by the imbalance of Bcl-2 family expression.16 Our results showed that Mag indeed led to a collapse of MMP and induced cytochrome c release, indicating that mitochondrial dysfunction was involved in Mag-induced apoptosis in HepG2 cells.
Mounting data indicate that ER stress plays an important role in the regulation of cell apoptosis.17 This study provides important evidence that Mag treatment concomitantly induces ER stress response, which is highlighted by increased expression of CHOP. CHOP is a marker of ER stress that induces apoptotic cell death,18 and in this study, we found that siRNA-mediated knockdown of CHOP significantly inhibits Mag-induced apoptosis in HepG2 cells, further indicating that the anti-HCC effect of Mag is partly regulated by the stimulation of ER stress-mediated apoptotic signaling.
Conclusion
In conclusion, we demonstrated that Mag possesses obvious anticancer activity in human HCC cells through inducing cell cycle arrest and apoptosis. Mag can activate ER stress response, thus leading to mitochondrial dysfunction and cell death. We believe our findings may be helpful for the development of Mag into a chemotherapeutic agent for HCC in the near future.
Disclosure
The authors report no conflicts of interest in this work.
References
Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992–2008. Hepatology. 2012;55(2):476–482. | ||
Scudellari M. Drug development: try and try again. Nature. 2014;516(7529):S4–S6. | ||
Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107(7):827–830. | ||
Bhat TA, Chaudhary AK, Kumar S, et al. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim Biophys Acta. 2017;1867(1):58–66. | ||
Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nature Rev Drug Discov. 2005;4(3):206–220. | ||
Hu Y, Wang S, Wu X, et al. Chinese herbal medicine-derived compounds for cancer therapy: a focus on hepatocellular carcinoma. J Ethnopharmacol. 2013;149(3):601–612. | ||
Lo YC, Teng CM, Chen CF, Chen CC, Hong CY. Magnolol and honokiol isolated from Magnolia officinalis protect rat heart mitochondria against lipid peroxidation. Biochem Pharmacol. 1994;47(3):549–553. | ||
Zhou Y, Bi Y, Yang C, et al. Magnolol induces apoptosis in MCF-7 human breast cancer cells through G2/M phase arrest and caspase-independent pathway. Pharmazie. 2013;68(9):755–762. | ||
Tsai JR, Chong IW, Chen YH, et al. Magnolol induces apoptosis via caspase-independent pathways in non-small cell lung cancer cells. Arch Pharm Res. 2014;37(4):548–557. | ||
Li M, Zhang F, Wang X, et al. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015;106(10):1341–1350. | ||
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG; NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med. 2010;12(7):561–563. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Lin SY, Liu JD, Chang HC, Yeh SD, Lin CH, Lee WS. Magnolol suppresses proliferation of cultured human colon and liver cancer cells by inhibiting DNA synthesis and activating apoptosis. J Cell Biochem. 2002;84(3):532–544. | ||
Gerl R, Vaux DL. Apoptosis in the development and treatment of cancer. Carcinogenesis. 2005;26(2):263–270. | ||
Elkholi R, Renault TT, Serasinghe MN, Chipuk JE. Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab. 2014;2:16. | ||
Harris MH, Thompson CB. The role of the Bcl-2 family in the regulation of outer mitochondrial membrane permeability. Cell Death Differ. 2000;7(12):1182–1191. | ||
Faitova J, Krekac D, Hrstka R, Vojtesek B. Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett. 2006;11(4):488–505. | ||
Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11(4):381–389. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.