Back to Journals » Infection and Drug Resistance » Volume 14
Magnitude of Mycobacterium tuberculosis Infection and Its Resistance to Rifampicin Using Xpert-MTB/RIF Assay Among Presumptive Tuberculosis Patients at Motta General Hospital, Northwest Ethiopia
Authors Demissie TA , Belayneh D
Received 5 January 2021
Accepted for publication 13 March 2021
Published 7 April 2021 Volume 2021:14 Pages 1335—1341
DOI https://doi.org/10.2147/IDR.S300585
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Tesfaye Andualem Demissie,1 Dereje Belayneh2
1Department of Medical Laboratory Science, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia; 2Shegaw Motta General Hospital, Amhara, Ethiopia
Correspondence: Tesfaye Andualem Demissie
Department of Medical Laboratory Science, College of Health Science, Debre Tabor University, P.O. Box 272, Ethiopia
Email [email protected]
Background: Mycobacterium Tuberculosis (MTB) and its drug resistance form are the devastating infectious diseases in the world. It is the major cause of morbidity and mortality in low-income countries with Ethiopia carrying a heavy burden. Data on the magnitude of MTB and rifampicin resistance using Xpert- MTB/RIF assay is limited in the study area. Therefore, this study aimed to assess the prevalence of Mycobacterium tuberculosis and rifampicin resistance among presumptive TB patients using GeneXpert at Motta General Hospital, North West Ethiopia.
Methods: A retrospective cross-sectional study was conducted from 1st October to 30 November 2020 among patients tested for GeneXpert at Motta General Hospital, Northwest Ethiopia. Data recorded on GeneXpert test results were collected on laboratory registration book in Microbiology laboratory. Data were analyzed by using the Statistical Package for Social Sciences (SPSS) version 20.
Results: A total of 4109 specimens were tested using the GeneXpert automated system. Of these, the majority 2148 (52.3%) of participants were males and 1961 (47.7%) were females. Similarly, about 1553 (37.8%) were in the age range of 25– 44 years followed by 1347 (32.8%) in 45– 64 years. Moreover, about 2486 (60.5%) participants were from rural. The overall prevalence of M. tuberculosis was 346 (8.4%) among these, the majority 222 (5.4%) had unknown HIV status, 48 (1.2%) were HIV positive, and 314 (7.6%) was new MTB cases. The overall prevalence of rifampicin resistance was 15 (4.3%) and 8(1.7%) were intermediate. Among rifampicin resistance, 10 (2.9%) were males, 8(2.3%) lived in rural, 9 (2.6%) had unknown HIV status, 13 (3.8%) were new TB patients, and 13 (3.8%) had pulmonary tuberculosis.
Conclusion: The prevalence of M. tuberculosis was 8.4% and relatively higher rate of rifampicin-resistant M. tuberculosis was found.
Keywords: drug resistance, GeneXpert, Mycobacterium tuberculosis, prevalence, rifampicin
Introduction
Tuberculosis (TB) remains one of the world’s dead list communicable diseases. It is caused by a bacterium called MTB which commonly affects the lung.1 It transmits from person to person via droplets from the infected individuals. Of infectious diseases in human beings record history, TB has been a great cause of morbidity and mortality. Even after introduction of directly observed treatment short courses (DOTS) strategy, TB still remains as a major problem.2,3
According to World Health Organization (WHO) TB report 2020, globally it causes an estimate of 10.0 million people and 1.3 million deaths among HIV negative and 208,00 deaths among HIV positive patients.4 In Africa, approximately 2.48 million cases were reported. Ethiopia is one of the 30 highly burden countries in the world. In Ethiopia, as WHO 2019 report, an estimate of 157,000 cases was developed TB. The estimated incidence rate was 164 cases per 1000 population and the mortality rate is 125 per 100,000 population per year from 2000–2017.4
Globally in 2019, an estimated 3.5% of new cases and 18% of previously treated cases had multi drug resistance tuberculosis/rifampicin resistance (MDR/RR-TB).4 The emergence and spread of multi-drug resistance tuberculosis/MDR has become a significance complex for TB control problem that cannot be treated by the currently available standard anti-TB drugs.5,6
Resistance to at least the two major anti-tuberculosis drugs, isoniazid and rifampicin has been termed as multidrug-resistant tuberculosis (MDR-TB).7,8 Treatment of MDR-TB requires prolonged and expensive chemotherapy using second line drugs of heightened toxicity and less effective.9,10 Drug resistances is mostly a manmade problem resulting from misuse and mismanagement of the drugs or combined.10–12
In Ethiopia, the burden of Both HIV and TB infections is relatively high while TB diagnostic are grossly inadequate and done mostly by conventional diagnosis methods. Along with the emergence of MDR and poor environmental and living conditions makes a significance threat to public health and safety in the country.13
In Ethiopia, since 2014 the GeneXpert assay was introduced in referral hospitals and regional laboratories and now it is going to be implemented in health facilities as per WHO recommendation. Early the conventional method sputum microscopy was the commonly used laboratory diagnostics techniques for TB.14 Moreover; to date the magnitude of MDR/RR-TB has not been addressed extensively using the newly diagnostic method GeneXpert in the study area up to 2015. Therefore, the aim of the study was to give information regarding the magnitude of Mycobacterium tuberculosis and rifampicin resistance among patients tested using GeneXpert method at Motta General Hospital from 2015–2019, Northwest Ethiopia.
Materials and Methods
Study Design, Period, and Area
Hospital based retrospective cross sectional study was conducted in Motta General Hospital, East Gojjam zone, Northwest Ethiopia from 1st October to 30 November 2020. Motta General Hospital is found in Amhara region which is located at a distance of120 Km from Bahir Dar and 370 Km away from capital city of the country, Addis Ababa. The hospital provided service for above 1.2 million populations. A total 4109 TB suspected patient were presumptively tested by GeneXpert in last five year from 2015–2019.
Source Population and Study Populations
All patients who suspected and diagnosis with TB at the study hospital during the study period were the source population. Patients with a recorded document in the microbiology laboratory logbook for TB investigation were our study populations.
Inclusion Criteria and Exclusion Criteria
All TB suspected patients who were tested by GeneXpert were included in the study whereas TB suspected individuals who were tested by GeneXpert had not completed records were excluded from the study.
Data Collection and Processing
Data Collection
From 2015 to 2019 GeneXpert results data were collected by the principal investigator from the log book. All the required information were collected by classifying their type of TB based on age, sex, residence, registration group and HIV status.
Data Processing and Analysis
Data was checked before entering for analysis for its completeness. Then, the data were entered and analysis using statistical package for social sciences (SPSS) statistical software version 20. Descriptive statistics were used and finally, the study findings were explained in words, tables and graphs. Odds ratios (OR) and their 95% confidence intervals (CI) were estimated using bivariate and multivariate logistic regression analysis to identify possible explanatory variables on occurrence of MTB and RR. The result at p-value < 0.05 was considered as statistically significant.
Operational Definitions
GeneXpert MTB/RIF Assay
It is an automated cartridge based nucleic acid amplification test which can identify MTB DNA and resistance to rifampicin.
Extra Pulmonary TB(EPTB)
An infectious disease of humans which is caused by MTB which infecting out of the lungs, such as brain, kidney, intestine, bone and others.
MDR
Resistance to at least the two major anti-tuberculosis drugs, isoniazid and rifampicin has been termed as multidrug-resistant tuberculosis (MDR-TB.
Pulmonary TB (PTB)
An infectious disease of humans which is caused by bacterium Mycobacterium tuberculosis mainly infecting the lungs.
Rifampicin Resistant TB
Resistance to Rifampicin detected using phenotypic or genotypic methods, with or without resistance to other anti-TB drugs.
Ethical Aspects
The ethical clearance approval was obtained from Department of Medical Laboratory Science, Collage of Health Science, Debre Tabor University. Permission letter was also obtained from Motta General Hospital to conduct the study. The confidentiality of each study subjects were kept as well. This study was conducted in accordance with the Declaration of Helsinki, and that patient informed consent was not required.
Results
Socio-Demographic Characteristics of Study Participants
In the present study, a total of 4109 study participants were recruited. Of those, the majority 2148 (52.3%) of participants were males and 1961 (47.7%) were females. Study participant`s age ranged from 1–90 years old with mean age 40.25, median 40.0 and standard deviation (SD = 17. 26). Majority of study participants were 1553 (37.8%) in 25–44 years followed by 1347 (32.8%) in 45–64 years age. A majority of, 2486 (60.5%) were urban inhabitants. Unknown HIV status of participants accounted as 2953 (71.9%), followed by 755 (18.4%) HIV negative, and 401 (9.8%) were HIV positive. Furthermore, the majority of 4027 (98.0%) were new presumptively diagnosed by GeneXpert (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Study Participants Tested by GeneXpert at Shegaw Motta General Hospital, Northwest Ethiopia, 2020 |
Prevalence of Mycobacterium tuberculosis
The overall prevalence of MTB was 346 (8.4%) at [95% CI; 7.6–9.4]. Of these, 323 (93.4%) were PTB, and 23 (6.6%) were EPTB. Accordingly, M. tuberculosis positive by GeneXpert were 176 (4.3%) males, and 170 (4.1%) were females. 163 (4.0%) were seen in the age groups of 25–44 years old. Moreover, 95 (2.3%) were urban residents, and 251 (6.1%) were in rural dwellers. 222 (5.4%) were unknown HIV status, 48 (1.2%) were HIV positive, 314 (7.6%) were new diagnosis, and 28 (0.6%) were relapse cases for MTB.
Year based prevalence of MTB was (51 (1.2%)) in 2015, (76 (1.8%)) 2016, (75 (1.8%)) 2017, (81 (2.0%)) 2018, and (63 (1.5%)) 2019 were varied and indicated differently as shown in Figure 1.
 |
Figure 1 Mycobacterium tuberculosis detection by GeneXpert at Motta General Hospital, 2020. |
Prevalence of Rifampin Resistance
Prevalence of rifampicin resistance among 346 MTB positive cases was 15 (4.3%), intermediate were 8(1.7%), and sensitive were 325 (93.9%). Of positive results, 10 (2.9%) were males, 8 (2.3%) were rural residents, 9 (2.6%) were unknown HIV status, 13 (3.8%) were new patients diagnosed presumptively, and 13 (3.8%) were PTB (Table 2).
 |
Table 2 Rifampicin Resistance Profile Detected Among MTB Positive at Shegaw Motta General Hospital, Northwest Ethiopia, 2020 |
In the final model multivariable analysis showed that sex were not found to be significantly associated with prevalence of MTB and RR at 95% confidence interval. Increased proportions of MTB were detected in males (OR= 1.04, 95% CI= 0.84–1.30, P=0.7). Being urban were significantly associated with prevalence of MTB (OR=1.79, 95% CI= 1.39–2.28, P < 0.001), but it was not significantly associated with MTB/RR (OR=1.44, 95% CI= 0.16–1.27, P =0.13). Moreover, HIV positive patients were also found to be significantly associated with the prevalence of MTB (OR=1.40, 95% CI= 1.06–1.84 P =0.017), but it was not significantly associated with the prevalence of MTB/RR (OR=1.12, 95% CI= 0.33–3.80, P =0.85) as shown below in (Tables 3 and 4).
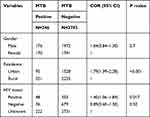 |
Table 3 Multivariate Analysis of Factors Associated with MTB Positive in Motta General Hospital, Ethiopia, 2020 |
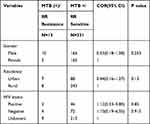 |
Table 4 Multivariate Analysis of Factors Associated with MTB/RR Positive Among MTB Positive Patients in Motta General Hospital, Ethiopia, 2020 |
Discussion
The rapid increment and the emergence of antibiotics resistance of MTB strain is a serious concern in many developing countries. In Ethiopia, laboratory investigation confirms that drug resistance of MTB is increasing. And many scholars agreed that one of the most important problems for unresolved MTB is increment of its drug resistance.15
The prevalence of MTB in the current study was 346 (8.4%) at [95% CI; 7.6–9.4]. Hence, the present finding was comparable with the previous studies reported as 8.9% in East part of Ethiopia.16 8.98% in Ataye district hospital, Northeast Ethiopia,17 and 7.9% in Tigray region, Ethiopia.18
In other way, the current study was higher than a study done in spiritual holy water in Northwest Ethiopia (2.9%).19 In contrast to this, the current study was less than as compared to the previous studies reported in Nepal (13.8%),20 Zimbabwe (11%),21 and Ethiopia: Metehara sugar factory (14.2%).22 Western Oromia (21.3%),23 Addis Ababa (15.11%),24 Gambella regional state (20.0%),25 Dubti Hospital in Afar region (24.5%),26 Western Oromia (21.3%),27 at University of Gondar (24.6%),16 Debre Markos Referral Hospital (23.2%),28 Hiwot fana Hospital Harar (15.7%),29 and Djibouti Hospital (24.5%).26 The variations may be due to geographical variation, climatic condition and diagnostic methodology.
According to rifampicin resistance in the current study, the overall prevalence of rifampicin resistance was 15 (4.3%). Relatively low as compared with others studies were done in Nepal (10.2%),20 Saudi Arabia (15%),30 KwaZulu-Natal South Africa (8.8%),31 Zimbabwe (4.5%),21 and similar higher rifampicin resistance reports were also seen in Ethiopia as: Debre Markos (10.3%),28 Gondar (15.8%),16 Gambella regional state, Southwest Ethiopia (4.9%),25 Afar region (4%),26 Western Oromia state (25.9%),23 Addis Ababa (9.9%),24 and Tigray, Northern Ethiopia (7.9%).18 In the present study, the proportion of rifampicin-resistant M. tuberculosis was significantly lower among previously treated patients compared to treatment naive patients that may be due to low failure and relapse from previous treatment and contact with drug resistant TB patients.
In contrast, the present study RR-TB was significantly higher compared to the previous studies reported as 4.3% in Dubti Hospital, Afar Ethiopia (4.3%),26 Ambo Town, Central Ethiopia (1.2%),27 East Gojjam Zone, Northwest Ethiopia (3.89%),32 and was in line with study done in Ataye, Amhara Northeast Ethiopia (5.3%).17 In the present study, the proportion of rifampicin-resistant M. tuberculosis was significantly higher among previously treated patients compared to treatment naive patients that might be due to failure from previous treatment and contact with drug resistant TB patients. The high prevalence of RR TB among new TB cases in the current showed there may be an existence of active transmission of the bacteria or the existence of new undiagnosed RR-TB cases. Moreover, drug resistance among previously untreated cases showed that the performance of TB control program in the past. In Ethiopia, the strict practice of direct observed therapy (DOTS) program is currently implemented is questionable.
The present study indicated that TB/HIV co-infection occurs with 1.2% of TB infected individuals co-infected with HIV as presented in Table 1. HIV positive patients were statistically associated with the development of MTB. The finding was supported by studies were done in India33 and WHO guide lines.34 This may be due low immune statues of the patients by HIV. But HIV positive patients were not significantly associated with the development of MTB/RR as found in other study Debre Markos Ethiopia.28 This might have been due to ART decrease the chances of drug resistance among HIV positive PTB patients.
Limitation of the Study
This study could not do the level of resistance to other anti-TB drugs.
Conclusion and Recommendation
In our study, the prevalence of M. tuberculosis was 8.4% and relatively higher rate of rifampicin-resistant M. tuberculosis was observed, and the use of GeneXpert should be scaled up across the country for rapid diagnosis, management and expanded surveillance of drug-resistant M. tuberculosis.
Data Sharing Statement
All data analyzed in this study can access from the corresponding author.
Ethics Approval and Consent
Study protocol was approved by the research committee of Debre Tabor University, College of Health Science. This study was conducted in accordance with the Declaration of Helsinki, and that patient informed consent was not required.
Acknowledgment
We would like to thank Shegaw Motta General Hospital SMGH for giving permission to conduct this study. We are very grateful to all laboratory staffs of SMGH for their support during data collections.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grange JM, Zumla A. The global emergency of tuberculosis: what is the cause? J R Soc Promot Health. 2002;122(2):78–81. doi:10.1177/146642400212200206
2. Pai M, Behr MA, Dowdy D, et al. Tuberculosis. Nat Rev Dis Primers. 2016;2(1):16076. doi:10.1038/nrdp.2016.76
3. Adejumo OA, Daniel OJ, Otesanya AF, Salisu-Olatunj SO, Abdur-Razzaq HA. Evaluation of outcomes of tuberculosis management in private for profit and private-not-for profit directly observed treatment short course facilities in Lagos State, Nigeria. Niger Med J. 2017;58(1):44–49. doi:10.4103/0300-1652.218417
4. World Health Organazation (WHO). Global Tuberculosis Report. WHO; 2020.
5. Zignol M, Dean AS, Falzon D, et al. Twenty years of global surveillance of antituberculosis-drug resistance. N Engl J Med. 2016;375(11):1081–1089. doi:10.1056/NEJMsr1512438
6. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi:10.1016/S1473-3099(17)30753-3
7. Drobniewski F, Nikolayevskyy V, Balabanova Y, Bang D, Papaventsis D. Diagnosis of tuberculosis and drug resistance: what can new tools bring us? [State of the art series. New tools. Number 1 in the series]. Int J Tuberc Lung Dis. 2012;16(7):860–870. doi:10.5588/ijtld.12.0180
8. Lawn SD, Wilkinson R. Extensively drug resistant tuberculosis. BMJ. 2006;333(7568):559–560. doi:10.1136/bmj.38971.587222.AB
9. Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med. 2015;5(9):a017863. doi:10.1101/cshperspect.a017863
10. Soini H, Vasankari T. MDR tuberculosis. Duodecim. 2014;130(16):1599–1605.
11. Raviglione MC. The new stop TB strategy and the global plan to stop TB, 2006–2015. Bull World Health Organ. 2007;85(5):327. doi:10.2471/BLT.06.038513
12. Dessalegn M, Daniel E, Behailu S, Wagnew M, Nyagero J. Predictors of multidrug resistant tuberculosis among adult patients at Saint Peter Hospital Addis Ababa, Ethiopia. Pan Afr Med J. 2016;25(Suppl 2):5.
13. Gelaw YA, Assefa Y, Soares Magalhaes RJ, et al. TB and HIV epidemiology and collaborative service: evidence from Ethiopia, 2011–2015. HIV/AIDS. 2020;12:839–847.
14. Derbie A, Worku S, Mekonnen D, et al. Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and its rifampicin resistance at Felege Hiwot and Debre Tabor Hospitals, Northwest Ethiopia: a preliminary implementation research. Ethiop J Health Dev. 2016;30(2):60–66.
15. Li D, Song Y, Yang P, Li X, Zhang AM, Xia X. Genetic diversity and drug resistance of Mycobacterium tuberculosis in Yunnan, China. J Clin Lab Anal. 2019;33(5):e22884. doi:10.1002/jcla.22884
16. Jaleta KN, Gizachew M, Gelaw B, Tesfa H, Getaneh A, Biadgo B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, northwest Ethiopia. Infect Drug Resist. 2017;10:185–192. doi:10.2147/IDR.S135935
17. Gebretsadik D, Ahmed N, Kebede E, Mohammed M, Belete MA. Prevalence of tuberculosis by automated GeneXpert rifampicin assay and associated risk factors among presumptive pulmonary tuberculosis patients at Ataye District Hospital, North East Ethiopia. Infect Drug Resist. 2020;13:1507–1516. doi:10.2147/IDR.S248059
18. Wasihun AG, Dejene TA, Hailu GG, Anupurba S. Frequency of MTB and rifampicin resistance MTB using Xpert-MTB/RIF assay among adult presumptive tuberculosis patients in Tigray, Northern Ethiopia: a cross sectional study. PLoS One. 2020;15(11):e0240361. doi:10.1371/journal.pone.0240361
19. Derseh D, Moges F, Tessema B. Smear positive pulmonary tuberculosis and associated risk factors among tuberculosis suspects attending spiritual holy water sites in Northwest Ethiopia. BMC Infect Dis. 2017;17(1):100. doi:10.1186/s12879-017-2211-5
20. Sah SK, Bhattarai PR, Shrestha A, Dhami D, Guruwacharya D, Shrestha R. Rifampicin-resistant Mycobacterium tuberculosis by GeneXpert MTB/RIF and associated factors among presumptive pulmonary tuberculosis patients in Nepal. Infect Drug Resist. 2020;13:2911–2919. doi:10.2147/IDR.S263795
21. Charambira K, Ade S, Harries AD, et al. Diagnosis and treatment of TB patients with rifampicin resistance detected using Xpert(®) MTB/RIF in Zimbabwe. Public Health Action. 2016;6(2):122–128. doi:10.5588/pha.16.0005
22. Yohanes A, Abera S, Ali S. Smear positive pulmonary tuberculosis among suspected patients attending metehara sugar factory hospital; eastern Ethiopia. Afr Health Sci. 2012;12(3):325–330. doi:10.4314/ahs.v12i3.12
23. Zewdie O, Dabsu R, Kifle E, Befikadu D. Rifampicin-resistant multidrug-resistant tuberculosis cases in selected hospitals in Western Oromia, Ethiopia: cross-Sectional Retrospective Study. Infect Drug Resist. 2020;13:3699–3705. doi:10.2147/IDR.S274589
24. Arega B, Menbere F, Getachew Y. Prevalence of rifampicin resistant Mycobacterium tuberculosis among presumptive tuberculosis patients in selected governmental hospitals in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19(1):307. doi:10.1186/s12879-019-3943-1
25. Ejeta E, Beyene G, Bonsa Z, Abebe G. Xpert MTB/RIF assay for the diagnosis of Mycobacterium tuberculosis and Rifampicin resistance in high human immunodeficiency virus setting in Gambella regional state, southwest Ethiopia. J Clin Tuberc Other Mycobact Dis. 2018;12:14–20. doi:10.1016/j.jctube.2018.06.002
26. Gebrehiwet GB, Kahsay AG, Welekidan LN, Hagos AK, Abay GK, Hagos DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti Hospital, Afar, Ethiopia. J Infect Dev Ctries. 2019;13(1):21–27. doi:10.3855/jidc.10462
27. Tilahun M, Ameni G, Desta K, et al. Molecular epidemiology and drug sensitivity pattern of Mycobacterium tuberculosis strains isolated from pulmonary tuberculosis patients in and around Ambo Town, Central Ethiopia. PLoS One. 2018;13(2):e0193083. doi:10.1371/journal.pone.0193083
28. Mulu W, Abera B, Yimer M, Hailu T, Ayele H, Abate D. Rifampicin-resistance pattern of Mycobacterium tuberculosis and associated factors among presumptive tuberculosis patients referred to Debre Markos Referral Hospital, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10(1):8. doi:10.1186/s13104-016-2328-4
29. Bodena D, Ataro Z, Tesfa T. Trend analysis and seasonality of tuberculosis among patients at the Hiwot Fana Specialized University Hospital, Eastern Ethiopia: a Retrospective Study. Risk Manag Healthc Policy. 2019;12:297–305. doi:10.2147/RMHP.S228659
30. Jarallah JS, Elias AK, Al Hajjaj MS, Bukhari MS, Al Shareef AH, al-Shammari SA. High rate of rifampicin resistance of Mycobacterium tuberculosis in the Taif region of Saudi Arabia. Tuber Lung Dis. 1992;73(2):113–115. doi:10.1016/0962-8479(92)90065-R
31. Coovadia YM, Mahomed S, Pillay M, Werner L, Mlisana K, Nicol MP. Rifampicin mono-resistance in Mycobacterium tuberculosis in KwaZulu-Natal, South Africa: a significant phenomenon in a high prevalence TB-HIV region. PLoS One. 2013;8(11):e77712. doi:10.1371/journal.pone.0077712
32. Adane K, Ameni G, Bekele S, Abebe M, Aseffa A. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Public Health. 2015;15(1):572. doi:10.1186/s12889-015-1933-9
33. Swaminathan S, Paramasivan CN, Ponnuraja C, Iliayas S, Rajasekaran S, Narayanan PR. Anti-tuberculosis drug resistance in patients with HIV and tuberculosis in South India. Int J Tuberc Lung Dis. 2005;9(8):896–900.
34. WHO. Guidelines Approved by the Guidelines Review Committee. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: World Health Organization; 2014.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
