Back to Journals » Infection and Drug Resistance » Volume 14
Magnitude of Intestinal Parasite Infection and Associated Factors Among Pregnant Women Attending Antenatal Care Service in Shewarobit Town Health Facilities, North Shoa Zone, Amhara Region, Ethiopia
Authors Dagnaw A , Sahlie M , Mulugeta H , Shine S , Bediru W, Zebene A, Weldetensay Y, Abebe AM
Received 14 September 2021
Accepted for publication 4 November 2021
Published 24 November 2021 Volume 2021:14 Pages 4921—4930
DOI https://doi.org/10.2147/IDR.S338326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Abinet Dagnaw,1 Mamush Sahlie,2 Hailemichael Mulugeta,1 Sisay Shine,1 Woinshet Bediru,1 Asmare Zebene,3 Yitaferu Weldetensay,3 Ayele Mamo Abebe4
1Department of Public Health, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia; 2KNCV Tuberculosis Foundation, Addis Abeba, Ethiopia; 3Shewarobit Health Center, North Shoa Zone, Amhara Region, Ethiopia; 4Department of Nursing, College of Health Science, Debre Berhan University, Debre Berhan, Ethiopia
Correspondence: Abinet Dagnaw Debre Berhan
Tel +251913416934
Email [email protected]
Introduction: Intestinal parasites are the most common infectious gastrointestinal parasites in developing countries including Ethiopia. Globally, it remains a public health problem by affecting 3.2 billion people, of which 10% were pregnant women. In Sub-Saharan Africa, pregnant women are the risky group next to children for this infection. This study aimed to assess the magnitude and associated factors of intestinal parasite infection among pregnant women.
Methods: Facility-based cross-sectional study was conducted among 365 pregnant women attending antenatal care service in Shewarobit town health facilities, North Shoa Zone, Amhara Region, Ethiopia. Data were collected using an interview questionnaire and laboratory microscopic stool examination from February 1, 2020, to March 30, 2020. Descriptive statistics and multivariable analyses were used to characterize the data and to identify the associated factors with the outcome variable at a p-value < 0.05, respectively.
Results: A total of 347 (95.1%) pregnant women participated in this study. The magnitude of intestinal parasite infection was 27.7% during the study period. Among the parasites, G. lamblia and S. mansoni were the most prevalent identified parasites. Pregnant mother, who did not have handwashing practice after using the toilet [AOR: 3.89, 95% CI (1.86– 8.13)], had a habit of walking on barefoot [AOR: 5.65, 95% CI (1.72, 18.56)], had uncooked food meal habit [AOR: 5.12, 95% CI (1.24, 21.14)], use of water in unimproved water source [AOR: 3.20, 95% CI (1.11– 9.24)], lack of health education [AOR: 4.08, 95% CI (2.01– 8.27)], and not dewormed [AOR: 3.09, 95% CI (2.01– 7.94)] were predictors for parasitic infection.
Conclusion: High prevalence of intestinal parasite infection is observed in pregnant women. Personal hygiene practice, health education, and water quality were factors identified as contributors to intestinal parasite infection in pregnant women. Public health measures on water and environmental sanitation, health education for intestinal parasite infection and personal hygiene practices, and early deworming are vital to reduce the intestinal parasites’ infection and assure safe pregnancy.
Keywords: pregnant women, intestinal parasitic infection, health facilities
Introduction
Intestinal Parasitic Infection (IPI) is a condition in which a parasite infects the gastrointestinal tract of humans.1 It is the most abundant and common infectious microorganism in developing countries. Globally, it remains a public health problem by affecting 3.2 billion people, of which 10% were pregnant women. In Sub-Saharan Africa, pregnant women are the most at-risk group next to children for this infection.2
Even though the infection occurred worldwide, it created a considerable public health burden among populations in low-income countries with poor hygiene and sanitation practices.3,4 Most IPI occur due to situations of poverty, poor sanitation, and poor hygiene practice in the tropical and subtropical region’s communities of sub-Saharan countries.3 In Africa, intestinal parasites have a high load and distribution in different regions of the continent and more affecting especially children and pregnant.5
Pregnancy affects the mother’s body physically, physiologically, and immunologically.6 This burden is aggravated when combined with parasite infection. IPI in pregnancy is associated with serious adverse outcomes for the mother and the unborn baby.7 Untreated mothers with drugs for intestinal parasites have a negative health impact, including anemia, electrolyte imbalance, malabsorption, premature delivery, low birth weight of the infant, and impaired lactation.6–8
About 114 studies comprising 98, 342 pregnant women from across 35 countries globally indicate prevalent intestinal parasites of helminth infection include Hookworm (19%), A. lumbricoides (17%), and T. trichiura (11%); and of protozoan infections including Blastocystis sp. (21%), E. histolytica/dispar (9%), and G. lamblia (8%).9
Sanitation practices, availability of latrine, lack of proper use of latrines, handwashing practices, water quality, barefooted, receiving deworming, use of untreated water, eating raw vegetables, and health education are reported as associated factors in a different study.10−14
Intestinal helminthic (like Hookworm) infection causes severe anemia in up to one-third of pregnant women in sub-Saharan Africa, resulting in an increased likelihood of premature births, babies with low birth weight, and impaired lactation.15,16 Additionally, protozoan (like E. histolytica and G. lamblia) infection causes bloody stool and diarrhea, which causes secondary effects of fluid loss, malabsorption, and electrolyte imbalance, which may adversely affect the nutrition status of women and the outcome of pregnancy including asphyxia, underweight neonate.7,17
Ethiopia is one of the countries where intestinal parasites are endemic. The number of people living in soil-transmitted diseases endemic areas is estimated at 81 million and living in schistosomiasis-endemic areas is estimated at 38.3 million.18 Different intervention activities have been implemented to prevent IPI in the country despite its women are the most affected group by IPI among neglected tropical diseases.18,19 Standard guidelines for antenatal care in Ethiopia emphasize that every pregnant mother should receive services including health education, physical examination, blood tests for infection screening, urine test, tetanus toxoid injections, iron folate supplements, and deworming medications.19 National Neglected Tropical Diseases master plan emphasized on children and adults as whole communities, but the 2016 Ethiopian Health and Demographic Survey report indicated only 5.7% of women dewormed during the pregnancy period.18–20
In the country, there is a paucity of information about intestinal parasite infection among pregnant women.19 The study aimed to determine the magnitude and associated factors of intestinal parasites among pregnant women attending antenatal care services.
Methods and Materials
Study Setting and Design
An institution-based cross-sectional study was conducted among three health facilities, namely, Shewarobit health center, Shewarobit district hospital, and Yifat hospital from February 1, 2020, to March 30, 2020. The facilities are providing an antenatal care service, and the town of Shewarobit is located 225 km from the nation's capital city, Addis Ababa, with an elevation of 1280 m above sea level. The town is in Shewarobit district. The district is one of the 24 districts in the North Shoa zone, Amhara region, Ethiopia.
Selected pregnant women attending an antenatal care service during the study period were included and pregnant women who had taken anti-helminthic and/or anti-protozoan drugs in the last 1 month were excluded from the study.
Sample Size Determination and Sampling Procedure
The sample size was determined using a single population formula with an assumption of 95% confidence level, 5% marginal error, and 31.5% proportion of intestinal parasites in pregnant women.13 After adding 10% for the nonresponse rate, the final sample size was 365. The sample size was allocated proportionally to health facilities based on the previous 2 months’ data of mothers enrolled in an antenatal care service. A total of 750 pregnant women were attending the service. The study participants were selected through systematic sampling techniques with an interval of 2. At each health facility, the first participant was selected by a lottery method. The remaining participants were enrolled in every second sample interval.
Data Collection Tools and Laboratory Diagnosis
Data were collected using an interviewer-administered questionnaire and laboratory stool examination. The questionnaire was adapted from CDC Water Safety Survey, UNICEF Water and Sanitation survey, 20,210, and the previously performed study questionnaires.21–23 It consists of socio-demographic characteristics, hygiene practices (availability of toilet, toilet utilization, anal cleaning materials, hand washing, cutting fingernails, eating uncooked food, water source, barefooted, disposing waste), and health service (health education and deworming status). The questionnaire was reviewed for coherence, and pretest was conducted in a similar population and study setup just to check the reliability and validity of the questionnaire using Cronbach's alpha.
Intestinal parasite infection status of the participants was examined using laboratory stool wet mount microscopic examination, and formalin-ether concentration techniques.1 A labeled, leakproof, and screw-capped container with a clean applicator stick was given to each participant. All study participants were asked to provide a sufficiently large stool sample,24 after instruction was informed. The specimen was checked based on the acceptance criteria.
Laboratory sample processing was performed by laboratory technicians and technologists. The direct wet smear was prepared through mixing matchstick head amount of fresh, unpreserved stool with a few drops of 0.85% saline and added similar amount iodine to the edge of the coverslip for color contrast to identify the protozoan cysts/oocysts. The direct smear was examined by 10x and 40x microscopic magnifications with standard procedures for the identification of helminths and protozoans. Some part of the specimen was preserved by 10% formalin for the concentration process by formol ether concentration technique to detect missed parasites, which were processed within the same day of sample collection and direct wet mount processing. Using a stick, emulsify an estimated 1 g (pea-size) of feces in about 4 mL of 10% formol water contained in a screw-cap tube, add a further 3–4 mL of 10% formol water, cap the bottle, and then mix well by shaking. Sieve emulsified feces, collected the sieved suspension in a beaker. Then, transfer the suspension to a centrifuge tube and add 3–4 mL of diethyl ether. The test tubes were mixed well and centrifuged at 1000 revolutions for 3 min. Fecal debris, ether, and formol water were removed. Finally, the sediment was mixed well and transferred to a slide covered with a covered glass, and detected under a microscope. One public health profession supervisor, three medical laboratory profession, and five midwifery participated in the data collection.
Data Quality Assurance
Before actual data collection, the data collectors and supervisors were trained about study objectives, tools, approaches, and ethical issues. Structured questionnaire was adopted from different published literatures. The questionnaire was translated from English to Amharic (local language), and reverse translation to the English language was done by language experts to check the consistency. Furthermore, the translated questionnaire and the original English questionnaire were compared and analyzed to identify discrepancies in words, meanings, and contents of the items. A pretest study was conducted at another district health facility on 5% of the total sample size before the actual data collection. Based on the results of the pretest, essential modifications to the tool and approach were done.
The supervisor collected the filled questionnaires after checking for consistency and completeness. Stool specimen was collected with appropriate instructions and properly labeled, and laboratory investigation was performed based on standard operating procedures (SOP). Before the examination of the sample, internal quality control was performed to assure the materials and reagents. Completeness and correctness of the data were checked before data entry. Data cleaning was managed through sort, frequency, and lists.
Data Processing and Analyzing
Data were entered by using Epidata software version 3.1.1 and then exported to the Statistical Package for Social Sciences (SPSS) software version 23.0 for cleaning and analysis. Descriptive statistics were used to summarize the data. Logistic regression analysis was used to see an association between intestinal parasite infection and factors. Hosmer-Lemeshow test was performed (Chi-square=7.36, Df=8, Sig=0.50) to check the model fitness and collinearity statistics were checked (Tolerance > 0.3, Variance inflation factor <3) to see the presence of multicollinearity. Bivariate analysis was performed to see the candidate variables for multivariable logistic regression, and variables with a p-value less than 0.05 were analyzed by using multivariate analysis to control confounders. Adjusted odds ratios with 95% confidence intervals were calculated and a P-value less than 0.05 was considered to be statistically significant.
Operational Definition
Accessible distance from water source: water source within 1 km distance from home.25
Barefooted: A habit of walking on without shoe-wearing.
Deworming status: Took deworming from the last 1 month to the last 1 month.
Hand washing: the practice of washing hands after toilet or before the meal by water with soap/ash.
Health educated: pregnant women who have got health education by the health profession related to the intestinal parasite infection.
Intestinal parasitic infection: include protozoan and helminth infections which hatched in the intestine and are found on stool examination.
Unimproved water sources: water source which includes unprotected dug wells, unprotected springs, and surface water (rivers, dams, lakes, ponds, streams, canals, irrigation channels).25
Result
Socio-Demographic Characteristics
From the 365 pregnant women recruited for the study, 347 (95%) pregnant women participated in the study. The mean age of the participants was 25.70 (with a standard deviation of 4.98) years old and the majority (90.2%) of the respondents were married. About one-third (33.1%) of participants’ residential areas were rural and 149 (42.9%) participants’ educational status was a primary school (Table 1).
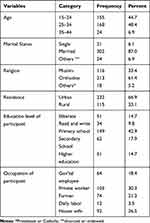 |
Table 1 Socio-Demographic Characteristics of Pregnant Women in Shewarobit Health Facilities, North Shoa, Amhara Region, Ethiopia, 2020 (N = 347) |
Hygiene Practice and Health Service
Among the participants, almost all (92.8%) had a toilet, 309 (89%) were using the toilet for defecation, and 293 (84.4%) practiced hand washing after the toilet. Few participants, 71 (20.5%), were dewormed in the last month (Table 2).
 |
Table 2 Hygiene, Environmental, and Health-Related Determinants of Intestinal Parasites Among Pregnant Women in Shewarobit Health Facilities, North Shoa, Amhara Region, Ethiopia, 2020 (N = 347) |
Magnitude of Intestinal Parasite Infection
From a total respondent, 96 (27.7%) participants were infected by any intestinal parasites. Out of 96 positive results, 56 (58.3%) were protozoans’ infections, whereas 40 (41.7%) were helminth infections, and 23 participants had been double infected. Of intestinal parasites, G. lamblia accounted the highest infection 43 (36.1%) and followed by S. mansoni that covered 27.7% of the total infections (Figure 1).
 |
Figure 1 Frequency and type of intestinal parasites infection among pregnant women in Shewarobit health facilities, North Shoa, Amhara region, Ethiopia, 2020. |
Associated Factors of Intestinal Parasite Infection
Each variable was analyzed using bivariate logistic regression. Age, marital status, distance from water source, and disposing of solid waste were variables excluded from the multivariable logistic regression due to a P≥0.05 in bivariate logistic regression, not model fitting and collinearity. Other variables with a P-value < 0.05 were a candidate for the multivariable logistic regression. Hygiene practices, eating uncooked food and health service status were significantly associated with intestinal parasite infection in the multivariable logistic regression (Table 3).
 |
Table 3 Multiple Logistic Regression for Selected Factors of Intestinal Parasite Infection Among Pregnant Women at Shewarobit Health Facilities, North Shoa, Amhara Region, Ethiopia, 2020 (N = 347) |
Discussion
This study focused on the magnitude of intestinal parasite infection and its possible associated factors among pregnant women in Shewarobit town Health facilities, North Shoa, Amhara Region, Ethiopia. A total of 96 (27.7%) [95% CI (23.1, 32.6)] pregnant women had at least one type of intestinal parasite infection. Selected hygiene practice and health service delivery status were factors associated with intestinal parasite infection.
The magnitude of intestinal parasite infection in this study was in line with the studies conducted in Bahir Dar, Ethiopia (31.7%), iratnagar, Nepal (29%).13,26 However, our finding was observed to be lower than studies in Northwest Ethiopia, 37.3%, in western Ethiopia, 43.8%, Bogota, Colombia (41%), and Venezuela (73.9%); and higher than other studies reported in Northwest Ethiopia (14.3%), North Ethiopia (17.7%), and Ghana (14.3%).1,11,27–30 This difference might be due to study area physical environment variation and study populations` socioeconomic status.4 Humidity and temperature status of any geographic location affect the viability of most parasites, ova, and cysts.32 Also, among a population with low socioeconomic status, high parasitic infection prevalence is known.3,4 Furthermore, the stool examination methods and the time of study might be contributed to the variation.
Out of all positive for IPI in this study, 56 (16.1%) were protozoans and 40 (11.6%) were helminths. G. lamblia was the leading parasite observed. This finding is in line with a study at Felege Hiwot Hospital, Amhara region, Ethiopia.13 However, this finding is in contrast with other studies in which Hookworm in western Ethiopia, Northwest Ethiopia, and Nepal; E. histolytica in Ghana and A. lumbricoides in Venezuela were reported as the predominant parasites.1,11,26,28,31 This disparity might be due to the warm climate of the current study area in the study period (24–29 Co) making a suitable setting for the survival of G. lamblia cysts in contaminated water, participants shoe-wearing habits, and socio-cultural differences between study participants.1,11,32–35
Some parasites have adverse consequences for pregnant women (causing anemia, weight loss, malabsorption) and pregnancy outcome (underweight of neonate, preterm delivery asphyxia). From these parasites, Hookworm is one and investigated in this study that accounted for 27.7% of the total infection. Of the total participants, 4.3% were infected by this parasite, which is comparable with the finding in Nepal that showed 7.9% infection of Hookworm.36 The other parasite that harms pregnant women is S. mansoni which showed 9.5% infection from total participants. This finding is similar to the review finding that S. mansoni had 8.7% prevalence.37
Some factors like hand hygiene, eating uncooked food, water source type, bared foot, health education, and deworming were significantly associated with the risk of IPI in the multivariable logistic regression.
Pregnant mothers, who did not have washing practice after using the toilet more than three times [AOR: 3.89, 95% CI (1.86–8.13)] were more likely to have IPI than respondents who had washing practice with soap and water. This finding is consistent with similar studies conducted in western and Northwest Ethiopia.1,11,13 The reason might be due to Ethiopian communities’ handwashing practice with soap and water being much lower (urban households, 28%, and rural households, 7%) and such situation might increase due to ingesting of the infectious agent in food. Proper handwashing practice is a prevention mechanism to break the chain of intestinal parasite transmission.19,38
A habit of walking barefoot increases the odds of IPI among pregnant women by 5.65 folds [AOR: 5.65, 95% CI (1.72, 18.56)]. This result was in accordance with previous studies conducted in Ethiopia.1,39 This may be due to shoe-wearing preventing the infection intensity for parasites transmitted directly through the feet.40 Participants who had uncooked food meal habits were more than two times odd for IPI [AOR: 5.12, 95% CI (1.24, 21.14)] than those who had the opposite character. A similar previous finding was reported in Ethiopia.11,39 These findings were justified by the fact of raw food like fruit and vegetables are highly contaminated from the source or in the path of transportation and that food acts as a vehicle for transporting intestinal parasites.41–44 Use of water from an improved water source increased the odds of IPI in pregnant women by 3.20 folds [AOR: 3.20, 95% CI (1.11–9.24)]. Which agreed with study report from other areas in Ethiopia.11,39 Improved water sources have a chance of contamination with intestinal parasites, ova, and cysts; and the individuals who used water from such sources might have a chance of acquiring IPI compared with the individuals who use water to improve the source.14,33,45,46
In this study, IPI was 4.08 folds higher among pregnant people in the lack of health education [AOR: 4.08, 95% CI (2.01–8.27)]. This finding was in line with previous studies conducted in Ethiopia.11,39 This might be due to the health-seeking behavior of educated pregnant women.38,47 Pregnant women who did not deworm had more than three times the probability to have intestinal parasites [AOR: 3.09, 95% CI (2.01–7.94)] when compared with those who did deworm. This was comparable to studies done in Ethiopia and Colombia.12,27 Parasite might be riding out from their body after being dewormed, and pregnant women health-seeking behavior could be due to preventive chemotherapy.47–50
This study had its strengths and limitations. The study focused on increasing the quality from data collection to analysis. However, due to financial constraints, the current study was institutional-based, restricted to cross-sectional study design, and laboratory diagnosis using less sensitivity than polymerase chain reaction (PCR) in the identification and confirmation of numerous parasites.
In conclusion, high prevalence of intestinal parasite infection is observed in pregnant women. Personal hygiene practices, uncooked food, health education, and water quality were factors identified as contributors to intestinal parasite infection in pregnant women. Public health measures on water quality, environmental sanitation, health education on intestinal parasite infection, uncooked meat and personal hygiene practices, and periodic chemotherapy are vital to reduce intestinal parasite and ensure safe pregnancy. Stool examination should be also performed for early detection and treating of infected pregnant women.
Abbreviation
AOR, Adjusted Odds Ratio; CI, Confidence Interval; IBR, Institutional Review Board; IPI, Intestinal Parasitic Infection; SOP, Standard Operating Procedure; SPSS, Statistical Package for Social Science.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
We declare that the research is governed by the Declaration of Helsinki. The study was conducted after ethical clearance was obtained from the Institutional Review Board (IRB) of the College of Health Sciences, Debre Berhan University, and the health facilities allowed to conduct the study. As verbal consent is allowed for the diagnosis, and this study was on the diagnosis process and no risk to pregnant women (participants), the IRB of the college approved the verbal consent on the IRB’28 decision as Informed Consent Version No: 16/2020 with the protocol No of 16/02/SPH. Before enrolling each participant in this study, the detailed purpose of the study was explained for each participant. They were also informed that free treatment service (if their sample becomes positive for intestinal parasites) is their benefit from this study, and as they had the full right to refuse at any time of the study. The principal investigator’s address was given for each participant to contact for any questions and ambiguity. Withdrawal from the study was clarified that it did not affect their health-care services. Finally, verbal consent was obtained from each study participant. Participants who had positive lab results were linked to a health-care provider for treatment, and they had been treated based on the guideline.
Acknowledgment
We would like to thank Debre Berhan Health Science College, Alem Ketema Enat Hospital, Shewarobit health center for supporting us with materials. Our gratitude also extended to data collectors in health facilities and all participants for their kind collaboration.
Author Contributions
All authors contributed to the data analysis, revising the article, have agreed to the journal in which the article will be submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Yesuf DA, Abdissa LT, Gerbi EA, Tola EK. Prevalence of intestinal parasitic infections and associated factors among pregnant women attending antenatal care at public health facilities in Lalo Kile district, Oromia, Western Ethiopia. BMC Res Notes. 2019;12(1):1–6. doi:10.1186/s13104-019-4781-3
2. World Health Organization WHO. Intestinal worms. [Updated March 28, 2017; cited 2019 October 20]. Available from: http://www.who.int/intestinal_worms/more/en/.
3. World Health Organization (WHO). Soil-transmitted helminth infections: key facts. [cited July 7, 2019]. Available from: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections.
4. Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infections and disease burden of soil-transmitted helminth infections in 2010. Parasit Vectors. 2014;7(1):1–9. doi:10.1186/1756-3305-7-37
5. Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412. doi:10.1371/journal.pntd.0000412
6. Tsoka-Gwegweni JM, Ntombela NP. A double load to carry: parasites and pregnancy. Southern Afr J Infect Dis. 2014;29(2):52–55. doi:10.1080/23120053.2014.11441569
7. Reynolds SJ, Robert CB, Thomas CQ. Parasitic Diseases During Pregnancy; 2008. Available from: . Accessed November 11, 2021.
8. Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: a community-based cross-sectional study. PLoS One. 2017;12(12):e0188783. doi:10.1371/journal.pone.0188783
9. Taghipour A, Ghodsian S, Jabbari M, Olfatifar M, Abdoli A, Ghaffarifar F. Global prevalence of intestinal parasitic infections and associated risk factors in pregnant women: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2021;115(5):457–470. doi:10.1093/trstmh/traa101
10. Mengist HM, Zewdie O, Belew A. Intestinal helminthic infection and anemia among pregnant women attending ante-natal care (ANC) in East Wollega, Oromia, Ethiopia. BMC Res Notes. 2017;10(1):1–9. doi:10.1186/s13104-017-2770-y
11. Hailu T, Kassa S, Abera B, Mulu W, Genanew A. Determinant factors of anemia among pregnant women attending antenatal care clinic in Northwest Ethiopia. Trop Dis Travel Med Vaccines. 2019;5(1):1–7. doi:10.1186/s40794-019-0088-6
12. Shiferaw MB, Zegeye AM, Mengistu AD. Helminth infections and practice of prevention and control measures among pregnant women attending antenatal care at Anbesame health center, Northwest Ethiopia. BMC Res Notes. 2017;10(1):1–5. doi:10.1186/s13104-017-2609-6
13. Derso A, Nibret E, Munshea A. Prevalence of intestinal parasitic infections and associated risk factors among pregnant women attending antenatal care center at Felege Hiwot Referral Hospital, northwest Ethiopia. BMC Infect Dis. 2016;16(1):1–7. doi:10.1186/s12879-016-1859-6
14. Fuhrimann S, Winkler MS, Pham-Duc P, et al. Intestinal parasite infections and associated risk factors in communities exposed to wastewater in urban and peri-urban transition zones in Hanoi, Vietnam. Parasit Vectors. 2016;9(1):1–4. doi:10.1186/s13071-016-1809-6
15. Hotez PJ. The neglected tropical diseases and the neglected infections of poverty: overview of their common features, global disease burden and distribution, new control tools, and prospects for disease elimination. Causes Impacts Negl Trop Zoonotic Dis. 2011;31:221–236.
16. Relman DA, Choffnes ER, editors. The Causes and Impacts of Neglected Tropical and Zoonotic Diseases: Opportunities for Integrated Intervention Strategies. National Academies Press; 2011.
17. Dwedar LM, ABD El-hamid AA, El-batae HE, Abdl-monem AS. Relationship between intestinal parasitic infections and pregnancy outcome in Kafer El-Shiekh Governorate, 2017. Med J Cairo Univ. 2017;85(1):185–194.
18. Federal Democratic Republic of Ethiopia Ministry of Health. National Neglected Tropical Diseases Master Plan 2015/16 – 2019/20. Addis Ababa; 2016.
19. Federal Democratic Republic of Ethiopia Ministry of Health. Demographic and Health Survey 2016. Addis Ababa, Ethiopia; 2017.
20. Federal Democratic Republic of Ethiopia Ministry of Health. National NTD Master Plan for the Control, Elimination, and Eradication of Targeted NTDs in Ethiopia. Adiss Ababa; 2012.
21. Center for Disease Control and Prevention CDC. A Guide to Conducting Household Surveys for Water Safety Plans. Vol. 2; 2008: 2017
22. United Nations Children’s Fund UNICEF. Water, Sanitation and Hygiene Household Survey Gaza. UNICEF; 2010.
23. Mahande AM, Mahande MJ. Prevalence of parasitic infections and associations with pregnancy complications and outcomes in northern Tanzania: a registry-based cross-sectional study. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-1413-6
24. Monica C. District Laboratory Practice in Tropical Countries, Ed. S. Edition. Cambridge University Press; 2009.
25. World Health Organization. Guidelines for Drinking Water Quality. World Health Organization; 1993.
26. Chaudhary M, Maharjan M. Association of anaemia with parasitic infection in pregnant women attending antenatal clinic at Koshi Zonal Hospital. Nepalese J Zool. 2014;2(1):1–2.
27. Aranzales AF, Radon K, Froeschl G, Rondón ÁM, Delius M. Prevalence and risk factors for intestinal parasitic infections in pregnant women residing in three districts of Bogotá, Colombia. BMC Public Health. 2018;18(1):1–5.
28. Rodríguez-Morales AJ, Barbella RA, Case C, et al. Intestinal parasitic infections among pregnant women in Venezuela. Infect Dis Obstet Gynecol. 2006;2006:1–5. doi:10.1155/IDOG/2006/23125
29. Alem M, Enawgaw B, Gelaw A, Kena T, Seid M, Olkeba Y. Prevalence of anemia and associated risk factors among pregnant women attending antenatal care in Azezo Health Center Gondar town, Northwest Ethiopia. J Interdiscipl Histopathol. 2013;1(3):137–144. doi:10.5455/jihp.20130122042052
30. Berhe B, Mardu F, Legese H, et al. Prevalence of anemia and associated factors among pregnant women in Adigrat General Hospital, Tigrai, northern Ethiopia, 2018. BMC Res Notes. 2019;12(1):1–6.
31. Abaka-Yawson A, Sosu SQ, Kwadzokpui PK, Afari S, Adusei S, Arko-Mensah J. Prevalence and determinants of intestinal parasitic infections among pregnant women receiving antenatal care in Kasoa Polyclinic, Ghana. J Environ Public Health. 2020;2020:1–7. doi:10.1155/2020/9315025
32. United States Environmental Protection Agency, EPA. Office of Water 4304. Giardia: Drinking water fact sheet;2000.
33. Ouattara M, N’Guéssan NA, Yapi A, N’Goran EK. Correction: prevalence and spatial distribution of Entamoeba histolytica/dispar and Giardia lamblia among Schoolchildren in Agboville Area (Côte d’Ivoire). PLoS Negl Trop Dis. 2010;4(2). doi:10.1371/annotation/a28fd8eb-b0a9-47f1-935c-7912050dc19b
34. Wasonga J, Okowa M, Kioli F. Sociocultural determinants of the adoption of safe water, sanitation, and hygiene practices in Nyakach, Kisumu County, Kenya: a descriptive qualitative study. J Anthropol. 2016;2016:1–5. doi:10.1155/2016/7434328
35. Dittmer A. Towards total sanitation: socio-cultural barriers and triggers to total sanitation in West Africa. Water Aid. 2009. Available from: https://washmatters.wateraid.org/publications/towards-total-sanitation-socio-cultural-barriers-and-triggers-to-total-sanitation-in. Accessed November 11, 2021.
36. Navitsky RC, Dreyfuss ML, Shrestha J, Khatry SK, Stoltzfus RJ, Albonico. Ancylostoma duodenale is responsible for hookworm infections among pregnant women in the rural plains of Nepal. J Parasitol. 1998;84(3):647–651. doi:10.2307/3284746
37. Adam I, ALhabardi NA, Al-Wutayd O, et al. Prevalence of schistosomiasis and its association with anemia among pregnant women: a systematic review and meta-analysis. Parasites Vectors. 2021;14:133. doi:10.1186/s13071-021-04642-4
38. Hashi A, Kumie A, Gasana J. Hand washing with soap and WASH educational intervention reduces under-five childhood diarrhoea incidence in Jigjiga District, Eastern Ethiopia: a community-based cluster randomized controlled trial. Prev Med Rep. 2017;6:361–368. doi:10.1016/j.pmedr.2017.04.011
39. Feleke BE, Jember TH. Prevalence of helminthic infections and determinant factors among pregnant women in Mecha district, Northwest Ethiopia: a cross-sectional study. BMC Infect Dis. 2018;18(1):1–6. doi:10.1186/s12879-018-3291-6
40. Paige SB, Friant S, Clech L, et al. Combined footwear with public health iconography to prevent soil-transmitted helminth infections. Am J Trop Med Hyg. 2017;96(1):205–213. doi:10.4269/ajtmh.15-0910
41. Tefera T, Biruksew A, Mekonnen Z, Eshetu T. Parasitic contamination of fruits and vegetables collected from selected local markets of Jimma Town, Southwest Ethiopia. Int Scholarly Res Notices. 2014;2014. doi:10.1155/2014/382715
42. Alemu G, Nega M, Alemu M. Parasitic contamination of fruits and vegetables collected from local markets of Bahir Dar City, northwest Ethiopia. Res Rep Trop Med. 2020;11:17. doi:10.2147/RRTM.S244737
43. Al-Binali AM, Bello CS, El-Shewy K, Abdulla SE. The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia. Saudi Med J. 2006;27(5):613–616.
44. Omowaye OS, Audu PA. Parasites contamination and distribution on fruits and vegetables in Kogi, Nigeria. Cibtech J BioProtocols. 2012;1(1):44–47.
45. Erismann S, Diagbouga S, Odermatt P, et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasit Vectors. 2016;9(1):1–4. doi:10.1186/s13071-016-1835-4
46. Clasen TF, Alexander KT, Sinclair D, et al. Interventions to improve water quality for preventing diarrhoea. Cochrane Database Syst Rev. 2015; 10. doi:10.1002/14651858.CD004794.pub3
47. Li X, Yang H, Wang H, Liu X. Effect of health education on healthcare-seeking behavior of migrant workers in China. Int J Environ Res Public Health. 2020;17(7):2344. doi:10.3390/ijerph17072344
48. World Health Organization. Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups. World Health Organization; 2017.
49. Savioli L, Albonico M, Daumerie D, et al. Review of the 2017 WHO guideline: preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. An opportunity lost in translation. PLoS Negl Trop Dis. 2018;12(4):e0006296. doi:10.1371/journal.pntd.0006296
50. Bennett A, Eisele T, Keating J, Yukich J Global trends in care seeking and access to diagnosis and treatment of childhood illnesses. DHS Working Papers. 2015: 116.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
