Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 13
Magnitude of Anemia and Undernutrition Among Primary School Children in a Setting of Mass Deworming in Central Ethiopia
Authors Wordofa M , Abera D, Mesfin A, Desta K , Taye B, Tsegaye A
Received 29 July 2022
Accepted for publication 22 December 2022
Published 30 December 2022 Volume 2022:13 Pages 385—400
DOI https://doi.org/10.2147/PHMT.S381467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Moges Wordofa,1 Dessie Abera,1 Abiyot Mesfin,2 Kassu Desta,1 Bineyam Taye,3 Aster Tsegaye1
1Addis Ababa University, College of Health Sciences, Department of Medical Laboratory Sciences, Addis Ababa, Ethiopia; 2Pyramid Pharma Plc, Addis Ababa, Ethiopia; 3Biology Department, Colgate University, Hamilton, NY, USA
Correspondence: Moges Wordofa, Addis Ababa University, College of Health Sciences, Department of Medical Laboratory Sciences, Addis Ababa, Ethiopia, Email [email protected]
Background: Undernutrition and anemia in children continue to be a public health problem in developing countries. Besides, intestinal parasitic infection among school children is common in developing countries. World Health Organization (WHO) recommends periodic deworming of children who live in endemic areas. The aim of this study was to determine the magnitude of anemia and undernutrition among school children in a setting of mass deworming.
Methods: A cross-sectional study was conducted among 510 school children aged 5– 14 years from three randomly selected governmental schools in Sululta town, central Ethiopia. Socio-demographic variables were assessed using interviewer administered structured questionnaire. Anthropometric data were obtained and analyzed using WHO Anthroplusv1.0.4. Venous blood samples were collected using EDTA vacutainers. Hemoglobin level was determined by Sysmex KX-21N automated hematology analyzer and stool samples were processed using direct wet mount, formol-ether concentration and Kato-Katz methods. Data were entered and analyzed using SPSS version 21. Logistic regression analysis was performed to determine the association of anemia and undernutrition with the independent variables.
Results: The overall magnitude of anemia was 3.7%. Among anemic individuals, 84.2% and 15.8% of participants had mild and moderate anemia, respectively. The magnitude of stunting and thinness was 16.9% and 10.8%, respectively. Of them, 18.6% of stunting and 14.5% of thinness were severe. Of factors related to undernutrition, children from large families (≥ 5) were less likely to be stunted (AOR=0.38, 95% CI=0.2– 0.7, P=0.002) compared to small families.
Conclusion: The magnitude of anemia in the study area was considered as an insignificant public health problem and none of the socio-demographic variables of participants were significantly associated with anemia and likewise with undernutrition except for family size. Further studies are required to clearly understand the impact of mass deworming on the magnitude of anemia and undernutrition.
Keywords: anemia, undernutrition, deworming, school children
Introduction
Anemia is functionally defined as insufficient red blood cell mass to adequately deliver oxygen to peripheral tissue to meet the body’s physiologic need that varies according to several factors including person’s age, gender, altitude and physiological state.1
Despite many efforts, anemia is still a global public health concern with a major impact on human health, social as well as economic development. Even if the problem was common in all age groups, pregnant women and young children were the most vulnerable.2 Globally, it affects 1.62 billion people and 305 million school age children.3 In 2011, World Health Organization (WHO) stated that South-East Asia, Eastern Mediterranean and African regions had the highest prevalence of anemia and severe anemia was the highest in African regions, with 3.6% children affected.4 In 2013, a community study in southwest Ethiopia showed the magnitude of anemia was 43.7% and considered a severe public health problem.5
Anemia has adverse effects; mainly on social, economic and health related aspect of school children. Children with anemia have lower school performance with poor psychomotor development, growth retardation, impaired immune system with increased risk of exposure to comorbid disease as well as negatively affected behavioral development. In addition, anemia is also responsible for low economic development by reducing working capacity as well as impairing physical, mental and social health of children, and low hemoglobin concentration in children is related to a higher risk of death later.6,7
The causes of anemia are diverse, multidimensional, and interrelated. A systematic analysis of the global burden of anemia from 1990 to 2010 revealed iron deficiency and parasitic infection such as Hookworm, Schistosomiasis and malaria as the most common causes in both sexes.8 Deficiency of other key micronutrients such as folic acid, vitamin B12 and high burden of parasitic infection are also associated with anemia.5,9
Malnutrition continues to be a public health concern among school children in developing countries such as South East Asia and Africa with an adverse effect on their physical and mental development which then has a paramount effect on their school performance.10,11 Globally, there were 165 million stunted (low height-for-age), 99 million underweight (low weight-for-age), and 51 million wasted (low weight-for-height)12 school children. In Ethiopia, undernutrition was associated with 24% child mortality; with an estimated 379,000 deaths within the period 2004–2009.13
The cause of children’s malnutrition is multifactorial. Generally, the main attributes are infections and inadequate intake and/or availability of nutritious food.14 Anthropometric measurements such as height and weight are considered very useful in determining the nutritional status of a community, particularly school children.15
Infection with intestinal parasites is common among Ethiopian school children and the magnitude in some parts of the country goes up to 83%.16 WHO advocates periodic deworming of school children in areas where helminths infection is common. This will enhance nutritional status and cognitive skills as well as increase hemoglobin level thereby improving health, intellect and school performance.17 Thus, the country launched a mass deworming program in November, 2015.18 However, there are limited data on whether the deworming program has brought a change in reducing the prevalence of helminths as well as related problems such as anemia and undernutrition among school children as expected, particularly in the study area. There are only a few studies in a developing country with a contradictory finding on the effect of school deworming on the prevalence of anemia among school children. Some studies have reported improvement in child growth, anemia, cognitive skills, and school attendance following deworming program, while others indicated lack of a significant impact of periodic deworming on the prevalence of anemia among school children.19 Thus, the current study provides information on the magnitude of anemia and undernutrition in the setting of mass deworming. The study also aimed to determine the associated risk factors and thus, may provide input for policy makers and other stake holders to design effective strategies to address those problems in the community.
Materials and Methods
Study Design, and Setting
A cross-sectional study was conducted from October-December, 2017 in Sululta town among 510 school children. The town is located in Oromia region, 21 Km North West of the capital city, Addis Ababa, Ethiopia. The site has an altitude of 2450 meters above sea level with average temperature ranging from 15°C to 18°C. The total population of the town is estimated to be 49,000. The town is semi-urban with a portion of its population relying on agricultural activities while larger proportions are also working as daily laborers.
Source and Study Population
The source population was students in all governmental primary schools in Sululta town during the study period, while school children aged 5–14 years in the selected primary public schools were the study population.
Eligibility Criteria
School children under the age of fifteen who volunteered and whose parents/guardians gave consent to participate, were included in the study. On the other hand, children under five years, currently taking iron supplement drugs (ferrous sulphate, ferrous gluconate and Heam up syrup) as well as drugs for H. Pylori infection (triple therapy: Amoxicillin, Omeprazole and metronidazole) and children with hematological malignancies were also excluded.
Study Variables
Magnitude of anemia and undernutrition were our dependent variables while socio-demographic characteristics, dietary information, H. Pylori and intestinal parasite infection, deworming status were our independent variables.
Sampling Technique and Sample Size Determination
There was a total of 6922 students in nine public primary schools. Three schools (Laga Dima, Wasarbi and Abdi Boru) were randomly selected from the nine schools in the town. We used a proportional allocation method to select participants from each school. To recruit participants from each class, the students were stratified based on their educational level (KG to grade 8) and systematic random sampling was used to select student from each class by using a class roster as a sampling frame. Accordingly, we selected 203, 109 and 198 students who fulfilled inclusion criteria from Laga dima, Abdi boru and Wasarbi primary schools, respectively. In this study, participants 5–14 years of age were considered as school children based on WHO age category criteria. Prior to data collection, we went to local health authorities and to each school to explain the purpose of the study. Investigators, the school principal and teachers explained the objective of the study to parents/legal guardians of selected children.
Single population proportion was used to determine the sample size(n). By taking 43.7% prevalence of anemia from a study in Jimma, southwest of Ethiopia,5 with margin of error(d)=0.05 and 95% CI, the calculated sample size was 378. Considering the possibility of 10% non-compliance, the minimum required sample size was 416. However, we enrolled 510 participants.
As part of the national mass deworming program, 500 mg of Mebendazole was given at schools by trained health extension workers to school children to treat soil-helminths (eg, Ascariasis, trichuriasis and hookworm disease). The mass deworming program was the first round at the study site. The data on deworming status of participants were obtained from school records and Sululta health bureau. Samples were collected 2–3 months after program commencement.
Data Collection
Socio-demographic data of participants were obtained using interviewer administered questionnaire. The questionnaire was designed in English and translated into local language (Amharic and Afan Oromo) for better understanding and to obtain reliable information. Before the actual data collection, the translated questionnaire was pretested among non-participants at the study site. Dietary pattern was assessed using food frequency questionnaire (FFQ) that was developed for this study based on food based dietary guidelines (FBDGs) developed in Ethiopia.20 The food items were categorized into eight groups (root and tubers, legumes, cereals, vegetables, fruits, milk and milk products and eggs) based on a preliminary assessment of the types of food commonly consumed in the study area. Data regarding family background were obtained from parents/legal guardians while information about types and consumption frequency of food was provided by school children.
Anthropometric Measurements
The measured anthropometric parameters were height and weight. Body weight was measured to an approximation of 0.1kg using digital calibrated weight scale and height was measured to nearest 0.1cm using measuring device (wooden device with sliding bar at the top and fixed at the base). The standard measurements were carried out on site while the children were barefoot and wearing light clothes.21 Anthropometric indices such as height for age z-score (HAZ) and body mass index for the age z-score (BAZ) were calculated using WHO Anthroplusv1.0.4 and children were categorized as, stunted: - HAZ< −2SD of WHO growth standard median; severely stunted: -HAZ< −3SD of WHO growth standard median; thinned: - BAZ<-2SD of WHO growth standard median, and severely thinned: - BAZ<-3SD of WHO growth standard median.14
Blood and Stool Collection and Analysis
Five milliliters (mL) of whole blood were collected using a vacutainer test tube containing ethylene diamine tetra-acetic acid (EDTA) for complete blood count (CBC) and analyzed with Sysmex Kx-21 automated analyzer (Sysmex Corporation, Kobe, Japan). In addition, about 4 mL of whole blood was drawn by trained laboratory personnel using serum separator test tube for C-reactive protein (CRP) qualitative and semi-quantitative test. School children were provided with a small leak-proof plastic cup and clean wooden applicator stick and instructed to bring sufficient stool sample free from contamination with urine and other materials. Samples were then processed immediately for the direct wet mount examination. In addition, on site kato-katz microscopic analysis was performed to determine the intensity of helminths infection by counting the number of helminths eggs excreted in feces (expressed egg per gram, epg.) and using WHO threshold value to report the intensity as light, moderate and heavy infection for each parasite.22 Moreover, H. Pylori stool antigen test was performed. The remaining stool sample was preserved using acetate-acetic acid-formalin (SAF) and transported to the Medical Laboratory Science department of Addis Ababa University and analyzed using formol-ether concentration technique for further parasitological examination.
Definition of Anemia
Before setting hemoglobin cut-off value to define anemia, adjustment for altitude is mandatory to consider reduction in oxygen saturation of blood. Accordingly, the following formula was used to adjust hemoglobin (Hgb) to the cut-off value.
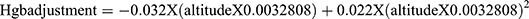 .23 Since the study site is at an altitude of 2450m above sea level, the result of hemoglobin adjustment based on the above formula is 1.16. Thus, 1.16 value was added to hemoglobin cut-off value of 11.5g/dl for 5–11 years and 12 g/dl for 12–14 years.1 After adjusting for the altitude, school children with hemoglobin value below 12.66 g/dl for 5–11 years and those with value less than 13.16 g/dl for aged 12–14 years were considered as anemic. Regarding severity, Hgb value of 12.16–12.6 g/dl for school children aged 5–11 years and 12.16–13.16 g/dl for the age 12–14 years were categorized as mild whereas, 9.16–12.15g/dl and lower than 9.16g/dl for children aged 5–14 years were considered as moderate and severe anemia, respectively.
.23 Since the study site is at an altitude of 2450m above sea level, the result of hemoglobin adjustment based on the above formula is 1.16. Thus, 1.16 value was added to hemoglobin cut-off value of 11.5g/dl for 5–11 years and 12 g/dl for 12–14 years.1 After adjusting for the altitude, school children with hemoglobin value below 12.66 g/dl for 5–11 years and those with value less than 13.16 g/dl for aged 12–14 years were considered as anemic. Regarding severity, Hgb value of 12.16–12.6 g/dl for school children aged 5–11 years and 12.16–13.16 g/dl for the age 12–14 years were categorized as mild whereas, 9.16–12.15g/dl and lower than 9.16g/dl for children aged 5–14 years were considered as moderate and severe anemia, respectively.
Statistical Methodology
The data were entered and analyzed by SPSS version 21. Hosmer-Lemeshow test and Omnibus test model coefficient were used to assess the fitness of model for logistic regression. The normality of data for was assessed by Kolmogorov–Smirnov and Shapiro-Wilk. Descriptive statistics were used to determine frequency of socio-demographic variables. Bivariate logistic regression was performed to determine the association of anemia and undernutrition with the independent variables. Multivariate logistic regression analysis was done for those variables with P<0.25 in bivariate logistic regression to identify the independent predictors of anemia and undernutrition and the cut off < 0.25 is supported by literature.24,25 P-value less than 0.05 was considered as statistically significant. The strength and the direction of association were determined by an odds ratio (OR) and 95% confidence interval (CI). The anthropometric data were entered and analyzed by WHO Anthroplusv1.0.4 software.
Ethical Clearance
This study was approved by the Research and Ethics Review Committee of the Department of Medical Laboratory Science, College of Health Science, Addis Ababa University. Written informed consent was obtained from all participants and/or their legally authorized representative after they were clearly briefed and understood the objective of the study. Children aged 12–14 years were also requested to give assent and informed of their right to refuse to participate or withdraw from the study at any time. Confidentiality of the data was also maintained throughout the study. Children who were found to be infected with intestinal parasites were treated with appropriate dose of anthelmintic drugs and those with abnormal result of hematological parameters were referred to nearby health facility for possible intervention.
Results
Demographic Characteristics of Children and Their Parents/Guardians
A total of 510 school children aged 5–14 years were involved in this study. The mean age of participants was 10.86 (±2.5 SD) and 53.9% were in the age group of 5–11 years. About 60.2% were females and 82.7% of participants were dewormed. Most of the school children were urban dwellers (76.7%) and from small size family of <5 (65.9%). Regarding maternal occupation and education, 59.6% were housewives and 52.9% could not read and write. On the other hand, 25.7% of fathers were daily laborers and over one-third (35.3%) of them could only read and write (Table 1).
 |
Table 1 Socio-Demographic Characteristics of School Children Aged 5–14 Years and Their Parents/Guardians in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
Anthropometric Characteristics of Study Participants
The mean HAZ and BAZ of school children were −1.11(±0.98 SD) and −0.88(±1.0 SD), respectively. According to WHO growth reference standards, taking <-2SD as a cut-off point,15,26 the overall magnitude of stunting and thinness among school children were 16.9% and 10.8%, respectively. With regard to severity, 18.6% of stunting and 14.5% of thinness were severe (Table 2).
 |
Table 2 Magnitude and Severity of Undernutrition Among School Children by Age Group in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
Dietary Pattern
Regarding the type and frequency of food consumed, 51.4% and 75.5% of school children ate legumes and cereals, respectively once a day; while 39.8%, 60.2%, 72.1%, 75% and 82.9% of them occasionally ate roots and tubers, vegetables, milk and milk products, eggs and meat, respectively (Table 3).
 |
Table 3 Food Type and Consumption Frequency Among School Children in Three Selected Public Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
Magnitude and Severity of Anemia
The overall magnitude of anemia as defined by Hgb<12.66 g/dl for the age 5–11 years and Hgb <13.16g/dl for the age 12–14 years was 3.7%. The magnitude of anemia was comparable between the age groups (3.6% for 5–11 years and 3.8% for the 12–14 years age), males (3.4%) and females (3.9%) as well as between dewormed (3.6%) and non-dewormed (4.5%) participants. Of anemic individuals, 84.2% had mild anemia while 15.8% were moderate. There was no severe anemia among study participants during the study period (Table 4).
 |
Table 4 Magnitude of Anemia and Its Severity Among School Children by Age Group, Sex and Deworming Status in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
Association of Socio-Demographic Variables with Anemia
Binary logistic regression analysis showed that none of the socio-demographic characteristics of children and parents (age, sex and grade level of children, parent occupation and educational status, family size and residence) was associated with anemia (Table 5).
 |
Table 5 Socio-Demographic Variables of School Children and Their Parents/Guardians as Predictors of Anemia in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017. (n=510) |
Association of Undernutrition and Intestinal Parasites with Anemia
The overall magnitude of helminthes infection was 15.7%. The magnitude of anemia among helminthes infected participants was 5.5%; while it was 3.5% among non-infected school children. The intensity of helminthic infection was assessed using Kato-Katz technique and there were 1.8%, 2.7%, and 1.2% mild infections of hookworm,Ascaris lumbricoides and Trichuris trichuria, respectively. There was no moderate and heavy helminths infection in the study area. Individuals with mild hookworm infection had 3.4 times increased odds of being anemic. However, the association was not statistically significant (P=0.27) (Table 6).
 |
Table 6 The Association of Anemia with Helminthes Infection Among School Children in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
In this study, only 4.7% of stunted and 5.5% of thinned participants had anemia. The association of anemia with stunting (P= 0.62) and thinness (P=0.48) was statistically insignificant (Table 7).
Association of Deworming Status with Anemia and Undernutrition
While assessing the study sites, some students were found not to be part of the deworming program for various reasons. The magnitude of anemia and undernutrition was compared between dewormed and non-dewormed study participants in a logistic regression analysis. The result indicated that deworming status was not associated with anemia (P=0.66), thinness (P=0.85), and stunting (P=0.36) (Table 8).
 |
Table 8 The Association of Deworming Status with Anemia and Undernutrition Among School Children in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017 (n=510) |
Factors Associated with Undernutrition
In binary logistic regression analysis, children whose fathers were merchants (OR= 2.3, 95% CI=1.05–4.99, P=0.038) and illiterate (OR=8.4, 95% CI=1.09–65.5, P=0.04) had 2.3 and 8.4-fold increased odds of being stunted, respectively. Family size was also significantly (P=0.001) associated with stunting. Children from a small family size (<5) were more likely to be stunted than those with a large family (≥5). On the other hand, intestinal helminths (P=0.014) were significantly associated with thinness (Table 9).
In the multivariable regression analysis, children whose fathers were illiterate had 7.2 times odds of being stunted but the association was marginally significant (P=0.07). However, family size was the only independent predictor of stunting with a statistically significant level (P=0.002) when adjusted for all the variables stated in Tables 9 and 10.
 |
Table 10 Multivariable Regression Analysis of Associated Risk Factors for Stunting Among School Children in Selected Governmental Schools in Sululta Town, Oromia Region, Ethiopia, 2017(n=510) |
Discussion
This cross-sectional study was aimed at determining the magnitude of anemia and undernutrition among selected governmental schools in a setting of mass deworming in Sululta town, northwest of Addis Ababa. It also investigated predictors of anemia and undernutrition. Evaluation of the magnitude of anemia and undernutrition among children is one of the most important health indicators in the community and thus, considered vital for design and implementation of possible interventions.
The study revealed that the overall magnitude of anemia among school children was 3.7% when adjusted for altitude. The magnitude observed in the current study is considered as insignificant (not in the public health problem category) based on WHO public health problem category. According to WHO, the magnitude of anemia as a public health problem is categorized as follows: <5%, no public health problem; 5–19.9%, mild public health problem; 20–39.9%, moderate public health problem; ≥40%, severe public health problem.2
The magnitude of anemia in this study is lower compared to a study by Hall et al, 2008, who in their nutrition and health survey in Ethiopian school children reported 9.8%; when adjusted for altitude.27 The anemia in their case was categorized as a mild public health problem. However, relatively high magnitude in the moderate and severe public health significance range (27.1% to 43.7%) was observed in other related studies in Ethiopia by Mesfin et al, 2012;28 Assefa et al, 2011;29 Desalegn et al, 20145 and neighboring African countries like Kenya, 28.8%,30 and Uganda 37.7%.31 It is also lower than reports from Nepal, 37.9%32 and Malaysia, 26.2%.33 The difference in socio-economic factors, health-related awareness, dietary practice, and sample size and anemia assessment methods could account for the discrepancies.
Low prevalence with mild intensity of parasite infection as well as relatively high consumption of legumes (51.4% participants ate once a day while 23.1% of them ate two/three times a week) could explain the very low magnitude of anemia observed in this study; while Mesfin et al28 revealed irregular consumption of legumes with increased risk of anemia. Phytoferritin iron from legumes serves as an excellent source of iron absorption compared to other non-heme iron sources.34
When anemia was categorized by severity based on hemoglobin levels, 84.2% and 15.8% of anemic participants had mild and moderate anemia, respectively. No severe anemia (Hgb<9.6g/dl) was observed in this study. However, unlike the current finding, Desalegn et al5 reported 15.6% severe anemia, which was due to high burden of parasitic infection and dietary deficiency in their study.
Of the factors associated with anemia, in contrast to other studies which showed significant association of anemia among school children with age groups, father’s education level (Mesfin et al, 2012), mother’s education level and average monthly income (Assefa et al, 2011) as well as consumption of less than two meals per day (Turyashemererwa et al),28,29,31 the current study indicated that none of the socio-demographic characteristics of children and parents were associated with anemia. Similarly, a lack of association was also observed in a study by Alelign et al, 2010.35 This could be due to homogeneity of respondents’ socio-economic characteristics in this study and differences in lifestyle, culture and environmental condition which contribute to the difference in risk factors in different communities.
Similar to a study by Assefa et al,29 the magnitude of anemia in this study among stunted participants was low (4.7%) with no significant association. Only 5.5% of thinned participants had anemia; which is contrary to Assefa et al’s report of 59.1% with a significant association. Although nutritional factors are thought to be the most important contributing factors to anemia in children, the exact contribution to the risk of anemia is not well established and varies with level of infection and diet quality.
The current study also showed similar magnitude of anemia between helminths infected (5.0%) and non-infected (3.5%) participants with insignificant association. However, Ngui et al indicated a significant association of anemia with Trichuris trichuria and Ascaris lumbricoides.33 Alelign et al also illustrated that the risk of anemia was approximately nine times higher in children who were infected with hookworm compared to children who were not infected with any helminthes species.35 A very low intensity of infection in this study might partly explain the absence of association between anemia and parasitic infection.
Nutritional status of children is a good gauge to assess undernutrition in a community since children are vulnerable to adverse environment and respond rapidly to dietary change.15 In the population investigated, the overall magnitude of stunting (16.9%) and thinness (10.8%) was low. The finding was comparable with other studies by Senbanjo et al, 201136 and Mukharje et al, 2008.37 However, compared to the current study, Mohammed et al38 reported relatively higher magnitude of thinness (23.1%) and lower magnitude of stunting (7.1%). In Ethiopia, the majority of malnourished children live in a rural area.39 A twelve-months longitudinal study in rural Bangladesh demonstrated the effect of mild intensity helminth infection as insignificant factor in poor growth status.40 Thus, relatively lower magnitude of stunting and thinness observed in the current study could be due to urban (76.7% of participants were urban dwellers) nature of study area as well as low burden of parasitic infection.
Of the factors associated with undernutrition, children from small size family (<5) were more likely to be stunted compared to large family. The current finding is consistent with a result obtained by Turyashemereraw et al.31 It is, however, expected that children from smaller families have a better chance of having a balanced diet and thus, a lower magnitude of undernutrition.37,41 According to resource dilution hypothesis, large family size will be related to higher cost of caring for children, reducing the parent probability of equal resource distribution. In addition, hygiene hypothesis also suggests that, as family size increases, the likely occurrence of health problems such as infectious diseases also increases, which in turn negatively affects child nutrition.42,43 Hence, the discrepancy cannot be explained and further studies may be required to explore this. However, the assumption that parents are the only family income source may not be the case and children from the extended family may not rely only on their parents. Besides, lower diet quality might not necessarily be due to lack of money or income, but also, lack of adequate knowledge of nutrition could contribute to this finding. It is also possible that Ethiopians’ tradition of dining together may encourage children to eat and thus, may favor better nutritional status in the family.
In the current study, socio-demographic characteristics of parents were not significantly associated with stunting and thinness of the school children. The finding is in line with a study by Turyashemereraw et al31 and Alelign et al.35 However, a low educational level of mothers was an important determinant of children’s undernutrition in many studies.34,35 As mothers’ education level increases, so does her financial contribution to the total family income. This empowers women to make a decision to improve nutrition and health of their children. With the current finding, even though over half of mothers were illiterate, they probably have good knowledge on child nutrition and besides, these mothers are most likely to be housewives (considering that 59.6% were housewives in the study) with the opportunity to stay at home and care for their children; thus, reducing the risk of undernutrition.41
As it was observed in this study, helminthes infection was not associated with stunting and thinness. The intensity of helminthes infection was mild. As a result, the infection may not have had a significant impact on the nutritional status of children. The finding is consistent with Alelign et al’s work.35
The magnitude of thinness and anemia was similar between dewormed and non-dewormed study groups. However, a relatively high magnitude of stunting was observed in dewormed (90.7%) compared to non-dewormed (9.3%) study groups. Nevertheless, no significant association of deworming with anemia and undernutrition was reported after two months of school deworming. The finding is inconsistent with Yimam et al.44 Contrary to the current study, a high prevalence and intensity of helminthes infection in their finding could have contributed to pronounced effect of anthelmintic treatment after one month in those with low hemoglobin value at baseline. In addition, selective treatment of only infected individuals in their study could explain the discrepant finding.
A national helminths control program study in Uganda among school children aged 6–14 years showed a decrement in parasitic infection and increment in hemoglobin concentration.45 As opposed to this study, the longitudinal nature of their study and two years follow-up as well as a combined treatment with albendazole and praziquantel could be responsible for the different outcome. Similarly, Stolzus et al46 observed a significant effect of deworming in the prevention of moderate to severe anemia in children with heavy hookworm infection at baseline.
Regarding the effect of deworming on undernutrition, our result is consistent with Watkins et al.47 Even though the burden of Ascaris was high in their study, a shorter follow-up period (six months) and less frequent deworming could have contributed to a relatively lower impact of albendazole on growth status of Guatemalan school children. On the other hand, only one round of deworming and two months of follow-up in the current study could possibly elucidate the lack of significant impact of intervention on undernutrition. Our result is also in line with a randomized controlled trial in Peru by Joseph et al, 2015.48 A low prevalence and intensity of helminths infection in both studies could explain the consistency of the results and would make the effect of deworming less evident. On the other hand, the current finding contradicts a double blind randomized controlled trial in urban slum of India by Sur et al 2002, which indicated the significant impact of albendazole on growth and also induction of diarrhea after three, six and nine months of deworming.49
WHO recommends periodic deworming as a means of controlling morbidity from helminths infection which indirectly improves nutritional status through maintaining low level of parasitic infections in childhood. As discussed, both supporting and contradicting evidence is available from different countries employing different study designs.
As a limitation, the cross-sectional nature of the study makes it difficult to establish cause-effect relationships between study variables as well as anemia because micronutrient deficiency such as iron, folate and vitamin B12 deficiency were not assessed.
Conclusion and Recommendation
The magnitude of anemia in the study area was considered an insignificant public health problem. None of the socio-economic variables were associated with anemia and likewise with undernutrition except for family size. The magnitude of anemia was comparable between dewormed and non-dewormed individuals. Deworming program alone may not bring a significant change in a short period of time in terms of reducing the magnitude of anemia and undernutrition. Rather, integration of iron supplement and nutrition program along with frequent deworming could drastically improve children’s nutritional status and anemia. Further studies are required to clearly understand the impact of mass deworming and different socio-economic factors on anemia and undernutrition.
Data Sharing Statement
All the data-sets used or analyzed during the current study are available in the manuscript.
Ethics Approval and Consent to Participate
Ethical clearance was obtained from Addis Ababa University, College of Health Science, Department of Medical Laboratory Science, Research and Ethics Review Committee. Written informed consent was obtained from all participants and/or their legally authorized representative after they were clearly briefed and understood the objective of the study. Children aged 12-14 years were also requested to give assent and all the methods were performed in accordance with the principles of the Helsinki II Declaration.
Acknowledgment
The authors would like to thank study participants for their willingness to take part in the study and school teachers for their support. Our sincere thanks go to Sululta district education and health bureau as well as the schools involved and their staff for their cooperation.
Funding
This study was financially supported by Colgate University research council and Addis Ababa University, College of Health Science, department of Medical Laboratory Science. The funders have no role in study design, data collection and analysis, decision to publish or prepare manuscript.
Disclosure
The authors declared that they have no competing interest. The manuscript was prepared from the primary author’s (Moges Wordofa) thesis which is uploaded on Addis Ababa University institutional repository and available on http://etd.aau.edu.et/bitstream/handle/123456789/12998/Moges%20Wordofa.pdf.
References
1. World Health Organization. Hemoglobin Concentrations for the Diagnosis of Anemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011.
2. United Nations Children’s Fund/United Nations University/WHO: Iron deficiency anemia. Assessment, Prevention and Control. A Guide for Program Managers (WHO/NHD/01.3). Geneva: World Health Organization; 2001:15–31.
3. Benoist B, McLean E, Cogswell M, Egli I, Wojdyla D. Worldwide Prevalence of Anemia 1993–2005. World Health Organization Global Database on Anemia. Geneva: World Health Organization; 2008:7–13.
4. World Health Organization. The Global Prevalence of Anemia in 2011. Geneva: World Health Organization; 2015.
5. Desalegn A, Mossie A, Gedefaw L, Schooling CM. Nutritional iron deficiency anemia: magnitude and its predictors among school age children, southwest Ethiopia: a community based cross-sectional study. PLoS One. 2014;9(12):12. doi:10.1371/journal.pone.0114059
6. Dary O, Hurrell R. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization, Food and Agricultural Organization of the United Nations; 2006.
7. Scott SP, Chen-Edinboro LP, Caulfield LE, Murray-Kolb LE. The impact of anemia on child mortality: an updated review. Nutrients. 2014;6(12):5915–5932. doi:10.3390/nu6125915
8. Kassebaum JN, Regan M, Brooker JS, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. doi:10.1182/blood-2013-06-508325
9. Ciesla B. Hematology in Practice. Philadelphia: FA Devis Company; 2007.
10. Best C, Neufingerl N, Geel VL, Briel DVT, Osendarp S. The nutritional status of school-aged children: why should we care? Food Nutr Bull. 2010;31(3):400–417. doi:10.1177/156482651003100303
11. Perignon M, Fiorentino M, Kuong K. Stunting, poor iron status and parasite infection are significant risk factors for lower cognitive performance in Cambodian school-aged children. PLoS One. 2014;9(11):e112605. doi:10.1371/journal.pone.0112605
12. Black ER, Victora GC, Walker PS, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi:10.1016/S0140-6736(13)60937-X
13. Policy and practice information for action. Federal democratic republic of Ethiopia ministry of health. Q Health Bulletin. 2014;6:27–35.
14. Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66(2):464–477. doi:10.1093/ajcn/66.2.464S
15. CDC and World Food Program (WFP). Manual: measuring and interpreting malnutrition and mortality; 2005.
16. Mengistu L, Berhanu E. Prevalence of intestinal parasites among schoolchildren in a rural area close to the southeast of Lake Langano, Ethiopia. Ethiop J Health Dev. 2004;18:116–120.
17. WHO Expert Committee on the Control of Schistosomiasis. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organization; 2002. Available from: https://apps.who.int/iris/handle/10665/42588.
18. Ending neglected diseases. Ethiopia’s national school-based deworming program to treat 16.5 million children. Available from: http://www.end.org/blogs/engagingnoteworthy-dialogue.
19. Girum T, Wasie A. The effect of deworming school children on anemia prevalence: a systematic review and meta-analysis. Open Nurs J. 2018;12:155–161. doi:10.2174/1874434601812010155
20. Bekele TH, deVries JJHM, Trijsburg L, et al. Methodology for developing and evaluating food-based dietary guidelines and a healthy eating index for Ethiopia: a study protocol. BMJ Open. 2019;9(7):e027846. doi:10.1136/bmjopen-2018-027846
21. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. National Center for health statistics. Vital Health Stat. 2012;11(252):1.
22. World Health Organization. Elimination of Soil-Transmitted Helminthiasis as Public Health Problem in Children. Progress Report 2001–2010 and Strategic Plan 2011–2020. World Health Organization; 2012.
23. Sullivan KM, Mei Z, Grummer-Strawn L, Parvanta I. Hemoglobin adjustments to define anemia. Trop Med Int Health. 2008;13:1267–1271. doi:10.1111/j.1365-3156.2008.02143.x
24. Bendal R, Afifi A. Comparison of stopping rules in forward regression. J Am Stat Assoc. 1977;72(357):46–53.
25. Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi:10.1093/oxfordjournals.aje.a115101
26. World Health Organization. Nutrition Landscape Information System (NLIS). Country Profile Indicators. Interpretation Guide. World Health Organization; 2010.
27. Hall A, Kassa T, Demissie T, Deggife T, Lee S. National survey of health and nutrition of school children in Ethiopia. Trop Med Int Health. 2008;13:1518–1526. doi:10.1111/j.1365-3156.2008.02168.x
28. Mesfin F, Berhane Y, Worku A, Cardoso MA. Anemia among primary school children in eastern Ethiopia. PLoS One. 2015;10(4):4–10. doi:10.1371/journal.pone.0123615
29. Assefa S, Mossi A, Hamza L. Prevalence and severity of anemia among school children in Jimma Town, Southwest Ethiopia. BMC Hematol. 2014;14:3–9. doi:10.1186/2052-1839-14-3
30. Ngesa O, Mwambi H. Prevalence and risk factors of anemia among school children aged between 6 months and 14 years in Kenya. PLoS One. 2014;9(11):5–10. doi:10.1371/journal.pone.0113756
31. Turyashemererwa FM, Kikafunda J, Annan R, Tumuhimbise GA. Dietary patterns, anthropometric status, prevalence and risk factors for anemia among school children aged 5–11 years in central Uganda. J Hum Nutr Diet. 2013;26:73–81. doi:10.1111/jhn.12069
32. Khatiwada S, Tamang KM, Gelal B, et al. Anemia among school children in eastern Nepal. J Trop Pediatr. 2015;61:231–233. doi:10.1093/tropej/fmv016
33. Ngui R, Lim YAL, Chong Kin L, SekChuen C. Association between anemias, iron deficiency anemia, neglected parasitic infections and socioeconomic factors in rural children of west Malaysia. PLoS Negl Trop Dis. 2012;6(3). doi:10.1371/journal.pntd.0001550
34. Liao X, Yun S, Zhao G. Structure, function and nutrition of phytoferritin: newly functional factor for iron supplement. Crit Rev Food Sci Nutr. 2014;54(10):1342–1352. doi:10.1080/10408398.2011.635914
35. Alelign T, Degarege A, Erko B. Prevalence and factors associated with undernutrition and anemia among school children in Durbe Town, Northwest Ethiopia. Arch Public Health. 2015;73:34–41. doi:10.1186/s13690-015-0084-x
36. Senbanjo OI, Oshikoya AK, Odusanya OO, Njokanma FO. Prevalence and risk factors for stunting among school children and adolescents in Abeokuta, Southwest Nigeria. J Health Popul Nutr. 2011;29(4):364–370. doi:10.3329/jhpn.v29i4.8452
37. Mukherjee RM, Chaturvedi SL, Bhalwar RC. Determinants of nutritional status of school children in Pune. MJAFI. 2008;64:227–231.
38. Mohamed S, Hussein DM. Prevalence of thinness, stunting and anemia among rural school-aged Sudanese children: a cross-sectional study. J Trop Pediatr. 2015;61:260–265. doi:10.1093/tropej/fmv028
39. Herrador Z, Sordo L, Gadisa E, et al. Cross-sectional study of malnutrition and associated factors among school aged children in rural and urban settings of fogera and LiboKemkem districts, Ethiopia. PLoS One. 2014;9:3–9.
40. Northrop-Clewes AC, Rousham KE, Mascie-Taylor NGC, Lunn GP. Anthelmintic treatment of rural Bangladeshi children: effect on host physiology, growth, and biochemical status. Am J Clin Nutr. 2001;73:53–60. doi:10.1093/ajcn/73.1.53
41. Degarege D, Degarege A, Aminut A. Undernutrition and associated risk factors among school age children in Addis Ababa. BMC Public Health. 2015;15:375–384. doi:10.1186/s12889-015-1714-5
42. Lundborg P, Ralsmark H, Rooth D. The More the Healthier? Health and Family Size. Mimeo: Lund University; 2015.
43. Horton S. Birth order and child nutritional status: evidence from the Philippines. Econ Dev Cult Change. 1988;36:341–354. doi:10.1086/451655
44. Yimam Y, Degarege A, Erko B. Effect of anthelmintic treatment on helminthes infection and related anemia among school-age children in northwestern Ethiopia. BMC Infect Dis. 2016;16:613. doi:10.1186/s12879-016-1956-6
45. Kabatereine BN, Brooker S, Koukounari A, et al. Impact of a national helminthes control program on infection and morbidity in Ugandan school children. Bull World Health Organ. 2007;85:91–97. doi:10.2471/BLT.06.030353
46. Stoltzfus JR, Albonico M, Chwaya MH, Tielsch MJ, Schulze JK, Savioli L. Effects of the Zanzibar school-based deworming program on iron status of children. Am J Clin Nutr. 1998;68:179–186. doi:10.1093/ajcn/68.1.179
47. Watkins WE, Pollit E. Effect of removing Ascaris on the growth of Guatemalan schoolchildren. Pediatrics. 1996;97(6 Pt 1):871–876.
48. Joseph SA, Casapía M, Montresor A, et al. The effect of deworming on growth in one- year-old children living in a soil-transmitted helminthes-endemic area of Peru: a randomized controlled trial. PLoS Negl Trop Dis. 2015;9(10):8–20. doi:10.1371/journal.pntd.0004020
49. Sur D, Saha RD, Manna B, Rajendran K, Bhattacharya KS. Periodic deworming with albendazole and its impact on growth status and diarrheal incidence among children in an urban slum of India. Trans R Soc Trop Med Hyg. 2005;99:261–267. doi:10.1016/j.trstmh.2004.08.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


