Back to Journals » Infection and Drug Resistance » Volume 16
Magnetic Resonance Imaging Manifestations in 13 Cases of Seminal Vesicle Tuberculosis
Authors Gan W, Bi Y, Fu X, Wei J, Qi M, He J, Li X
Received 13 July 2023
Accepted for publication 7 September 2023
Published 26 October 2023 Volume 2023:16 Pages 6871—6879
DOI https://doi.org/10.2147/IDR.S427561
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Wei Gan,1,* Yan Bi,2,* Xuwen Fu,1 Jialu Wei,1 Min Qi,1 Jintang He,3 Xiang Li1
1Department of Radiology, Kunming Third People’s Hospital/Yunnan Clinical Medical Center for Infectious Diseases, Kunming, 650041, People’s Republic of China; 2Department of Radiology, The People’s Hospital of Lincang, Lincang, Yunnan, 677000, People’s Republic of China; 3Department of Surgery, Kunming Third People’s Hospital/Yunnan Clinical Medical Center for Infectious Diseases, Kunming, 650041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiang Li, Department of Radiology, Kunming Third People’s Hospital /Yunnan Clinical Medical Center for Infectious Diseases, No. 319 of Wujing Street, Guandu District, Kunming, 650041, People’s Republic of China, Tel +86 871 6352 3507, Fax +86 871 6351 4717, Email [email protected] Jintang He, Department of Surgery, Kunming Third People’s Hospital/Yunnan Clinical Medical Center for Infectious Diseases, No. 319 of Wujing Street, Guandu District, Kunming, 650041, People’s Republic of China, Tel +86 871 6842 3689, Fax +86 871 6351 4717, Email [email protected]
Objective: This study aimed to examine the radiographic manifestations of seminal vesicle tuberculosis (SVT) on magnetic resonance imaging to gain a deeper understanding of this disease.
Methods: The clinical symptoms, general conditions, relevant laboratory tests and radiological data of 13 patients diagnosed with SVT were collected through bacteriological examination. A descriptive analysis was used to explore the composition ratio and rate values of the collected data.
Results: All 13 cases (100.0%) showed isointense signals on T1WI and hypointense signals on T2WI in the affected seminal vesicles, with the disappearance of the multi-chambered high signal on T2WI in normal seminal vesicles. Eight cases (61.5%) showed diffusion restriction on DWI of the affected seminal vesicle and significant enhancement on the contrast scan, whereas five cases (38.5%) showed unrestricted diffusion and mild enhancement on the contrast scan. Patients with significant enhancements exhibited higher counts and neutrophil percentages than patients with mild enhancements, with statistically significant differences (Z = 2.196, P = 0.030; Z = 2.781, P = 0.003, respectively). The counts and percentage of lymphocytes, CD3+T cells and CD4+T cells were significantly lower in patients with significant enhancements than in those with mild enhancements, with statistically significant differences (Z = − 2.196, P = 0.030; Z = − 2.928, P = 0.002; Z = − 2.928, P = 0.002; Z = − 2.928, P = 0.002, respectively). Patients with significant enhancements were more likely to have active pulmonary tuberculosis than those with mild enhancements, with a statistically significant difference (P = 0.035).
Conclusion: Magnetic resonance imaging reveals distinct radiographic features of SVT, and variations in imaging presentations can indicate a patient’s immune status.
Keywords: seminal vesicle tuberculosis, magnetic resonance imaging, genitourinary tuberculosis
Introduction
Tuberculosis is a multisystemic disease that affects multiple organs. It most commonly occurs in the lungs but can also affect extrapulmonary organs. However, the latter receives little attention in public health.1 Genitourinary tuberculosis (GUTB) accounts for 14–41% of extrapulmonary tuberculosis,2 and male genital tuberculosis constitutes approximately 34–43% of extrapulmonary tuberculosis.3 It can be sexually transmitted to women.4 Epididymal and prostate tuberculosis are the most common types, and tuberculosis of the prostate, epididymis or urinary tract can lead to seminal vesicle tuberculosis (SVT).5 However, there are relatively few reports on the imaging manifestations of SVT, especially those of magnetic resonance imaging (MRI), and there is a lack of clinical reference. Moreover, there is a significant deficiency in the knowledge of tropical urological diseases among European urologists, which may be related to the fact that genital tuberculosis receives relatively little attention in Europe.6 Therefore, we analysed the MRI manifestations of a case series of patients with SVT treated in Kunming Third People’s Hospital and the People’s Hospital of Lincang, with the aim of enhancing medical practitioners’ understanding of this disease worldwide.
Materials and Methods
Study Participants
This study collected and evaluated the clinical symptoms, relevant laboratory examinations and imaging data of a case series of patients diagnosed with SVT through bacteriological examination, effective anti-tuberculosis treatment or post-operative pathology from between January 2018 and March 2023 in Kunming Third People’s Hospital and the People’s Hospital of Lincang.
Imaging and Image Analysis
Imaging Examination
All 13 patients underwent chest CT scans. The scanning equipment used at Kunming Third People’s Hospital was a uCT510 scanner (United Imaging, Shanghai, China). The scan extended from the thoracic inlet to the level of the diaphragm, using a CT scan tube voltage of 120 kV and a tube current with automatic milliampere technology. The scanning equipment used at the People’s Hospital of Lincang was the Aquilion ONE TSX-301C (Canon, Japan) scanner, with the same scanning range and CT scan parameters. The 13 patients also underwent pelvic MRI scans and enhancements. At Kunming Third People’s Hospital, a uMR588 instrument (United Imaging, Shanghai, China) with a 12-channel abdominal coil was used. The plain scan sequence included coronal, sagittal and axial T2WI fat-suppressed sequences (TE = 90 ms, TR = 2800 ms, slice thickness = 3 mm), an axial T2WI non-fat-suppressed sequence (TE = 100 ms, TR = 3200 ms, slice thickness = 3 mm), axial DWI (TE = 80 ms, TR = 4300 ms, slice = thickness 3 mm, B = 800) and an axial T1WI fat-suppressed sequence (TE = 2 ms, TR = 5 ms, slice thickness = 3 mm). Gadodiamide (Hengrui, Jiangsu, China) at a dose of 0.2 mL/kg was used for the enhancement scan, with the enhanced scan covering coronal, sagittal and axial T1WI fat-suppressed sequences (TE = 2 ms, TR = 5 ms, slice thickness = 3 mm). At the People’s Hospital of Lincang, a Vantage Elan WRT-2020 system (Canon, Japan) was used for pelvic MRI scans and enhancements, with a 16-channel abdominal coil. The plain scan sequence included coronal and axial T2WI sequences (TR = 3600 ms, TE = 90 ms, slice thickness = 4 mm), coronal and axial T2WI fat-suppressed sequences (TR = 4000 ms, TE = 75 ms, slice thickness = 4 mm), axial T1WI sequence (TR = 670 ms, TE = 10 ms, slice thickness = 4 mm) and axial DWI (TR = 3400 ms, TE = 80 ms, slice thickness = 4 mm, B = 1000). Gadodiamide (Hengrui, Jiangsu, China) at a dose of 0.2 mL/kg was used for the enhancement scan, with the enhanced scan covering coronal, sagittal and axial T1WI fat-suppressed sequences (TE = 15 ms, TR = 560 ms, slice thickness = 5 mm).
Image Analysis
The images were downloaded from the hospitals’ Picture Archiving and Communication Systems and interpreted in a blinded manner by two physicians with many years of experience in the radiological diagnosis of tuberculosis. In the case of disagreement between the two primary readers, a third physician was brought in to review the images until a consensus was reached through joint consultation.
Statistical Analysis
A statistical analysis was performed using SPSS 26.0 software. Quantitative data were first tested for a normal distribution. If the data were normally distributed, they were described as x (±) s, and an independent-samples t-test was used for comparison. If the data were not normally distributed, they were described as M (Q1, Q3), and a non-parametric test was used to compare two independent samples. Count data were described as frequency (percentage, %), and Fisher’s exact probability test was used for comparisons. A value of P < 0.05 was considered statistically significant.
Results
General Patient Characteristics
The ages of the 13 patients ranged from 24 to 60 years, with a median age of 35 (29, 57) years; among them, 10 (76.9%) had concurrent pulmonary tuberculosis. All 13 patients (100.00%) had GUTB in organs other than the seminal vesicles. Specifically, 13 patients (100%) had epididymal tuberculosis, 7 (53.8%) had prostate tuberculosis, and 2 (15.4%) had kidney tuberculosis. Among these 13 patients, one (7.7%) had intracranial tuberculosis, two (15.4%) had adrenal tuberculosis and two (15.4%) had peritoneal tuberculosis.
Clinical Symptoms
The onset of symptoms ranged from 0.2 to 12 months, with a median time of 1 month. Twelve patients (92.3%) presented with scrotal pain and swelling, two of whom (15.4%) had scrotal skin ruptures with purulent discharge; three patients (23.1%) presented with urinary frequency and dysuria, nine patients (69.2%) presented with cough and sputum production and two patients (15.4%) presented with abdominal pain and bloating.
Laboratory Examination
Of the 13 patients, one (7.7%) had a reduced total white blood cell count (normal range 4–10 × 109/L), two (15.4%) had an elevated neutrophil count and two (15.4%) had a decreased neutrophil count (normal range 2–7 × 109/L). Additionally, five (38.5%) had an increased neutrophil percentage, and three (23.1%) had a decreased percentage (normal range 50–70%). Three patients (23.1%) exhibited reduced lymphocyte counts (normal range 0.8–4.0 × 109/L), with eight (61.5%) demonstrating a decreased lymphocyte percentage and one (7.7%) showing an increased percentage (normal range 20–40%). Four patients (30.8%) had an elevated erythrocyte sedimentation rate (normal range 0–20 mm/h), and nine (69.2%) patients had an elevated C-reactive protein level (normal range <3 mg/L). Seven patients (53.8%) had a reduced total T lymphocyte count, with six (46.2%) having decreased helper/inducer T lymphocytes (CD4+T cells) and eight (61.5%) demonstrating a reduction in suppressor/cytotoxic T lymphocytes (CD8+T cells). Tuberculosis infection T-cell spot tests were positive in 12 (92.3%) patients, urine GeneXpert MTB/RIF tests were positive in 7 (53.8%) patients, and 3 (23.1%) patients showed mycobacterial growth in urine BACTEC MGIT960 liquid culture.
Magnetic Resonance Imaging Findings
Location and Size of the Affected Seminal Vesicles
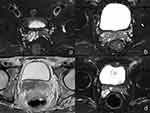
Among the 13 cases of SVT, 12 (92.3%) were located in a unilateral seminal vesicle, with 8 (61.5%) on the left (Figure 1a) and 4 (30.8%) on the right. Bilateral seminal vesicles were involved in one case (7.7%) (Figure 1b). Nine patients (69.2%) demonstrated a decrease in the size of the affected seminal vesicle (Figure 1c), whereas four (30.8%) patients presented with an enlargement of the affected seminal vesicle (Figure 1d).
Magnetic Resonance Imaging Signals and Enhancement
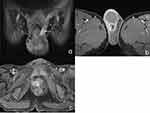
In the MRI plain scans, all 13 cases (100.0%) showed isointense signals on T1WI and hypointense signals on T2WI in the affected seminal vesicles, with the disappearance of the multi-chambered high signal on T2WI in normal seminal vesicles. Diffusion restriction on DWI in the affected seminal vesicles occurred in eight cases (61.5%), and the diffusion restriction cases showed significant enhancement on MRI-enhanced scans (Figure 2a and b). On DWI, the diffusion was unrestricted in the affected seminal vesicles in five cases (38.5%), and slight enhancement appeared on the enhanced scans (Figure 2c and d). All 13 patients (100.0%) had concurrent ipsilateral epididymal tuberculosis and varying degrees of thickening of the spermatic cord (Figure 3a), with epididymal tuberculosis presenting as iso-to-hypointense nodules on T1WI and mixed signals on T2WI, with nodular or ring-shaped enhancement on the enhanced scans (Figure 3b). Seven cases (53.8%) were accompanied by prostate tuberculosis, which manifested as hypointense signals on T1WI and hyperintense signals on T2WI within the prostate, with ring-shaped enhancement on the enhanced scans (Figure 3c).
Variations in Laboratory Examinations and Concomitant Tuberculosis in Other Organs Based on Different Enhancement Methods
Patients with diffusion restriction on DWI show significant enhancement on enhancement scanning, whereas those without diffusion restriction on DWI display mild enhancement. Among these, patients with significant enhancement had higher counts and percentages of neutrophils than those with mild enhancement, and the differences were statistically significant (Z = 2.196, P = 0.030; Z = 2.781, P = 0.003, respectively). Patients with significant enhancement had significantly lower counts and percentages of lymphocytes and lower levels of CD3+T cells and CD4+T cells than those with mild enhancement, and the differences were statistically significant (Z = −2.196, P = 0.030; Z = −2.928, P = 0.002; Z = −2.928, P = 0.002; Z = −2.928, P = 0.002, respectively) (see Table 1). The proportion of patients with significant enhancement who also had active pulmonary tuberculosis was higher than that of patients with mild enhancement, and the difference was statistically significant (P = 0.035) (see Table 2).
 |
Table 1 Laboratory Examination of Patients with Seminal Vesiculitis Showing Significant and Mild Enhancement |
 |
Table 2 Laboratory Examination and Concomitant Tuberculosis in Other Organs in Patients with Seminal Vesiculitis Showing Significant and Mild Enhancement |
Discussion
Genitourinary tuberculosis is a common type of extrapulmonary tuberculosis. About two-thirds of reproductive system tuberculosis is associated with urinary system tuberculosis, but it can also occur independently.7 Tuberculosis of the male reproductive system can occur in the prostate, seminal vesicles, vas deferens, epididymis, testes and penis. Epididymal tuberculosis and prostatic tuberculosis are the most common, and seminal vesiculitis is often found during examinations for prostatic and urinary system tuberculosis.8 Among the 13 patients in this study, 12 were discovered due to scrotal pain or urinary system symptoms, and imaging examinations were conducted. Therefore, during the clinical diagnosis and treatment process, attention should be paid to observing abnormalities in the seminal vesicles of patients with epididymis or testicular tuberculosis.
The seminal vesicles are located behind the base of the prostate, on the outside of the ampulla of the vas deferens. The distal end merges with the vas deferens to form the ejaculatory duct, which opens into the prostate and is then connected to the epididymis and testes. Therefore, epididymal tuberculosis, prostatic tuberculosis or urinary system tuberculosis can directly spread or infect the seminal vesicles via the vas deferens.5 All 13 patients in this study also had ipsilateral epididymal tuberculosis and thickening of the ipsilateral spermatic cord, which also explains this pathogenesis.
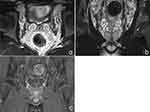
Magnetic resonance imaging has a high soft-tissue resolution and can be imaged in any plane, making it the preferred method for evaluating the seminal vesicles.9 Normal seminal vesicles present an oval and grape-like structure. Because the seminal vesicles secrete a viscous fluid, they appear slightly hypointense on T1WI and hyperintense on T2WI.10 The folds within the seminal vesicles present slightly hypointense signals on T2WI against a backdrop of high T2WI signals from seminal fluid. In enhancement scans, the folds of the seminal vesicles show mild enhancement, but the seminal fluid does not enhance. Although the size of normal seminal vesicles is affected by the duration of abstinence and age,11,12 they are symmetrical and consistent in size on both sides (Figure 4a–c).
Magnetic resonance imaging can depict the pathological process of seminal vesiculitis, including inflammatory thickening, granuloma formation and abscesses.13 Seminal vesiculitis can affect one or both seminal vesicles. Among the 13 cases in this group, 12 cases (92.3%) showed unilateral seminal vesicle involvement, and only one case (7.7%) had bilateral seminal vesicle involvement.
The mechanism of more frequent unilateral involvement is not yet clear. Seminal vesiculitis can manifest as shrinkage or enlargement of the affected seminal vesicles. The shrinkage of the seminal vesicles relates to the fusion and thickening of the folds, leading to structural collapse,14 whereas enlargement may be associated with acute dilation caused by infection. In terms of MRI signals, the condition often presents as a decrease in T2WI signals and an increase in T1WI signals on the affected side; this is associated with the destruction of the seminal vesicular structure, leading to a decrease in seminal fluid and oxidative stress in macrophages within the tuberculous granuloma.15 In terms of DWI and enhancement performance, lesions with restricted diffusion show significant enhancement, whereas lesions with non-restricted diffusion present mild enhancement. This is also related to the pathological process of tuberculous lesions. Restricted diffusion is often seen in abscess formation or liquefactive necrosis within granulomas, and non-restricted diffusion is usually associated with immature tuberculomas or caseous necrosis within tuberculomas.16
Tuberculosis is not only an infectious disease but also an immune disease. Its occurrence, development and outcome are closely related to the immune status of the host.17 When the quantity of Mycobacterium tuberculosis is small, its virulence is low or the immune response is strong, the tuberculosis pathology primarily exhibits a proliferative reaction. However, when the bacterial count is high, the virulence is strong, the host’s immunity is compromised or a hypersensitive reaction occurs, the tuberculosis pathology then mainly presents necrotic lesions.18 Necrotic lesions appear on DWI as restricted diffusion and significant enhancement, whereas proliferative reactions on DWI appear as non-restricted diffusion and mild enhancement. In this study, the lymphocyte levels and CD4+T cell levels in the significant enhancement group were significantly lower than those in the non-enhancement group. The reduction in lymphocyte count and CD4+T cell count represents a decline in the host’s anti-tuberculosis immune function.19 The proportion of patients with active pulmonary tuberculosis was higher in patients who demonstrated significant enhancement than in those with mild enhancement in our study. Therefore, it is hypothesised that significant enhancement in seminal vesiculitis may represent a decline in the patient’s immune function. Whether such patients are more prone to disseminated tuberculosis requires further observation and study.
Aside from seminal vesiculitis, all 13 cases in this group also exhibited unilateral epididymal tuberculosis and an increase in the thickness of the spermatic cord. Epididymal tuberculosis may present on CT as an enlarged epididymis with nodular or annular enhancement.20 Its MRI manifestation is similar to its CT presentation. The treatment for epididymal tuberculosis often involves the surgical removal of the epididymis and scrotal nodules, combined with anti-tuberculosis treatment.21 However, in patients with co-existing seminal vesiculitis, whether the pelvic segment of the vas deferens and seminal vesicle lesions also need surgical management requires further research.
In differential diagnosis, tuberculosis of the seminal vesicles needs to be distinguished from other conditions, such as acute or chronic seminal vesiculitis, seminal vesicle cysts, seminal vesicle stones and benign or malignant tumours of the seminal vesicles. Tuberculosis of the seminal vesicles often involves one side and presents as an increase or decrease in the volume of the seminal vesicles, characterised by elevated T1WI signals, reduced T2WI signals and mild or significant enhancement on enhanced scans. Seminal vesiculitis can be caused by Escherichia coli, Neisseria gonorrhoeae, Proteus mirabilis or Pseudomonas aeruginosa,12 and it may also coexist with prostatitis and epididymitis. Magnetic resonance imaging differentiation is difficult, and reliance on clinical symptoms and laboratory examinations is required for differentiation. Second, seminal vesicle cysts present as high T2WI signal shadows, but there is no enhancement on enhanced scans;22 however, seminal vesicle stones may present as nodular low-signal shadows in high T2WI signal seminal fluid, but typically, the form and signal of the seminal vesicle are normal.23 Finally, tumours of the seminal vesicle, including primary tumours and prostate cancer invasion into the seminal vesicles, can be identified. The former presents as nodular and mass shadows, whereas the latter has a history of prostate cancer and the two types can be differentiated in conjunction with imaging and tumour markers. Above all, the differential diagnosis of GUTB is vital to urologists. In today’s world, international travel is gradually leading to an increase in urological diseases that were previously only rarely encountered.24,25 Currently, GUTB, schistosomiasis, echinococcosis and HIV-related urological diseases have been reported in multiple case series.26,27 Therefore, if experienced doctors can achieve the goals of early and accurate diagnosis and treatment, the number of diagnostic examinations and length of hospital stay will be reduced.28 In addition, an increase in the number of cases–series reports will promote the exchange of experience among urologists around the world to tackle the increase in the number of case–series patients with GUTB.
There are some limitations to this study. First, the area of study was limited to Kunming and Lincang; multicentre research is therefore required. Second, the small cohort in this study might not be well suited to an assessment of our treatment policy, even if the number of this study appears adequate compared with that of other global and international studies recently published by the urological community.
Conclusion
In conclusion, epididymal tuberculosis can be associated with tuberculosis of the seminal vesicles. The fluid of the seminal vesicles is an important component of semen, and potentially, tuberculosis of the seminal vesicles can be sexually transmitted to women. Tuberculosis of the seminal vesicles has certain MRI characteristics, and the mode of MRI enhancement can, to some extent, represent the immune status of the patient. In the diagnostic and therapeutic process, combining epidemiological history, laboratory examinations, clinical features and imaging examinations can improve the diagnostic accuracy for this disease.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Kunming Third People’s Hospital. Informed consent was signed by all participants in this study.
Funding
Health Research Project of Kunming National Health Commission (2022-09-01-001).
Disclosure
Wei Gan and Yan Bi are co-first authors for this study. All authors declared no competing interests in this work.
References
1. Sanches I, Carvalho A, Duarte R. Who are the patients with extrapulmonary tuberculosis? Rev Port Pneumol. 2015;21(2):90–93. doi:10.1016/j.rppnen.2014.06.010.
2. Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system-Urinary tract tuberculosis: renal tuberculosis-Part I. Indian J Radiol Imaging. 2013;23(1):46–63. doi:10.4103/0971-3026.113615.
3. Kulchavenya E, Kim CS, Bulanova O, et al. Male genital tuberculosis: epidemiology and diagnostic. World J Urol. 2012;30(1):15–21. doi:10.1007/s00345-011-0695-y.
4. Kimura M, Araoka H, Baba H, et al. First case of sexually transmitted asymptomatic female genital tuberculosis from spousal epididymal tuberculosis diagnosed by active screening. Int J Infect Dis. 2018;73:60–62. doi:10.1016/j.ijid.2018.05.021.
5. Ramachandran A, Das CJ, Razik A. Male genital tract tuberculosis: a comprehensive review of imaging findings and differential diagnosis. Abdom Radiol. 2021;46(4):1677–1686. doi:10.1007/s00261-020-02811-0.
6. Mantica G, Van der Merwe A, Terrone C, et al. Awareness of European practitioners toward uncommon tropical diseases: are we prepared to deal with mass migration? Results of an international survey. World J Urol. 2020;38(7):1773–1786. doi:10.1007/s00345-019-02957-7.
7. Yadav S, Singh P, Hemal A, et al. Genital tuberculosis: current status of diagnosis and management. Transl Androl Urol. 2017;6(2):222–233. doi:10.21037/tau.2016.12.04.
8. Kumar R. Reproductive tract tuberculosis and male infertility. Indian J Urol. 2008;24(3):392–395. doi:10.4103/0970-1591.42624.
9. Ocal O, Karaosmanoglu AD, Karcaaltıncaba M, et al. Imaging findings of congenital anomalies of seminal vesicles. Pol J Radiol. 2019;84:e25–e31. doi:10.5114/pjr.2019.82711.
10. Zang Q, Ren ZJ, Cao DH, et al. MRI characteristics of distal genital tract region inrefractory hematospermia. Chongqing Medicine. 2020;49(4):577–581. doi:10.3969/j.issn.1671-8348.2020.04.015.
11. Yuruk E, Pastuszak AW, Suggs JM, et al. The association between seminal vesicle size and duration of abstinence from ejaculation. Andrologia. 2017;49(7):e12707. doi:10.1111/and.12707.
12. Catania R, Dasyam N, Furlan A, et al. Cross-sectional imaging of seminal vesicles and vasa deferentia. Abdom Radiol. 2020;45(7):2049–2062. doi:10.1007/s00261-019-02368-7.
13. Fraietta R, Mori MM, De Oliveira JM, et al. Tuberculosis of seminal vesicles as a cause of aspermia. J Urol. 2003;169(4):1472. doi:10.1097/01.ju.0000054926.03499.3e.
14. Reddy MN, Verma S. Lesions of the seminal vesicles and their MRI Characteristics. J Clin Imaging Sci. 2014;4:61. doi:10.4103/2156-7514.143734.
15. Qi LP, Chen KN, Zhou XJ, et al. Conventional MRI to detect the differences between mass-like tuberculosis and lung cancer. J Thorac Dis. 2018;10(10):5673–5684. doi:10.21037/jtd.2018.09.125.
16. Yin QH, Jiang ZS, Nie GJ, et al. Analysis of MRI characteristics of pleural tuberculoma. Chin J Antituberculosis. 2020;42(11):1153–1157. doi:10.3969/j.issn.1000-6621.2020.11.003.
17. Sia JK, Rengarajan J. Immunology of mycobacterium tuberculosis infections. Microbiol Spectr. 2019;7(4). doi:10.1128/microbiolspec.GPP3-0022-2018.
18. Chinese Medical Association Tuberculosis Branch. tuberculosis pathology diagnosis expert consensus writing group. Chinese tuberculosis pathology diagnosis expert consensus. Chin J Tuberc Respir Dis. 2017;40(6):419–425. doi: 10.3760/cma.j.issn.1001-0939.2017.06.005.
19. Tuberculosis Prevention and Control Key Laboratory/Beijing Key Laboratory of New Techniques of Tuberculosis Diagnosis and Treatment/Institute for Tuberculosis Research of the 8th Medical Center of Chinese PLA General Hospital, Editorial Board of Chinese Journal of Antituberculosis, Basic and Clinical Speciality Committees of Tuberculosis Control Branch of China International Exchange and Promotive Association for Medical and Health Care. Expert consensus on immune function assessment and immunotherapy in patients with active tuberculosis (2021 Edition). Chin J Antituberculosis. 2022;44(1):9–27. doi: 10.19982/j.issn.1000-6621.20210680.
20. Li X, Ma ZX, Fu XW. et al. CT imaging of 56 cases of epididymal tuberculosis. Chin J Antituberculosis. 2022;44(10):1100–1103. doi:10.19982/j.issn.1000-6621.20220208
21. Du L. Effect of surgery combined with drug treatment on epididymal tuberculosis and its influence on reproductive function. Chin J Hum Sex. 2020;29(1):27–30. doi:10.3969/j.issn.1672-1993.2020.01.009.
22. Shebel HM, Farg HM, Kolokythas O, et al. Cysts of the lower male genitourinary tract: embryologic and anatomic considerations and differential diagnosis. Radiographics. 2013;33(4):1125–1143. doi:10.1148/rg.334125129.
23. Mittal PK, Camacho JC, Sahani DV. et al. Hematospermia Evaluation at MR Imaging. Radiographics. 2016;36(5):1373–1389. doi:10.1148/rg.2016150195.
24. Riccardi N, Nosenzo F, Peraldo F, et al. Increasing prevalence of genitourinary schistosomiasis in Europe in the migrant era: neglected no more? PLoS Negl Trop Dis. 2017;11(3):e0005237. doi:10.1371/journal.pntd.0005237
25. Lingscheid T, Kurth F, Clerinx J, et al. Schistosomiasis in European travelers and migrants: analysis of 14 Years TropNet surveillance data. Am J Trop Med Hyg. 2017;97(2):567–574. doi:10.4269/ajtmh.17-0034
26. Poddighe D, Castelli L, Pulcrano G, et al. Urinary schistosomiasis in an adolescent refugee from Africa: an uncommon cause of hematuria and an emerging infectious disease in Europe. J Immigr Minor Health. 2016;18(5):1237–1240. doi:10.1007/s10903-015-0272-3
27. Villasante Ferrer A, Iranzo Tatay A, Aznar Oroval E, Mollar Maseres J. Estudio de la situación actual de la infección por Schistosoma haematobium en la Unión Europea. Una aproximación al posible riesgo en España [Study of the current status of Schistosoma haematobium infection in the European Union. An approach to the possible risk in Spain]. Rev Esp Salud Publica. 2018;92:e201804010. Spanish.
28. Lionis C, Petelos E, Mechili EA, et al. Assessing refugee healthcare needs in Europe and implementing educational interventions in primary care: a focus on methods. BMC Int Health Hum. 2018;18(1):1–8.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.