Back to Journals » Journal of Pain Research » Volume 16
Magnetic Peripheral Nerve Stimulation (mPNS) for Chronic Pain
Received 6 April 2023
Accepted for publication 4 July 2023
Published 12 July 2023 Volume 2023:16 Pages 2365—2373
DOI https://doi.org/10.2147/JPR.S409331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Marshall Bedder,1,2 Lisa Parker1
1Department of Surgery, Pain Medicine Service, Charlie Norwood Veterans Administration Medical Center, Augusta, GA, USA; 2Department of Psychiatry and Health Behavior, Addiction Medicine Service, Medical College of Georgia at Augusta University, Augusta, GA, USA
Correspondence: Marshall Bedder, FRCPC Chief of Pain Medicine Service, Department of Surgery, Pain Medicine Service, Charlie Norwood Veterans Administration Medical Center, 950 15th Street, Augusta, GA, 30904 4C-125 USA, USA, Tel +1 706-733-0188 extension 33481, Fax +1-706-823-3983, Email [email protected]
Purpose: To assess magnetic peripheral nerve stimulation (mPNS) for the treatment of chronic or chronic and intractable neuropathic pain with a retrospective review case series.
Patients and methods: Twenty-four patients with predominantly neuropathic post-traumatic or postoperative pain were treated as per protocol and followed for 3 months.
Results: Data were analyzed as an observational, one-armed, convenience sample. Graphical evidence backed up by a mixed model for repeated measures statistical analysis showed a highly significant reduction of pain at one month out from initial treatment with mPNS. At one month, there was a 3.8 average reduction in pre-pain scores using a visual analogue scale (VAS), and that relief was generally durable measured out to three months. Two-thirds of patients, deemed responders, showed an 87% reduction in pain. Opioid reduction was seen in 58.3% of responders as well.
Conclusion: mPNS appears promising for the treatment of chronic or chronic and intractable neuropathic pain for many of the same indications as traditional electrical peripheral nerve stimulation (PNS). No invasive techniques or implants are needed for mPNS.
Keywords: neuropathic pain, noninvasive, cost effective, pain relief, neuropathy
Introduction
The International Association for the Study of Pain (IASP) has revised the definition of pain to “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage”, with six accompanying descriptors and the etymology of the word “pain”.1 One of the descriptors states that “pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors”,1 and as such may affect individuals in different ways. Neuropathic pain (NP) has been defined as “pain caused by a lesion or disease of the primary afferent neurons of the somatosensory nervous system”, which includes peripheral neuropathy, postherpetic neuralgia, trigeminal neuralgia, nerve root pain and phantom limb pain. Several recent studies have shown that NP can adversely affect patients’ overall health-related quality of life (HRQoL), including physical and emotional functioning2,3 and that it is associated with substantial societal costs.4
The purpose of this retrospective chart review series was to assess initial effectiveness of magnetic peripheral nerve stimulation (mPNS) on a wide range of chronic neuropathic pain conditions. One perceived initiation of chronic neuropathic pain is the loss of the inhibitory function of Aβ nerve fibers, often due to degradation or damage of the myelin sheath. Magnetic fields pass through soft-tissue unobstructed and, therefore, can be targeted directly at the Aβ nerves. Delivering stimulation at 0.2–5Hz with a precise pulse-width (240–290us) specifically activates Aβ nerve fibers. A signal with a frequency and pulse-width outside of this range cannot recruit Aβ. The equipment utilized consists of a high current pulse generator able to produce a large electric discharge current (several thousand amperes). The current flows through a stimulating coil, generating magnetic pulses with field strength up to several Tesla. Heat is an unavoidable by-product derived from magnetic pulse generation; therefore, the coil must be contained in a liquid-cooled system. Many types of coils have been manufactured. Two frequently used are the round coil and figure of eight coil. Axon TherapyTM utilizes a figure of eight coil which produces a stronger magnetic field at the center with an accurate focus.
The primary objective of this retrospective chart review series is to evaluate the initial effectiveness of magnetic peripheral nerve stimulation (mPNS) in the management of a wide range of chronic neuropathic pain conditions, and potentially, contribute to the development of more effective, targeted therapies for neuropathic pain.
Methods
The current study was received by our Institutional Review Board-Panel A, Augusta University (Approval Date: December 20, 2022, IRB study ID #1945823). Based on the retrospective, deidentified nature of the study design, the current study was deemed to have exempt status and the requirement for consent from individuals was not required. For this study, the Veterans Administration (VA) electronic medical record, Computerized Patient Record System (CPRS), was queried. All data utilized in this study were fully de-identified prior to analysis.
Patients were selected as they presented at a busy Pain Clinic with chronic unresolved pain from a multitude of diagnoses. These were mainly neuropathic in nature but also involved centralized pain such as Phantom limb and osteoarthritic pain such as degenerative shoulder pain. See “Table 1”.
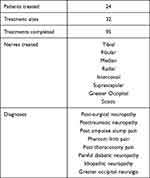 |
Table 1 Patient Selection and Diagnoses |
Treatment was administered using the Axon TherapyTM (NeuraLace Medical) mPNS device, see “Figure 1”. The treatment protocol consisted of three daily sessions in a row during the first week of therapy. This was followed by a weekly treatment for the remainder of the month totaling 6 treatments. There were treatments every second week in the second month. Monthly treatments were continued as needed for pain exacerbations.
 |
Figure 1 Magnetic Peripheral Nerve Stimulation Device. This is an image of the Axon Therapy mPNS Device. |
Each treatment involved mapping the putative nerve in question using the handheld coil. Using increasing stimulation thresholds, paresthesia was elicited in the painful anatomic area of pain and energy setting was backed off from the maximum attained to just below that level where a clear paresthesia was maintained so as not to overstimulate the nerve. Stimulation frequency is limited to a maximum of 2 Hz per pulse in the whole range of stimulation intensity (1–100% of magnetic stimulator output). Stimulation pulse waveform (electrical stimulation in the coil, from which the magnetic field corresponds) is approximately one harmonic wave with a total duration of 280–290 μs. The patient receives approximately 400 pulses in the 13.33-minute treatment. Technical specifications of the Axon TherapyTM device are described in Table 2.
 |
Table 2 Technical Specifications of the Axon TherapyTM Device |
Outcome measures included VAS pretreatment and post treatment using the VAS. Opioid use was determined by inquiry of the Georgia PDMP website (GA PMP AWARE) for Morphine Milligram Equivalents (MME) from the first date of treatment to the final treatment recorded.
Descriptive statistics are calculated for central tendencies with means and standard deviations for continuous data, while percentages are calculated for dichotomous data. Change in pre-pain scores over time were evaluated with a mixed model for repeated measures analysis.
Results
Data were analyzed as an observational, one-armed, convenience sample. Outcome measures included VAS and opioid MME. There was a striking immediate response seen on first time treatment in a majority of patients as you can see in “Figure 2”. Graphical evidence showed a highly significant reduction of pain at 1 month out from initial treatment with mPNS. There was a 3.8 average reduction in pre-pain scores using VAS. A mixed model for repeated measures was utilized to estimate the relationship between pre-pain score and treatment number. The model estimated a −0.273 (95% CI = −0.141 to −0.405) pre-pain score reduction per treatment (p-value <0.0001 for test of coefficient significance) as shown in “Figures 3” and “4”. Two-thirds of patients, deemed responders, showed an 87% average reduction in pain as seen in “Figures 5” and “6”. Opioid reduction was seen in 29% of patients looking at starting morphine milligram equivalents (MME) and three-month follow-up MME. The original 24 patient population was reviewed using data from Georgia PDMP. Of the original 16 pain responders, 12 were on prescription opioids 90 day data from each patients Initial to Current Rx in MME units. 58.3% of the responders (7 responders) with opioid Rx’s showed opioid reduction while undergoing Axon TherapyTM. The average opioid reduction for the 7 responders was 51%. The absolute reduction is MME for the 7 responders was 169.1 MME or 24.1 MME per patient. Durability of pain relief was demonstrated at 3 month follow-up as seen in “Figure 7”. We compared typical costs for the first year of therapy comparing mPNS (using the electronic catalogue (E-Cat) pricing from the VHA), the 60-day treatment PNS, implanted PNS systems and SCS. This analysis included 10 additional monthly mPNS treatments which may or may not be required. It did not include the professional fees for the 60-day PNS, implanted PNS and spinal cord stimulation (SCS) systems or the costs of any revisions to these systems. The mPNS system represents the lower end of the cost scale, see “Figure 8”.
 |
Figure 5 Number of Treatment Responders. This pie chart shows 2/3 of patients saw ≥50% reduction in pain. |
Discussion
The concept of generating a magnetic field by running electricity through a coil goes back to Michael Faraday. Faraday (1791 –1867) was an English scientist who contributed to the study of electromagnetism, and electrochemistry. His main discoveries include the principles underlying electromagnetic induction, diamagnetism, and electrolysis. All magnetic stimulation operates based on Faraday’s law of electromagnetic induction which describes the process by which a changing magnetic field induces the flow of electric current in a nearby conductor preferentially standing at 90 degrees to the magnetic field. The use of a time-varying magnetic field to induce a sufficiently strong current to stimulate living tissue was first reported by d’Arsonval in 1896.5 Jacques-Arsène d’Arsonval (1851–1940) was a French physician, physicist, and inventor of the moving-coil D’Arsonval galvanometer and the thermocouple ammeter. D’Arsonval was an important contributor to the emerging field of electrophysiology. Magnetic stimulation of nerve tissue was demonstrated by Oberg (1973).6 The first magnetic stimulation of peripheral nerves was reported by Polson in 19827 He established that magnetic stimulation as compared to electrical stimulation was pain free and could reach deep nerves. Another advantage over electrical stimulation is that higher current densities near the surface of the skin which can cause tissue damage are not seen in magnetic stimulation. Polson and Baker stated in their 1982 paper that “the potential advantages of pain-free, noninvasive magnetic stimulation of deep nerves suggest that further development of this technique will prove valuable”. The current report bears witness to this thesis and shows an advantage in cost, comfort, safety, and efficacy which can be investigated for many more applications. Professor Anthony Barker, along with Mike Polson and Ian Freeston, combined efforts earlier in 1978 to form a research team at Sheffield University to find an alternative to electrical nerve stimulation.8 To quote from this article: While at a neurophysiology/clinical meeting showcasing the magnetic stimulator, Anthony Barker and Reza Jalinous were asked, “What happens if you put the coil on your head?” Barker placed the coil on his head, fired the stimulator and subsequently elicited a motor response. This on-the-spot experiment led to the suggestion that Transcranial Magnetic Stimulation would replace Transcranial Electric Stimulation in the breakthrough publication of “Magnetic stimulation of the human brain”.9 Their pioneering transcranial magnetic stimulation work was then taken up commercially by Novametrix Medical Systems Inc. In 1987, Novametrix Model 200 Magstim (first-generation Magstim 200) received FDA clearance. In 2014,10 Leung et al reported a case series using an mPNS device in alleviating post-traumatic peripheral neuropathic pain states.
The perceived sustained relief of chronic pain follows the concepts and tenets as developed by Deer et al11 in their landmark paper on peripherally induced reconditioning of the central nervous system. Chronic pain is well known to affect peripheral and central sensitization. Activation of the large-diameter fibers has the potential to attenuate nociceptive signaling in the spinal dorsal horns. mPNS enjoys the benefits of remote selective targeting as described by Deer et al. It also produces activation of efferent fibers in mixed nerves resulting in strong, physiologic contraction without discomfort. Almost all studies used for suprathreshold stimulation are based on the rationale that muscle contraction would produce proprioceptive afferents to induce neuroplasticity.12 Seeing that mPNS can produce far more robust stimulation of the peripheral and central nervous systems, it suggests that at least the same reconditioning of the CNS can be expected and possibly to a greater effect than electrical stimulation, given the field properties and power outputs described earlier. Standard electrical PNS devices have been utilized for both acute and chronic pain conditions. Electrical PNS has been utilized to offer substantial analgesia in pain conditions including complex regional pain syndrome, postherpetic neuralgia, cranial neuralgias, migraines and cluster headaches, amputee pain, back pain, cancer-related pain8 as well as painful diabetic neuropathy (PDN) and knee osteoarthritis13–15 related pain. It appears that our patients represented a wide array of similar patients and further studies will validate the similarly expanded role for mPNS.
This study has potential limitations. The effects of the therapy on pain scores is limited by the retrospective nature of the study. In addition, the sample size is small and may not reflect the population as a whole. The collection of pain scores by VAS is subjective by nature. It is recommended that multicenter prospective RCTs be conducted to further understand the effect of this therapy in a more robust fashion.
Conclusion
mPNS generates lower electric fields at the surface of the body, resulting in greater penetration and the ability to stimulate deep nerves without pain. It has both practical and theoretical advantages over conventional electrical PNS as described herein which is the current standard of care (no pun intended). It is delivered as a standard protocol with the flexibility to deliver as-needed treatments over a longer-term course of therapy. Multiple nerves can be treated within each session. mPNS is capable of delivering higher intensities at and above motor threshold leading to maximal recruitment of the Aα & Aβ nerve fibers.
mPNS would appear to have the ability to mimic similar applications of standard PNS without invasive techniques or the need for any short-term or long-term implants. This renders it more cost-effective and moves it farther up the treatment algorithm. mPNS is currently FDA cleared to stimulate peripheral nerves for relief of chronic intractable, post-traumatic and post-surgical pain for patients 18 and older. A multicenter, randomized, clinical trial comparing the safety and effectiveness of Axon TherapyTM plus conventional medical management (CMM) versus CMM for the treatment of post-traumatic and post-operative neuropathic pain (SEAT Study) is currently underway. In addition, a multicenter, randomized, clinical trial comparing the safety and effectiveness of Axon TherapyTM and CMM for the treatment of painful diabetic neuropathy to Sham and CMM is currently underway.
Acknowledgments
Joe Milkovits COO, CTO, NeuraLace Medical for technical support in the production of this manuscript. Peter B. Rosenquist MD. Professor and Executive Vice Chair of the Dept. of Psychiatry and Health Behavior at Augusta University, Director of Therapeutic Neurostimulation. Many thanks for his thorough review of manuscript drafts and suggested revisions.
Funding
No funding was received for this study or the publication of this article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Raja SN, Carr DB, Cohen M. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi:10.1097/j.pain.0000000000001939
2. Oster G, Harding G, Dukes E, Edelsberg J. PD ClearyPain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6(6):356–363. doi:10.1016/j.jpain.2005.01.359
3. Gore M, Brandenburg NA, Hoffman DL, Tai KS, Stacey B. Burden of illness in painful diabetic peripheral neuropathy: the patients’ perspectives. J Pain. 2006;7(12):892–900. doi:10.1016/j.jpain.2006.04.013
4. O’Connor AB. Neuropathic Pain. Pharmacoeconomics. 2009;27:95–112. doi:10.2165/00019053-200927020-00002
5. Geddes LA. History of magnetic stimulation of the nervous system. J Clin Neurophysiol. 1991;8(1):3–9. doi:10.1097/00004691-199101000-00003
6. Malmivuo J, Plonsey R, editors. Magnetic Stimulation of Neural Tissues, Chapter 22. In: Bioelectromagnetism. Oxford University Press; 1995.
7. Polson MJR, Barker AT, Freeston IL. Stimulation of nerve trunks with time varying magnetic fields. Med Biol Eng Comput. 1982;20:243–244. doi:10.1007/BF02441362
8. Lucinda D. Who invented TMS? Brainclinics Foundation; 2021.
9. Barker AT, Jalinous R, Freeston IL. Noninvasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi:10.1016/S0140-6736(85)92413-4
10. Albert Leung MD, Amir Fallah BS, Shivshil Shukla BS. Transcutaneous Magnetic Stimulation (tMS) in Alleviating Post-Traumatic Peripheral Neuropathic Pain States: a Case Series. Pain Medicine. 2014;15(7):1196–1199. doi:10.1111/pme.12426
11. Deer TR, Eldabe S, Falowski SM, et al. Peripherally Induced Reconditioning of the Central Nervous System: a Proposed Mechanistic Theory for Sustained Relief of Chronic Pain with Percutaneous Peripheral Nerve Stimulation. J Pain Res. 2021;14:721–736. doi:10.2147/JPR.S297091
12. Beaulieu LD, Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol Clin. 2015;45(3):223–237. doi:10.1016/j.neucli.2015.08.002
13. Hoffmann CM, D’Souza RS, Hagedorn JM. An Advanced Practice Provider Guide to Peripheral Nerve Stimulation. J Pain Res. 2022;15:2283–2291. doi:10.2147/JPR.S370037
14. Rao VP, Satyarengga M, Lamos EM, Munir KM. The Use of Transcutaneous Magnetic Stimulation to Treat Painful Diabetic Neuropathy. J Diabetes Sci Technol. 2021;15(6):1406–1407. doi:10.1177/19322968211026943
15. Bagnato GL, Miceli G, Marino N, Sciortino D, Bagnato GF. Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology. 2016;55:755–762. doi:10.1093/rheumatology/kev426
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






