Back to Journals » Drug Design, Development and Therapy » Volume 14
Magnesium Isoglycyrrhizinate Induces an Inhibitory Effect on Progression and Epithelial–Mesenchymal Transition of Laryngeal Cancer via the NF-κB/Twist Signaling
Authors Zhang J, Zhao R, Xing D, Cao J, Guo Y, Li L, Sun Y, Tian L, Liu M
Received 16 July 2020
Accepted for publication 18 November 2020
Published 22 December 2020 Volume 2020:14 Pages 5633—5644
DOI https://doi.org/10.2147/DDDT.S272323
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Jiarui Zhang, Rui Zhao, Dongliang Xing, Jing Cao, Yan Guo, Liang Li, Yanan Sun, Linli Tian, Ming Liu
Department of Otorhinolaryngology, Head and Neck Surgery, The Second Affiliated Hospital, Harbin Medical University, Harbin City, Heilongjiang Province 150086, People’s Republic of China
Correspondence: Linli Tian; Ming Liu
Department of Otorhinolaryngology, Head and Neck Surgery, The Second Affiliated Hospital, Harbin Medical University, No. 148 Baojian Road, Nangang District, Harbin City, Heilongjiang Province 150086, People’s Republic of China
Email [email protected]; [email protected]
Background: Magnesium isoglycyrrhizinate (MI) was extracted from roots of the plant Glycyrrhiza glabra, which displays multiple pharmacological activities such as anti-inflammation, anti-apoptosis, and anti-tumor. Here, we aimed to investigate the effect of MI on the progression and epithelial–mesenchymal transition (EMT) of laryngeal cancer.
Methods: Forty laryngeal cancer clinical samples were used. The role of MI in the proliferation of laryngeal cancer cells was assessed by MTT assay, Edu assay and colony formation assay. The function of MI in the migration and invasion of laryngeal cancer cells was tested by transwell assays. The effect of MI on apoptosis of laryngeal cancer cells was determined by cell apoptosis assay. The impact of MI on tumor growth in vivo was analyzed by tumorigenicity analysis using Balb/c nude mice. qPCR and Western blot analysis were performed to measure the expression levels of gene and protein, respectively.
Results: We identified that EMT-related transcription factor Twist was significantly elevated in the laryngeal cancer tissues. The expression of Twist was also enhanced in the human laryngeal carcinoma HEP-2 cells compared with that in the primary laryngeal epithelial cells. The high expression of Twist was remarkably correlated with poor overall survival of patients with laryngeal cancer. Meanwhile, our data revealed that MI reduced cell proliferation, migration and invasion and enhanced apoptosis of laryngeal cancer cells in vitro. Moreover, MI decreased transcriptional activation and the expression levels of NF-κB and Twist, and alleviated EMT in vitro and in vivo. MI remarkably inhibited tumor growth and EMT of laryngeal cancer cells in vivo.
Conclusion: MI restrains the progression of laryngeal cancer and induces an inhibitory effect on EMT in laryngeal cancer by modulating the NF-κB/Twist signaling. Our finding provides new insights into the mechanism by which MI inhibits laryngeal carcinoma development, enriching the understanding of the anti-tumor function of MI.
Keywords: laryngeal cancer, progression, EMT, magnesium isoglycyrrhizinate, Twist, NF-κB signaling
Introduction
Laryngeal cancer is one of the most prevalent malignancies of the respiratory system, accounting for 26% to 30% of head and neck cancers.1,2 Almost 95% of the histologic pathogeny of laryngeal cancer is laryngeal squamous carcinoma, and the survival incidence of patients is low.3 Despite the development of new strategies in surgery, chemotherapy, and radiation, the targeted treatment of laryngeal cancer is still a challenge.4 Thus, identification of safe and effective treatment candidates for laryngeal cancer is urgently needed. Epithelial–mesenchymal transition (EMT) serves as a cellular program, in which cells drop their epithelial features and gain mesenchymal characteristics.5,6 EMT is correlated with multiple tumor progressions, such as resistance to therapy, blood intravasation, tumor initiation, tumor cell migration, tumor stemness, malignant progression, and metastasis.7–9 As a critical process of cancer development, EMT contributes to the development of laryngeal cancer. It has been reported that the combination of photodynamic therapy and carboplatin suppresses the expression of MMP-2/MMP-9 and EMT of laryngeal cancer by ROS-inhibited MEK/ERK signaling.10 EZH2 increases metastasis and aggression of laryngeal squamous cell carcinoma through the EMT program by modulating H3K27me3.11 However, investigation about the inhibitory candidates of EMT in laryngeal cancer remains limited.
Nutraceutical agents display a unique therapeutic activity to treat diseases such as inflammation and cancer.12–17 Glycyrrhizic acid (GA) is extracted from the roots of licorice and serves as a major component of licorice, presenting multiple biomedical activities, such as anti-oxidant and anti-inflammatory.18 Magnesium isoglycyrrhizinate (MI), refined from GA, is a18-α-GA stereoisomer magnesium salt and demonstrates a better activity than 18-β-GA.19 As a natural and safe compound, MI shows many biomedical activities such as anti-inflammation capacities,20 anti-apoptosis21 and anti-tumor.22 It has been reported that MI inhibits fructose-modulated lipid metabolism disorder and activation of the NF-κB/NLRP3 inflammasome.23 MI reduces paclitaxel in patients with epithelial ovarian cancer managed with cisplatin and paclitaxel.24 Moreover, MI attenuates high fructose-induced liver fibrosis and EMT by upregulating miR-375-3p to overcome the TGF-β1/Smad pathway and JAK2/STAT3 signaling.25 However, the role of MI in cancer development is unknown. The effect of MI on the progression and EMT of laryngeal cancer remains unreported.
Many transcription factors, such as Twist, serve as molecule switches in EMT progression.26 Twist is a crucial transcription factor for the modulating of EMT, which advances cell invasion, migration, and cancer metastasis, conferring tumor cells with stem cell-like properties and providing therapy resistance.27,28 The function of Twist in EMT of cancer development has been well reported. Disrupting the diacetylated Twist represses the progression of Basal-like breast cancer.29 The activation of Twist promotes progression and EMT of breast cancer.30 The inhibition of Twist limits the stem cell features and EMT of prostate cancer.31 Twist serves as a critical factor in promoting metastasis of pancreatic cancer.32 Besides, tumor necrosis factor α provokes EMT of hypopharyngeal cancer and induces metastasis through NF-κB signaling-regulated expression of Twist.33 NF-κB/Twist signaling is involved in the mechanism of Chysin inhibiting stem cell characteristics of ovarian cancer.34 Repression of the NF-κB/Twist axis decreases the stemness features of lung cancer stem cell.35 Down-regulation of TWIST reduces invasion and migration of laryngeal carcinoma cells by controlling the expression of N-cadherin and E-cadherin.36 The expression of Twist displays the clinical significance of laryngeal cancer.37 However, whether NF-κB and Twist are involved in the biomedical activities of MI is unclear.
In this study, we aimed to explore the function of MI in the development and EMT process of laryngeal cancer. We identified a novel inhibitory effect of MI in the progression and EMT of laryngeal cancer by regulating the NF-κB/Twist signaling.
Methods
Laryngeal Cancer Clinical Samples
A total of 40 laryngeal cancer clinical samples used in this study were obtained from the Second Affiliated Hospital, Harbin Medical University between June 2016 and August 2018. All the patients were diagnosed by clinical, radiographic, and histopathological analysis. Before surgery, no systemic or local therapy was performed on the subjects. The laryngeal cancer tissues (n = 40) and adjacent normal tissues (n = 40) obtained from the patients were immediately frozen into liquid nitrogen and stored at −80 °C before use. The clinical laryngeal cancer samples were separated into two groups according to the mean expression of Twist. The overall survival was analyzed by Kaplan–Meier survival analysis. The patients and healthy controls provided written informed consent, and that the Ethics Committee of The Second Affiliated Hospital, Harbin Medical University approved this study.
Cell Culture and Treatment
The human laryngeal carcinoma cells HEP-2 and primary laryngeal epithelial cells LEC-P were purchased from American Type Tissue Culture Collection. Cells were cultured in DMEM (Solarbio, China) containing 10% fetal bovine serum (Gibco, USA), 0.1 mg/mL streptomycin (Solarbio, China) and 100 units/mL penicillin (Solarbio, China) at 37 °C with 5% CO2. The Magnesium isoglycyrrhizinate (MI) (purity > 98%) was obtained from Zhengda Tianqing Pharmaceutical Co., Ltd (Jiangsu, China). Cells were treated with MI of indicated dose before further analysis.
Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNAs were extracted using TRIZOL (Invitrogen, USA), followed by reverse transcription into cDNA. The qRT-PCR reactions were prepared using the SYBR Real-time PCR I kit (Takara, Japan). GAPDH was used as the internal control. The qRT-PCR experiment was conducted in triplicate. The primer sequences were as follows:
Twist forward: 5′-CGCTGAACGAGGCATTTGC-3′
Twist reverse: 5′-CCAGTTTGAGGGTCTGAATC-3′
Slug forward: 5′-GTGTTTGCAAGATCTGCGGC-3′
Slug reverse: 5′-GCAGATGAGCCCTCAGATTTGA-3′
ZEB1 forward: 5′-CCCCAGGTGTAAGCGCAGAA-3′
ZEB1 reverse: 5′-TGGCAGGTCATCCTCTGGTACAC-3′
Snail forward: 5′-CCACACTGGTGAGAAGCCTTTC-3′
Snail reverse: 5′-GTCTGGAGGTGGGCACGTA-3′
GAPDH forward: 5′-AAGAAGGTGGTGAAGCAGGC-3′.
GAPDH reverse: 5′-TCCACCACCCAGTTGCTGTA-3′
MTT Assays
MTT assays were conducted to measure cell viability of HEP-2 cells. Briefly, about 1 × 104 HEP-2 cells were put into 96 wells and cultured for 12 h. Cells were then added with 10 μL MTT solution (5 mg/mL) (Sigma, USA) and cultured for another 4 h. The culture medium was discarded, and 150 μL/well DMSO (Thermo, USA) was added to the wells. An ELISA browser was used to analyze the absorbance at 570 nm (Bio-Tek EL 800, USA).
EdU Assays
The cell proliferation was analyzed by EdU assays using EdU detecting kit (RiboBio, China). Briefly, HEP-2 cells were cultured with EdU for 2 h, followed by fixation with 4% paraformaldehyde at room temperature for 30 min. Then, cells were permeabilized with 0.4% Triton X-100 for 10 min and stained with staining cocktail of EdU at room temperature for 30 min in the dark. Next, nuclear of the cells was stained with Hoechst at room temperature for 30 min. Images were analyzed using a fluorescence microscope.
Colony Formation Assay
About 1 × 103 HEP-2 cells were layered in 6 wells and incubated in DMEM at 37 °C. After two weeks, cells were cleaned with PBS Buffer, made in methanol for 30 min, and dyed with 1% crystal violet. The number of colonies was then calculated.
Transwell Assays
Transwell assays were conducted to evaluate the impacts of MI on cell invasion and migration of HEP-2 cells using a Transwell plate (Corning, USA). Briefly, the upper chambers were plated with about 1 × 105 cells. Cells were then solidified using 4% paraformaldehyde and dyed with crystal violet. The invaded and migrated cells were recorded and calculated.
Analysis of Cell Apoptosis
Approximately 2 × 105 HEP-2 cells were plated on 6-well dishes. Cell apoptosis was detected using the Annexin V-FITC Apoptosis Detection Kit (CST, USA) following the manufacturer’s instructions. Briefly, cells were collected and washed with binding buffer (BD Biosciences, USA), and then dyed at 25 °C, followed by flow cytometry analysis.
Luciferase Reporter Gene Assay
Luciferase reporter gene assay was performed using the Dual-luciferase Reporter Assay System (Promega, USA). Briefly, cells were treated MI as indicated dose, followed by transfection with pGL3-NF-κB and pGL3-Twist using Lipofectamine 3000 (Invitrogen, USA). Luciferase activities were detected and Renilla was used as a normalized control.
Western Blot Analysis
Total proteins were extracted from cells or tumor tissues using RIPA buffer (CST, USA). Protein concentrations were measured using the BCA Protein Quantification Kit (Abbkine, USA). Same amount of protein samples was separated by SDS-PAGE (12% polyacrylamide gels), transferred to PVDF membranes (Millipore, USA) in the subsequent step. The membranes were blocked with 5% milk and incubated with the primary antibodies for Twist (1:1000) (Abcam, UK), E-Cadherin (1:1000) (Abcam, UK), occluding (1:1000) (Abcam, UK), vimentin (1:1000) (Abcam, UK), N-cadherin (1:1000) (Abcam, UK), Ki-67 (1:1000) (Abcam, UK), IKK (1:1000) (Abcam, UK), NF-κB p65 (1:1000) (Abcam, UK), NF-κB (1:1000) (Abcam, UK), and β-actin (1:1000) (Abcam, UK) at 4 °C overnight, in which β-actin served as the control. Then, the corresponding second antibodies (1:1000) (Abcam, UK) were used to incubate the membranes at room temperature for 1 h, followed by visualization using an Odyssey CLx Infrared Imaging System. ImageJ software was used to quantify the results.
Analysis of Tumorigenicity in Nude Mice
The effect of MI on tumor growth in vivo was analyzed in nude mice of Balb/c. Mice were randomly separated into two groups (n = 3). To establish the in vivo tumor model, HEP-2 cells were treated with MI (300 mg/kg) or equal volume of saline. And about 2 × 106 cells were subcutaneously injected into mice. After 7 days of injection, tumor growth was measured every 7 days. The mice were sacrificed after 35 days of injection and tumors were scaled. Tumor volume (V) was observed by estimating the length (L) and width (W) with calipers and measured with the formula (L ×W2) × 0.5. The expression levels of Ki-67 of tumor tissues were measured by immunohistochemical staining with the Ki67 antibody (1:1000) (Abcam, UK). Protein expression levels in tumor tissues were determined by Western blot analysis using Twist (1:1000) (Abcam, UK), E-Cadherin (1:1000) (Abcam, UK), occluding (1:1000) (Abcam, UK), vimentin (1:1000) (Abcam, UK), N-cadherin (1:1000) (Abcam, UK), Ki-67 (1:1000) (Abcam, UK), IKK (1:1000) (Abcam, UK), NF-κB p65 (1:1000) (Abcam, UK), NF-κB (1:1000) (Abcam, UK), and β-actin (1:1000) (Abcam, UK). Animal care and experimental procedures in this study were approved by the Animal Ethics Committee of the Second Affiliated Hospital, Harbin Medical University.
Statistical Analysis
Data were presented as mean ± SD, and statistical analysis was performed using SPSS software (version 18.0). The unpaired Student’s t-test was applied for comparing two groups, and one-way ANOVA was applied for comparing multiple groups. P < 0.05 was considered as statistically significant.
Results
Twist is Potentially Correlated with the Progression and Poor Prognosis of Laryngeal Cancer
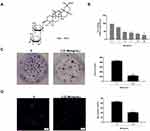
To assess the potential correlation of EMT-related transcription factors with laryngeal cancer, the expression of Twist, Slug, ZEB1, and Snail in the clinical laryngeal samples and laryngeal cells were measured by qPCR assays. The data showed that the expression levels of Twist, Slug, ZEB1, and Snail were significantly elevated in laryngeal cancer tissues (n = 40) compared to that in normal tissues (n = 40), among which Twist displayed the highest expression levels (P < 0.01) (Figure 1A). Meanwhile, the expression levels of Twist, Slug, ZEB1, and Snail were also enhanced in human laryngeal carcinoma HEP-2 cells compared with that in primary laryngeal epithelial cells (LEC-P), and Twist exerted the highest expression levels among these transcription factors (P < 0.001) (Figure 1B), implying that Twist is closely associated with the development of laryngeal cancer. To determine whether Twist was able to serve as the potential biomarker for patients with laryngeal cancer, we separated the clinical laryngeal cancer samples into two groups according to the mean expression of Twist. We observed that high expression levels of Twist was remarkably correlated with the poor overall survival (P < 0.01) (Figure 1C), suggesting that Twist may play a crucial role in the progression of laryngeal cancer.
Magnesium Isoglycyrrhizinate (MI) Attenuates Cell Proliferation of Laryngeal Cancer in vitro
Then, the role of MI in the modulation of laryngeal cancer proliferation was investigated, and the structure formula of MI is shown in Figure 2A. To evaluate the effect of MI on the progression of laryngeal cancer, MTT assay, colony formation assay, and EdU assay were performed in HEP-2 cells treated with MI. MTT assay demonstrated that MI significantly inhibited cell viability in a dose-dependent manner, and the half-maximal inhibitory concentrations (IC50) of MI in HEP-2 cells was 3.22 mg/mL (P < 0.01) (Figure 2B). Hence, we selected the MI dose of 3.22 mg/mL in the following experiments. Similarly, colony formation assay showed that the colony numbers of HEP-2 cells were remarkably reduced by MI treatment (P < 0.01) (Figure 2C). Besides, EdU assay showed that MI notably decreased number of EdU-positive cells (P < 0.01) (Figure 2D). These data suggested that MI was able to inhibit cell proliferation of laryngeal cancer.
MI Inhibits Laryngeal Cancer Cell Migration and Invasion and Enhances Cell Apoptosis in vitro
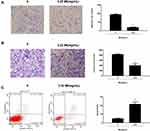
The role of MI in HEP-2 cell migration, invasion, and apoptosis was then evaluated. Transwell assays revealed that MI treatment remarkably reduced cell migration (P < 0.01) (Figure 3A). Similarly, cell invasion was significantly decreased by MI in HEP-2 cells (P < 0.01) (Figure 3B). Moreover, flow cytometry analysis showed that cell apoptosis was notably increased by MI treatment in the system (P < 0.01) (Figure 3C). These results indicated that MI inhibited laryngeal cancer cell migration and invasion and enhanced cell apoptosis in vitro.
MI Inhibits Transcriptional Activation and the Expression of NF-κB and Twist and Alleviates EMT in Laryngeal Cancer Cells
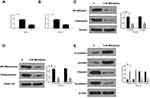
Next, the underlying mechanism of the effect of MI on the development of laryngeal cancer in HEP-2 cells was further explored. It showed that MI treatment significantly reduced the luciferase activities of NF-κB in the cells (P < 0.01) (Figure 4A), suggesting that MI may inhibit NF-κB at transcriptional level. Meanwhile, dual-luciferase reporter gene assays further revealed that MI treatment remarkably decreased the transcriptional activities of Twist in the cells (P < 0.01) (Figure 4B). Furthermore, Western blot analysis demonstrated that the total expression (Figure 4C) and nucleus expression (Figure 4D) of NF-κB and Twist were significantly down-regulated by MI in HEP-2 cells (P < 0.01), suggesting that MI may inhibit Twist by modulating NF-κB. Moreover, to assess the effect of MI on the EMT of laryngeal cancer, the expression of EMT markers, including E-Cadherin, occluding, vimentin, and N-cadherin, in HEP-2 cells treated with MI were measured. Our data showed that the expression levels of E-Cadherin and occluding were enhanced while the expression levels of vimentin and N-cadherin were reduced by MI treatment in HEP-2 cells (P < 0.01) (Figure 4E), suggesting that MI can inhibit EMT of laryngeal cancer.
MI Inhibits Tumor Growth and EMT of Laryngeal Cancer via the Twist/NF-κB Signaling in vivo
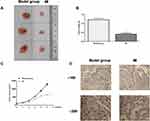
The effect of MI on laryngeal cancer development was further investigated in vivo. Tumorigenicity analysis was conducted in nude mice injected with HEP-2 cells, which were treated with MI or corresponding control. The MI treatment significantly reduced tumor size (Figure 5A), tumor weight (P < 0.01) (Figure 5B), tumor volume (P < 0.01) (Figure 5C), and the expression levels of Ki-67 in tumor tissues of the mice (Figure 5D), suggesting that MI inhibited tumor growth of laryngeal cancer in vivo.
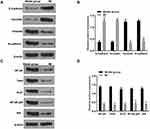
Moreover, our data showed that MI treatment significantly enhanced the expression levels of E-Cadherin and occluding, whereas reduced the expression levels of vimentin and N-cadherin in tumor tissues of the mice (P < 0.01) (Figure 6A and B), indicating that MI attenuated EMT of laryngeal cancer in vivo. Besides, the expression levels of Twist, Ki-67, and NF-κB signaling proteins containing p65 and IKK were significantly decreased by MI in tumor tissues of the mice (P < 0.01) (Figure 6C and D), implying that MI may inhibit EMT of laryngeal cancer via the Twist/NF-κB signaling.
Discussion
Laryngeal cancer is the second most prevalent head and neck malignancy.38 Laryngeal cancer patients hold poor survival incidences and low prognosis, and many cases still suffer from recurrence.39 Although improvements have been made in chemotherapy, radiotherapy, and surgery, invasion and metastasis are still the principal reasons for laryngeal cancer-related mortality for patients with advanced grade.40 Searching for more safe and practical treatment candidates for the effective therapy of laryngeal cancer is of great importance.41 As a natural compound, MI has exerted practical potential in the medical application. It has been reported that MI represses cardiac hypertrophy by modulating the TLR4/NF-κB signaling in mice.42 MI preserves against triptolide-produced hepatotoxicity through activating the Nrf2 signaling.43 MI relieves fructose-mediated apoptosis of podocyte by down-regulation of miR-193a to enhance WT1.44 However, the investigation of MI in cancer progression is limited. Previous study identified that the exposure-impact-toxicity was correlated with MI and docetaxel in non-small cell lung cancer mice.22 MI inhibits chemotherapy-caused damage to liver throughout the initial therapy of gastrointestinal tumor patients.45 MI decreases paclitaxel in patients with epithelial ovarian cancer treated with cisplatin and paclitaxel.24 The function of MI is associated with its activity to restrain several crucial pathways such as phospholipase A2/arachidonic signaling,46 STAT3 signaling,47 and NF-κB signaling.23,48 In this study, we firstly identified that MI inhibited cell proliferation, migration, invasion, and enhanced cell apoptosis in laryngeal cancer. MI reduced tumor growth of laryngeal cancer in vivo. Our data present a novel inhibitory function of MI in laryngeal cancer and provide valuable insights into the role of MI in cancer development.
EMT plays a critical role in the development of laryngeal cancer, especially in cancer cell metastasis progression by promoting resistance to apoptotic stimulation, invasion, and mobility.49 It was reported that TGF β-induced lncRNA MIR155HG promoted EMT of laryngeal squamous cell carcinoma by regulating the miR-155/SOX10 signaling.50 YAP modulates the Wnt/β-catenin signaling and EMT program of laryngeal cancer.51 Abnormal methylation and down-regulation of ZNF667 and ZNF667-AS1 enhance EMT of laryngeal carcinoma.52 Meanwhile, it was reported that MI alleviated high fructose-caused liver fibrosis and EMT by enhancing miR-375-3p to repress the TGF-β1/Smad signaling and JAK2/STAT3 signaling in rat.25 Our data demonstrated that MI attenuated EMT of laryngeal cancer via modulating the Twist/NF-κB signaling. It provides valuable information that MI exerts an inhibitory function of EMT in cancer progression.
As a critical EMT transcription factor, Twist regulates EMT program during cancer development.53 The correlation of Twist with NF-κB signaling in the modulation of EMT in cancer progression is well identified. It has been reported that presentation to TGF β combined with TNF α provokes tumorigenesis by modulating NF-κB/Twist signaling in vitro.54 Antrodia salmonea represses aggression and metastasis through modifying EMT via the NF-κB/Twist signaling in triple-negative breast cancer cells.55 Twist is involved in the mechanism that ursolic acid restrains EMT of gastric cancer through the Axl/NF-κB signaling.56 NF-κB activation by the RANKL/RANK pathway enhances the expression of snail and twist and promotes EMT of mammary cancer cells.57 Twist plays a crucial role in regulating the NF-κB and HIF-1α signaling on hypoxia-induced chemoresistance and EMT in pancreatic cancer.58 MiR-153 depletion down-regulates the expression of metastasis-associated family member 3 and Twist family BHLH transcription factor 1 in laryngeal squamous carcinoma cells.59 The expression of TWIST is remarkably down-regulated in response to paclitaxel, and TWIST may present a crucial function in paclitaxel-related laryngeal cancer cell apoptosis.60 TWIST is involved in the mechanism that TrkB elevates the metastasis of laryngeal cancer by activating the PI3K/AKT signaling.61 In the present study, we revealed that Twist was significantly elevated in laryngeal cancer tissues and human laryngeal carcinoma HEP-2 cells. It suggests that Twist may play a critical role in the development of laryngeal cancer. NF-κB/Twist signaling was involved in the mechanism of MI inhibiting EMT of laryngeal cancer. It provides new evidence of the role of Twist in cancer progression.
Conclusion
In conclusion, we discovered that magnesium isoglycyrrhizinate attenuated the progression of laryngeal cancer in vitro and in vivo. Magnesium isoglycyrrhizinate induced an inhibitory effect on epithelial–mesenchymal transition in laryngeal cancer by modulating the NF-κB/Twist signaling. Our findings provide new insights into the mechanism by which magnesium isoglycyrrhizinate inhibits laryngeal carcinoma development. Magnesium isoglycyrrhizinate may be applied as a potential anti-tumor candidate for laryngeal cancer in clinical treatment strategy.
Abbreviations
EMT, epithelial–mesenchymal transition; MI, Magnesium isoglycyrrhizinate; GA, Glycyrrhizic acid; LEC-P, primary laryngeal epithelial cells; OS, overall survival; IC50, half-maximal inhibitory concentration.
Ethics Approval and Consent to Participate
The patients and healthy controls provided written informed consent, and that the Ethics Committee of The Second Affiliated Hospital, Harbin Medical University approved this study. All animal experiments in this study were approved according to the standards of Care and Use enacted by Laboratory Animals of The Second Affiliated Hospital, Harbin Medical University, and was approved by the Animal Ethics Committee of The Second Affiliated Hospital, Harbin Medical University.
Funding
This work was supported by the Natural Science Foundation of Heilongjiang Province [grant numbers LH2019H014] and The Research Project Foundation Traditional Chinese Medicine Scientific of Heilongjiang Province (grant numbers 3-52).
Disclosure
All the authors have declared no potential conflicts of interest for this research.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An update on larynx cancer. CA Cancer J Clin. 2017;67(1):31–50. doi:10.3322/caac.21386
3. Su J, Lu E, Lu L, Zhang C. MiR-29a-3p suppresses cell proliferation in laryngocarcinoma by targeting prominin 1. FEBS Open Bio. 2017;7(5):645–651. doi:10.1002/2211-5463.12199
4. Tomeh C, Holsinger FC. Laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg. 2014;22(2):147–153. doi:10.1097/MOO.0000000000000032
5. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84.
6. Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29(3):212–226. doi:10.1016/j.tcb.2018.12.001
7. Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45. doi:10.1016/j.cell.2016.06.028
8. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110.
9. Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16(6):488–494. doi:10.1038/ncb2976
10. Mao W, Sun Y, Zhang H, Cao L, Wang J, He P. A combined modality of carboplatin and photodynamic therapy suppresses epithelial-mesenchymal transition and matrix metalloproteinase-2 (MMP-2)/MMP-9 expression in HEp-2 human laryngeal cancer cells via ROS-mediated inhibition of MEK/ERK signalling pathway. Lasers Med Sci. 2016;31(8):1697–1705. doi:10.1007/s10103-016-2040-6
11. Luo H, Jiang Y, Ma S, et al. EZH2 promotes invasion and metastasis of laryngeal squamous cells carcinoma via epithelial-mesenchymal transition through H3K27me3. Biochem Biophys Res Commun. 2016;479(2):253–259. doi:10.1016/j.bbrc.2016.09.055
12. Wang A, Leong DJ, Cardoso L, Sun HB. Nutraceuticals and osteoarthritis pain. Pharmacol Ther. 2018;187:167–179. doi:10.1016/j.pharmthera.2018.02.015
13. Pandey MK, Gupta SC, Karelia D, Gilhooley PJ, Shakibaei M, Aggarwal BB. Dietary nutraceuticals as backbone for bone health. Biotechnol Adv. 2018;36(6):1633–1648. doi:10.1016/j.biotechadv.2018.03.014
14. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274.
15. Prasad S, Gupta SC, Tyagi AK. Reactive oxygen species (ROS) and cancer: role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi:10.1016/j.canlet.2016.03.042
16. Zhang S, Xin H, Li Y, et al. Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013:819296.
17. Sen Z, Weida W, Jie M, Li S, Dongming Z, Xiaoguang C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. 2019;57:385–395. doi:10.1016/j.phymed.2018.12.045
18. Liang B, Guo XL, Jin J, Ma YC, Feng ZQ. Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol. 2015;21(17):5271–5280. doi:10.3748/wjg.v21.i17.5271
19. Sui M, Jiang X, Chen J, Yang H, Zhu Y. Magnesium isoglycyrrhizinate ameliorates liver fibrosis and hepatic stellate cell activation by regulating ferroptosis signaling pathway. Biomed Pharmacother. 2018;106:125–133. doi:10.1016/j.biopha.2018.06.060
20. Li L, Zhou J, Li Q, Xu J, Qi J, Bian H. The inhibition of Hippo/Yap signaling pathway is required for magnesium isoglycyrrhizinate to ameliorate hepatic stellate cell inflammation and activation. Biomed Pharmacother. 2018;106:83–91. doi:10.1016/j.biopha.2018.06.102
21. Zhao Z, Tang Z, Zhang W, Liu J, Li B. Magnesium isoglycyrrhizinate protects against renal ischemia reperfusion injury in a rat model via antiinflammation, antioxidation and antiapoptosis. Mol Med Rep. 2017;16(3):3627–3633. doi:10.3892/mmr.2017.6993
22. Li P, Li S, Gu H, et al. The exposure-effect-toxicity correlation of docetaxel and magnesium isoglycyrrhizinate in non-small cell lung tumor-bearing mice. Biomed Pharmacother. 2018;97:1000–1010. doi:10.1016/j.biopha.2017.10.158
23. Zhao XJ, Yang YZ, Zheng YJ, et al. Magnesium isoglycyrrhizinate blocks fructose-induced hepatic NF-kappaB/NLRP3 inflammasome activation and lipid metabolism disorder. Eur J Pharmacol. 2017;809:141–150. doi:10.1016/j.ejphar.2017.05.032
24. Chen KJ, Chen WY, Chen X, Jia YM, Peng GQ, Chen L. Increased elimination of paclitaxel by magnesium isoglycyrrhizinate in epithelial ovarian cancer patients treated with paclitaxel plus cisplatin: a pilot clinical study. Eur J Drug Metab Pharmacokinet. 2014;39(1):25–31. doi:10.1007/s13318-013-0136-y
25. Yang YZ, Zhao XJ, Xu HJ, et al. Magnesium isoglycyrrhizinate ameliorates high fructose-induced liver fibrosis in rat by increasing miR-375-3p to suppress JAK2/STAT3 pathway and TGF-beta1/Smad signaling. Acta Pharmacol Sin. 2019;40(7):879–894. doi:10.1038/s41401-018-0194-4
26. Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13(9):963–972. doi:10.2174/15680096113136660102
27. Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer. 2016;15:18. doi:10.1186/s12943-016-0502-x
28. Cao J, Wang X, Dai T, et al. Twist promotes tumor metastasis in basal-like breast cancer by transcriptionally upregulating ROR1. Theranostics. 2018;8(10):2739–2751. doi:10.7150/thno.21477
29. Shi J, Wang Y, Zeng L, et al. Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25(2):210–225. doi:10.1016/j.ccr.2014.01.028
30. Lee HJ, Li CF, Ruan D, et al. The DNA damage transducer RNF8 facilitates cancer chemoresistance and progression through twist activation. Mol Cell. 2016;63(6):1021–1033. doi:10.1016/j.molcel.2016.08.009
31. Ruan D, He J, Li CF, et al. Skp2 deficiency restricts the progression and stem cell features of castration-resistant prostate cancer by destabilizing Twist. Oncogene. 2017;36(30):4299–4310. doi:10.1038/onc.2017.64
32. Krebs AM, Mitschke J, Lasierra Losada M, et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19(5):518–529. doi:10.1038/ncb3513
33. Yu L, Mu Y, Sa N, Wang H, Xu W. Tumor necrosis factor alpha induces epithelial-mesenchymal transition and promotes metastasis via NF-kappaB signaling pathway-mediated TWIST expression in hypopharyngeal cancer. Oncol Rep. 2014;31(1):321–327. doi:10.3892/or.2013.2841
34. Li H, Chen A, Yuan Q, et al. NF-kappaB/Twist axis is involved in chysin inhibition of ovarian cancer stem cell features induced by co-treatment of TNF-alpha and TGF-beta. Int J Clin Exp Pathol. 2019;12(1):101–112.
35. Zakaria N, Mohd Yusoff N, Zakaria Z, Widera D, Yahaya BH. Inhibition of NF-kappaB signaling reduces the stemness characteristics of lung cancer stem cells. Front Oncol. 2018;8:166. doi:10.3389/fonc.2018.00166
36. Yu L, Li HZ, Lu SM, et al. Down-regulation of TWIST decreases migration and invasion of laryngeal carcinoma Hep-2 cells by regulating the E-cadherin, N-cadherin expression. J Cancer Res Clin Oncol. 2011;137(10):1487–1493. doi:10.1007/s00432-011-1023-z
37. Xiang Z, Li Q, Chang A, Zhuo X, Zhang X. Expression and significance of TWIST, a zinc finger transcription factor, in laryngeal carcinoma among Chinese population: a meta-analysis. Int J Clin Exp Med. 2015;8(10):18351–18358.
38. Yang T, Li S, Liu J, Yin D, Yang X, Tang Q. lncRNA-NKILA/NF-kappaB feedback loop modulates laryngeal cancer cell proliferation, invasion, and radioresistance. Cancer Med. 2018;7(5):2048–2063. doi:10.1002/cam4.1405
39. Aydil U, Akmansu M, Gumusay O, et al. Failure of concurrent chemoradiotherapy for organ preservation in laryngeal cancer: survival outcomes and recurrence patterns. Ear Nose Throat J. 2019;98(7):E92–E96. doi:10.1177/0145561319839788
40. Kitani Y, Kubota A, Furukawa M, et al. Impact of combined modality treatment with radiotherapy and S-1 on T2N0 laryngeal cancer: possible improvement in survival through the prevention of second primary cancer and distant metastasis. Oral Oncol. 2017;71:54–59. doi:10.1016/j.oraloncology.2017.05.017
41. Obid R, Redlich M, Tomeh C. The treatment of laryngeal cancer. Oral Maxillofac Surg Clin North Am. 2019;31(1):1–11. doi:10.1016/j.coms.2018.09.001
42. Ma D, Zhang J, Zhang Y, et al. Inhibition of myocardial hypertrophy by magnesium isoglycyrrhizinate through the TLR4/NF-kappaB signaling pathway in mice. Int Immunopharmacol. 2018;55:237–244. doi:10.1016/j.intimp.2017.12.019
43. Tan QY, Hu Q, Zhu SN, et al. Licorice root extract and magnesium isoglycyrrhizinate protect against triptolide-induced hepatotoxicity via up-regulation of the Nrf2 pathway. Drug Deliv. 2018;25(1):1213–1223. doi:10.1080/10717544.2018.1472676
44. Li TS, Chen L, Wang SC, et al. Magnesium isoglycyrrhizinate ameliorates fructose-induced podocyte apoptosis through downregulation of miR-193a to increase WT1. Biochem Pharmacol. 2019;166:139–152. doi:10.1016/j.bcp.2019.05.016
45. Yan Y, Mo Y, Zhang D. [Magnesium isoglycyrrhizinate prevention of chemotherapy-induced liver damage during initial treatment of patients with gastrointestinal tumors]. Zhonghua Gan Zang Bing Za Zhi. 2015;23(3):204–208. Chinese.
46. Xie C, Li X, Wu J, et al. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/Arachidonic acid pathway. Inflammation. 2015;38(4):1639–1648. doi:10.1007/s10753-015-0140-2
47. Tang GH, Yang HY, Zhang JC, et al. Magnesium isoglycyrrhizinate inhibits inflammatory response through STAT3 pathway to protect remnant liver function. World J Gastroenterol. 2015;21(43):12370–12380. doi:10.3748/wjg.v21.i43.12370
48. Jiang W, Liu J, Li P, et al. Magnesium isoglycyrrhizinate shows hepatoprotective effects in a cyclophosphamide-induced model of hepatic injury. Oncotarget. 2017;8(20):33252–33264. doi:10.18632/oncotarget.16629
49. Mezi S, Chiappetta C, Carletti R, et al. Clinical significance of epithelial-to-mesenchymal transition in laryngeal carcinoma: its role in the different subsites. Head Neck. 2017;39(9):1806–1818. doi:10.1002/hed.24838
50. Cui W, Meng W, Zhao L, Cao H, Chi W, Wang B. TGF-beta-induced long non-coding RNA MIR155HG promotes the progression and EMT of laryngeal squamous cell carcinoma by regulating the miR-155-5p/SOX10 axis. Int J Oncol. 2019;54(6):2005–2018.
51. Tang X, Sun Y, Wan G, Sun J, Sun J, Pan C. Knockdown of YAP inhibits growth in Hep-2 laryngeal cancer cells via epithelial-mesenchymal transition and the Wnt/beta-catenin pathway. BMC Cancer. 2019;19(1):654. doi:10.1186/s12885-019-5832-9
52. Meng W, Cui W, Zhao L, Chi W, Cao H, Wang B. Aberrant methylation and downregulation of ZNF667-AS1 and ZNF667 promote the malignant progression of laryngeal squamous cell carcinoma. J Biomed Sci. 2019;26(1):13. doi:10.1186/s12929-019-0506-0
53. Diaz VM, de Herreros AG. F-box proteins: keeping the epithelial-to-mesenchymal transition (EMT) in check. Semin Cancer Biol. 2016;36:71–79. doi:10.1016/j.semcancer.2015.10.003
54. Dong W, Sun S, Cao X, et al. Exposure to TNFalpha combined with TGFbeta induces carcinogenesis in vitro via NF-kappaB/Twist axis. Oncol Rep. 2017;37(3):1873–1882. doi:10.3892/or.2017.5369
55. Hseu YC, Lin YC, Rajendran P, et al. Antrodia salmonea suppresses invasion and metastasis in triple-negative breast cancer cells by reversing EMT through the NF-kappaB and Wnt/beta-catenin signaling pathway. Food Chem Toxicol. 2019;124:219–230. doi:10.1016/j.fct.2018.12.009
56. Li J, Dai C, Shen L. Ursolic acid inhibits epithelial-mesenchymal transition through the Axl/NF-kappaB pathway in gastric cancer cells. Evid Based Complement Alternat Med. 2019;2019:2474805.
57. Tsubaki M, Komai M, Fujimoto S, et al. Activation of NF-kappaB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell lines. J Exp Clin Cancer Res. 2013;32:62. doi:10.1186/1756-9966-32-62
58. Cheng ZX, Wang DW, Liu T, et al. Effects of the HIF-1alpha and NF-kappaB loop on epithelial-mesenchymal transition and chemoresistance induced by hypoxia in pancreatic cancer cells. Oncol Rep. 2014;31(4):1891–1898. doi:10.3892/or.2014.3022
59. Zhang B, Fu T, Zhang L. MicroRNA-153 suppresses human laryngeal squamous cell carcinoma migration and invasion by targeting the SNAI1 gene. Oncol Lett. 2018;16(4):5075–5083.
60. Yu L, Li HZ, Lu SM, et al. Alteration in TWIST expression: possible role in paclitaxel-induced apoptosis in human laryngeal carcinoma Hep-2 cell line. Croat Med J. 2009;50(6):536–542. doi:10.3325/cmj.2009.50.536
61. Jiang L, Wang Z, Liu C, et al. TrkB promotes laryngeal cancer metastasis via activation PI3K/AKT pathway. Oncotarget. 2017;8(65):108726–108737. doi:10.18632/oncotarget.21711
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.