Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach
Authors Zhang B, Shi H, Wang H
Received 15 March 2023
Accepted for publication 12 June 2023
Published 26 June 2023 Volume 2023:16 Pages 1779—1791
DOI https://doi.org/10.2147/JMDH.S410301
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bo Zhang,1 Huiping Shi,1 Hongtao Wang2
1Jinling Institute of Science and Technology, Nanjing City, Jiangsu Province, People’s Republic of China; 2School of Life Science, Tonghua Normal University, Tonghua City, Jilin Province, People’s Republic of China
Correspondence: Bo Zhang, Jinling Institute of Science and Technology, No. 99, Hongjing Avenue, Jiangning District, Nanjing City, Jiangsu Province, 211169, People’s Republic of China, Email [email protected]
Abstract: Cancer is a leading cause of morbidity and mortality worldwide. While progress has been made in the diagnosis, prognosis, and treatment of cancer patients, individualized and data-driven care remains a challenge. Artificial intelligence (AI), which is used to predict and automate many cancers, has emerged as a promising option for improving healthcare accuracy and patient outcomes. AI applications in oncology include risk assessment, early diagnosis, patient prognosis estimation, and treatment selection based on deep knowledge. Machine learning (ML), a subset of AI that enables computers to learn from training data, has been highly effective at predicting various types of cancer, including breast, brain, lung, liver, and prostate cancer. In fact, AI and ML have demonstrated greater accuracy in predicting cancer than clinicians. These technologies also have the potential to improve the diagnosis, prognosis, and quality of life of patients with various illnesses, not just cancer. Therefore, it is important to improve current AI and ML technologies and to develop new programs to benefit patients. This article examines the use of AI and ML algorithms in cancer prediction, including their current applications, limitations, and future prospects.
Keywords: machine learning, artificial intelligence, treatment selection, cancer diagnosis, cancer-related mortality
Introduction
Cancer is a significant public health issue globally, marked by an elevated incidence and mortality rate.1 According to the GLOBOCAN 2020 database, approximately 19.3 million new cases and 10 million deaths have been reported annually.2 Lung cancer remains the most common cause of cancer-related mortality, with expected 1.8 million fatalities, followed by stomach, liver, colorectal, and breast cancer.2 The prevention and treatment of cancer remain difficult.3 After heart disease, cancer remains the second leading cause of death in the United States. In 2023, there are projected to be 1.9 million new cancer cases (equivalent to around 5370 cases per day) and 609,820 deaths from cancer (equivalent to around 1670 deaths per day) in the US. The International Agency for Research on Cancer (IARC) has released a poster on known causes and prevention by organ site of human cancer. A description of known causes and prevention by organ site is provided in Figure S1.
The “Global Cancer Observatory” reports indicate that on a global scale, 37 individuals are diagnosed with cancer and over 19 individuals succumb to the disease every minute. Figure 1A shows the number of new cases in 2020 and Figure 1B shows the number of deaths in 2020 due to all cancers.
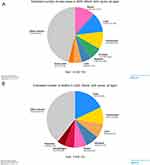 |
Figure 1 The number of new cancer cases reported in 2020 and the number of deaths caused by these cancers. (A). Estimated number of new cases worldwide in 2020 among both sexes. (B) Estimated number of deaths worldwide in 2020 among both sexes. Reprinted from World Health Organization. © International Agency for Research on Cancer, 2020. Cancer Today. Available from:https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0. [Accessed June 20, 2023].4 |
However, the introduction of machine learning and artificial intelligence positively supports cancer prevention and management.5 Artificial intelligence is commonly defined as a set of computer-coded programs or algorithms that use data analysis and pre-programmed instructions to make predictions and decisions about various aspects of a disease. Machine learning is a specialized field within AI that refers to a group of algorithms designed to automatically learn and improve from experience. In other words, machine learning is an AI subset that focuses on developing algorithms capable of learning from data and refining their performance over time.6,7 Deep Learning is a subfield of “Machine Learning” that employs neural network-based models to imitate the human brain’s capacity to analyze huge amounts of complicated data in areas such as language processing, drug discovery, and image recognition.8 An overview of AI, ML, and DL is provided in Figure 2.9
 |
Figure 2 (a) The concept of the AI, ML and DL. (b) Timescale of major breakthrough (c). Schematic of workflow of ML and (d). of DL. Notes: Reprinted from Cancer Letters, 471, Huang S, Yang J, Fong S, Zhao Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. 61–71, Copyright (2020), with permission from Elsevier.9 |
All of these computer algorithms use data such as investigations performed, scans conducted, patients’ medical histories, and other information to forecast or diagnose a cancerous condition. During surgical procedures, the use of cutting-edge imaging technology and artificial intelligence (AI) has enabled the real-time detection and diagnosis of brain tumors.10 Moreover, this approach demonstrated an exceptional ability to differentiate between malignant and normal tissue by accurately identifying cancerous tissue. The use of artificial intelligence (AI) to analyze the expression of specific genes enabled successful classification of cancer based on their activity levels, distinguishing between active, hyperactive, and quiet genes in both malignant and normal tissue.11 Similarly, machine learning algorithms have been applied to identify mismatch repair deficit (dMMR) in colorectal screening.12,13
AI techniques such as CS-SVM have been used on liver cancer rehabilitation groups and discovered that it can predict the timing and site of cancer recurrence.14 Studies have also reported that different ML and AI techniques serve as models for global practices allowing big data and cognition-capable computers to aid cancer research specialists in revolutionizing medicine by performing multifaceted tasks to improve clinical workflow, diagnostic accuracy, reduce human resource cost, increase efficiency of data, and enhance treatment.15,16 Therefore, researchers are increasingly discussing the use of AI and ML for cancer prognosis, diagnosis, and rehabilitation; assessing the broad scope, development, and accomplishments may serve as a benchmark for future studies and applications of breakthrough technology.17
Although AI has been quickly integrated into cancer research, artificial intelligence-based solutions are still in their early stages. Only a few applications based on AI have been authorized for usage in the real world, such as in drug firms, hospitals, and so on. It is still a matter of debate whether AI can replace medical practitioners as professionals.
Much of the popular discourse on artificial intelligence centers on AI’s use in cancer clinical research. As a result, research into AI technologies has accelerated and achieved performance similar to that of human biological experts. Moreover, AI will provide human decision-makers with more knowledge and may become an integral part of the health-care team. Figure 3 shows schematic representation of machine learning representative fields of health-care services. This article presents an introduction to AI and ML in cancer clinical research including its prediction, prognosis, and limitations.
 |
Figure 3 Machine learning representative fields of health care services. |
Machine Learning- An Introduction
Machine Learning (ML) is a subfield of artificial intelligence (AL) that allows computers to “learn” from training data and enhance their performance with time without being supervised learning.18 Machine learning can recognize patterns in data and obtain information from them in order to develop their own predictions.19 Generally, machine learning models and algorithms acquire knowledge through experience. These models and algorithms retrieve patterns in data and link those patterns to compact classes of samples in the data.20 For example, given a set of features discussing a person, an ML model with experience can predict whether that person is ill or healthy; given a set of parameters describing an animal, an ML model predicts whether that animal is being treated or under control; or given a set of features describing molecules, an ML model predicts whether those molecules are likely to interact or not. Such patterns may also be discovered using ML methods in an agnostic way, that is, without knowledge of the classes. These techniques are referred to as supervised and unsupervised machine learning, respectively.21 Reinforcement learning is a third form of ML that looks for a series of actions that help achieve a certain objective. All of these techniques are gaining popularity in biomedical research across a wide range of disciplines, including treatment outcome prediction, drug development, medical imaging analysis, patient stratification, molecular interactions, and many more.21
Furthermore, researchers describe ML as an academic field of study that incorporates computer science, statistics, and mathematics. Machine learning is the fundamental engine that is driving the development of artificial intelligence forward.22 It is remarkably being used in both business and academia to promote the creation of “intelligent products” that can generate accurate predictions from varied data sources.23 To date, the primary benefactors of the twenty-first-century surge in the availability of big data, data science, and machine learning have been companies that were able to acquire these data and pay the required personnel to transform their products. The learning methods created in and for these businesses have the potential to significantly improve clinical treatment and medical research, particularly as more and more providers adopt electronic health records. For example, the medical sector may benefit from the deployment of machine learning techniques in the areas of diagnostics and outcome prediction using electronic health records. This includes the prospect of identifying those at high risk for medical problems such as relapse or progression to a different illness state. Recently, ML algorithms have been used successfully in prognosis of skin cancer with equivalent accuracy to a skilled dermatologist,24 and to predict the development of pre-diabetes type 2 diabetes using routinely obtained electronic health record data of the patients.25 In medical sciences, one of the biggest advantages of using machine learning is that it is an automated technique that lets robots solve issues with minimal or no human input and respond based on previous observations.
An Introduction to AI in the Medical Field
In healthcare, artificial intelligence refers to the use of software, or “machine-learning algorithms” to simulate human “cognition” in the examination, presentation, and understanding of complicated health and medical care data.26 In particular, artificial intelligence can provide results based on input data alone. The basic objective of AI applications in the field of health care is to examine associations between patient outcomes and clinical procedures. But what differentiates artificial intelligence from previous traditional methods is its ability to collect data, process it, interpret it, and provide a definite output.27 This is accomplished by AI using deep learning methods and machine learning techniques. These systems can identify behavioral patterns and generate their own reasoning. Currently, AI-based information is being used in diagnostic procedures, medication development, customized medicine, patient monitoring, and treatment protocol formation.28
For example, it is being used in precisely identifying and risk-stratifying individuals with “coronary artery disease.” In terms of diagnostic accuracy of coronary artery disease, artificial intelligence algorithms have demonstrated potential as an early triage tool.29 Several aspects of gastroenterology are also taking advantage of the use of AI. For instance, endoscopic examinations, including colonoscopies and esophagogastroduodenoscopies rely on the quick diagnosis of abnormal tissue. Researchers are now believing that by incorporating AI into these endoscopic procedures, practitioners may diagnose illnesses, estimate their severity, and view blind regions more quickly.30
Additionally, AI has demonstrated great potential in the clinical and laboratory settings of “infectious disease medicine.”31 As the novel coronavirus infests the world, the United States is expected to spend more than $2 billion in AI-linked research studies by 2025, quadrupling the amount spent in 2019 ($463 million).32 AI-based Neural networks have been introduced to quickly and precisely identify a host response to Coronavirus on the basis of mass spectrometry samples. Other uses of AI in infectious diseases include “support-vector machines” for discovering antibiotic resistance, ML investigation of blood smears for malaria diagnosis, and enhanced point-of-care diagnostics for Lyme disease on the basis of antigen detection. AI has also been studied for refining the diagnosis of tuberculosis, meningitis, and sepsis for predicting treatment problems in hepatitis C and B patients.31
Uses of Machine Learning in Cancer Prediction
The ability to accurately predict which treatment regimens are best suited for each patient based on their distinct molecular, genetic, and tumor-based features is a challenging task in oncologic care that AI is intended to solve.33 To assess whether AI and its subfield including machine learning can help in oncology care, a large number of studies investigated the applications of AI in cancer risk stratification, diagnoses, cancer medication development, and molecular tumor characterization.34–36 According to these researches, ML can help in cancer prediction and diagnosis by analyzing pathology profiles, imaging studies, and its ability to convert pictures to “mathematical sequences.” In January 2020, researchers developed an artificial intelligence system based on a “Google DeepMind algorithm” capable of outperforming human “breast cancer” detection specialists.36,37 In July 2020, the University of Pittsburgh developed an AI system-based machine learning technique with the highest accuracy in diagnosing prostate cancer, with a specificity of 98% and sensitivity of 98%.38 A very recent study used an improved ViT (Vision Transformer) architecture, which they called ViT-Patch, is validated on a publicly available dataset, and the results of the experiments reveal that it is effective for both malignant detection and tumor localization.39
A study used machine learning techniques to classify data relevant to cancer and generated a diagnosis for breast cancer.40 In this study, different classification techniques were examined and applied to certain feature subsets, including support vector machine classifiers, probabilistic neural networks, and K-nearest neighbors. Support vector machine classifier models showed the maximum overall accuracy for the diagnosis of breast cancer. Rana et al used machine learning classification algorithms, which use stored historical data to learn from and forecast new input categories, benign and malignant tumors.41 According to this study, the random forest model demonstrated the highest accuracy of 96% to detect different cancers. This study served as the foundation for a thorough understanding of the random forest model and served as the basis for the suggested AI system’s implementation.
Observational research compared the accuracy of the support vector machine, artificial neural networks (ANN), Naive Bayes classifier, and AdaBoost tree to identify a potent model for breast cancer prediction.42 Principal component analysis was used to reduce dimensionality. The study found that, when compared to techniques like decision trees, regression trees, and so on, artificial neural network (ANN) was found to be the most popular one. The ANN method offered a reliable approach to making real-time predictions and prognoses.
Pulse-Coupled Neural Networks have been utilized in the field especially for image processing.43 A survey investigated the disadvantages and advantages of various neural network designs. According to the survey, multilayer auto-encoders probabilistic and neural networks both delivered a 96% accuracy for a given cancer dataset.44 On the basis of the Wisconsin Diagnostic Breast Cancer dataset, the research examined a variety of machine learning techniques, including support vector machines, linear regression, multilayer perceptron, and SoftMax regression. The results demonstrated that all of the machine language algorithms given completed the classification job successfully and with high test accuracy in the prediction of cancer. This study also proposed that more accurate feature selection techniques, which were used in the proposed model, can increase prediction accuracy.45
Use of AI in Cancer Prediction
Over the past few decades, caregivers from all fields, from experts to paramedics, have been inquired to predict cancer prognoses based on their professional experience. Clinicians realize the need to use AI innovations such as DL and ML as a result of the emergence of the digital data era.9 They believe that due to the complex and vast nature of statistical analysis it is difficult to anticipate how cancer will progress.6 Health-care experts are also concerned about the risk that a patient may contract a disease, can have a tumor recurrence after treatment, or die. These considerations have a substantial influence on treatment options and results. In reality, a large body of evidence on clinical cancer is concerned with predicting patient response to therapy or establishing prognosis. Patients with more accurate prognoses can get more effective therapies; in fact, these treatment options typically include personalizing or individualizing care for each patient. AI can evaluate and understand “multi-factor” data from several patient assessments and provide more precise information about the patient survival, prognosis, and disease progression predictions in order to predict cancer.9 Enshaei et al evaluated many strategies, integrating classifiers with traditional logistic regression analytic techniques to demonstrate that AI has a role in providing forecasting and predicting information to ovarian cancer patients.46
Algorithms based on artificial intelligence have been shown to be capable of analyzing unstructured data and correctly estimating the likelihood of patients getting different illnesses, including cancer.47 Accurately, agnostic AI algorithms can improve risk stratification criteria and influence cancer screening recommendation outcomes.48–52 For instance, an artificial “neural network model” for “colorectal cancer risk stratification” demonstrated the highest accuracy than “current screening guidelines.49
These AI algorithms might be used for a whole population. These algorithms can benefit those people who are at high risk of developing cancer or High-risk persons who are not covered in the existing screening criteria. Although traditional screening procedures for individuals with “early-onset sporadic colorectal cancer” are restricted, patients can benefit from the rigorous risk-based screening guidelines.49
For tumors without an established screening method that is primarily asymptomatic in their first stages, individualized risk prediction might assist in early detection and perhaps increase treatment rates. For instance, an “artificial neural network model” for predicting “pancreatic cancer” risk has attained an area under the “receiver operating characteristic curve” of 85%.53 In low-resource settings, individualized risk calculation algorithms can assist prioritize “screening for high-risk” people.
Which Types of Cancer are Easily Predicted?
Cancer is a genetic disorder and several types of cancers have been identified;54,55 therefore, it is no surprise that AI advancements have helped oncology in particular. For example, “DNA methylation analysis” in cancer has been shown to be useful for cancer categorization and prognosis.56 The “machine-determined DNA methylation” technique can reclassify more than 70% of human-labeled cancers, potentially leading to dramatically altered prognosis and therapy options.57 In a research MethylationEPIC (850 k) and Illumina HumanMethylation450 showed the highest 93% accuracy in categorizing 82 kinds of “brain tumors.”58 The authors’ reported the highest accuracy even greater than pathologists.
Deep Learning technique is effective in different industries and is used to detect a variety of chronic conditions and aid clinicians in making medical decisions.59 According to a review, the deep learning algorithm was able to classify five different forms of cancer, including prostate and colon adenocarcinoma, breast invasive carcinoma, kidney renal clear cell carcinoma (KIRC), and lung adenocarcinoma (LUAD).60
Dwivedi et al used microarray gene expression patterns to propose a system61 of supervised machine-learning approaches for differentiating acute lymphoblastic leukemia from acute myeloid leukemia. This classification was achieved using an “artificial neural network” (ANN).62 In 2020, Gupta et al predicted prostate cancer using multi-layer perceptrons and discovered multiple data-balancing procedures.63 Another recent study in 2021 using ANN predicted mesothelioma with 96% accuracy and provided a method for classifying cancer depending on gene expression data.64 In these studies, the logarithmic transformation was used to preprocess gene expression data in order to lessen the classification’s complexity, while the Bhattacharya distance was used to identify the most informative genes. In another research, An ML technique predicted blood and colon cancer on the basis of gene expression with a detection rate of 0.9666 and an accuracy of 0.9534.65
Using machine learning (ML), researchers achieved a 97% accuracy rate in diagnosing two common types of lung cancer by analyzing tissue sample slides. The algorithm studied cancer tissue imaging and detected genetic changes associated with the disease. It successfully distinguished between normal lung tissue and the two most prevalent types of lung cancer, adenocarcinomas, and squamous cell carcinomas, which are often challenging to differentiate even for experienced pathologists due to their distinct cell origins and different treatment requirements.66
Recently, a technique called radiomics has been introduced, a part of “Deep learning” techniques that can be “applied” to medical images in order to obtain a huge number of features that are invisible by humans and perhaps reveal disease-related patterns and characteristics.67 In medical field, radiomics is the study of these characteristics, and there is a rising interest in merging them with clinical genomic data.68 Radiomic techniques can inform models that accurately anticipate the treatment effectiveness or adverse effects of cancer therapy. Radiomics was found to be useful in predicting three different types of cancer such as lung, brain, and liver cancer69,70 Additionally, deep learning exploiting radiomic brain features MRI can distinguish between brain gliomas and brain metastases with the same accuracy as expert neuroradiologists.71
Currently, Al-based “cancer survival prediction” has been introduced for numerous cancer types, such as lung, prostate, and breast carcinomas.72–74 The survival prediction accuracy of AI-based systems is superior to that of conventional analytic methodologies.75 This could be due to their greater accuracy for variables having nonlinear relationships, making them more applicable to real-world situations. Predicting cancer survival can assist in customizing treatment plans. For patients at high risk, treatment planning can be strengthened, while therapies with limited effect can be avoided.72 Moreover, AI models can predict the risk of illness recurrence following a therapeutic option. The applications of AI for the prediction of cancer recurrence have demonstrated greater accuracy than standard statistical models,76,77 which will further facilitate the optimization of clinical follow-up plans.
Limitations
The use of artificially intelligent systems in any industry, including healthcare, has its limitations and obstacles. The moment has come to shift our perspective from being reactive to proactive in the face of emerging technology flaws. Here, we address various limitations of AI and machine learning with an emphasis on those that are particularly relevant to healthcare.
Data Privacy
Data accessibility and data gathering is the first step in developing an artificially intelligent system after problem selection and solution approach development. For the creation of effective models, it is necessary to have access to a large quantity of high-quality data. Due to patient privacy concerns and data breaches by prominent organizations, the issue of data collection is still in controversy. For example, patient confidentiality restricts the availability of data, which in turn limits model training; hence, a model’s full potential is not explored.
Fragmented Data
Another limitation of the deployment of artificial intelligence is that models that one organization designs and deploys for a specific job such as natural language processing, regression, classification, clustering, NLP cannot be effortlessly transferred to another organization for immediate use without recalculation. Due to privacy issues, data sharing between health-care organizations is frequently inaccessible or restricted, resulting in fragmented data that reduces the model’s dependability.78 A group of researchers have described a four-tier model; access, transitions, quality, and socioeconomic/environmental impact, which offers a pragmatic framework to establish measurements that may be helpful to advance equity for both the patients and the staff of the health-care provider organization.79 The use of blockchain technology in healthcare may help in reducing fragmentation.80 Data silos is another problem facing by the ML in health care. Researchers found that using mobile phones apps may help in data silos,81 while others focused on cloud computing.82 Similarly, the application of FAIR principles (Findable, Accessible, Interoperable, and Reusable) in health research data is a hot topic of the day. There have been several research groups working this aspects.83 The European Union has released a special project.84 An editorial has been published which is based on the challenges of the effectual implementation of FAIR Principles in biomedical research.85 Similarly, a Hybrid Hierarchical K-means (HHK) clustering machine learning algorithm was applied to group the data into homogeneous subgroups and ascertain the underlying structure of the data using a Nigerian-based FAIR dataset. The data contained economic factors, health-care facilities, and coronavirus occurrences in all the 36 states of the country. The model successfully interpreted the research data and it was obvious that the ML pipeline can be FAIRified, shared, and reused by implementing the proposed FAIRification workflow and the technical architecture.86
Knowledge Graphs
A knowledge Graph (KG) is a structured representation of facts that defines a set of interconnected entity descriptions, relationships, and entity semantic descriptions.87 Stokman FN and de Vries PH first proposed the concept of organized knowledge in a graph in 198888, and it gained popularity in 2012 after being used in Google’s search engine. A Knowledge Graph is generally defined as “A multi-relational graph composed of entities as nodes and relations as different types of edges.”89 Besides its uses in other data sciences and social sciences,90 the knowledge graphs have been used in medical field and health-care science. Traditional graphs and networks used for biomedical data integration only contain one type of relation (eg, interactions between proteins), whereas the Knowledge Graphs provides diverse information, including multiple entities (eg, proteins, targets, and drugs) and multiple types of relations (eg, interactions between drugs or drug-target pairs).91 Complex relations between entities in biological systems can be easily modeled by a Knowledge Graphs.92 The knowledge graph-based works that instrument drug repurposing and hostile drug reaction projection for drug discovery has been reviewed somewhere else.93 A knowledge graph-based approach was used in organizing cancer registry data. This knowledge graph approach semantically augments the data, and easily enables linking with third-party data, which can help explain variation in cancer incidence patterns, disparities, and outcomes.94 Another team have used the knowledge graphs based approach to discover unobvious genes that drive drug resistance in lung cancer.95 Medical Knowledge Graph Deep Learning for cancer phenotyping has been established.96
Scale
The volume of data created by and about patients is expanding exponentially and is becoming increasingly challenging to manage within the necessary timeframes for therapeutic usefulness. Patient data are being generated at a rate that is beyond our capacity to collect and analyze them.97 However, what may be more troublesome is the fact that practically all medical data are unavailable for analysis, regardless of how many are generated. Medical imaging systems are exclusive and prohibit interaction with other systems, rendering the information they contain relatively useless and incapable of being used for machine learning.
Data Normalization
The initial step in data analysis is the processing of a specified data set beforehand (s). Combining several datasets requires feature selection, noise filtering, and normalization. Merging various data sets necessitates normalization to reduce bias while studying the resulting data set. The pattern recognition method is the selection of defined features important to the effectiveness of classification and regression. Integrating data obtained from many types of “omics” and assorted information sources to foresee clinical conclusions and biomarkers is an additional significant problem in precision oncology.98
Complicated Data
Due to the complexity of the mathematical methods used, Artificial Intelligence systems have a reputation for being black boxes. Models should be made more accessible and easier to interpret. While there has been significant effort in this area, there is still a need for improvement.99
What are the Future Prospects?
As soon as obstacles are resolved and “AI algorithms” are confirmed by future research, AI-based models will be integrated into all aspects of healthcare. In the coming years, “oncology AI applications” will be realized through “data intelligence”, a better knowledge of tumors, more accurate therapy alternatives, and enhanced “decision-making processes.” The field of Oncology will become a more specialized field, and individuals will receive treatment more frequently than ever.
Additionally, risk assessment tools integrated into smartphone applications will give the general public an immediate cancer risk estimate. The provision of high-risk estimates can drive patients to receive medical help and comply with medical advice. In addition, risk reduction estimates might drive individuals to adopt healthier behaviors, such as stopping smoking or being physically active. Algorithms will assist physicians in determining whether to refer people to high-complexity healthcare centers in a primary care setting. Algorithm integration with EHR systems can help health-care facilities by providing an alternative for improved resource allocation based on the information of the individual subgroups with a greater risk of cancer growth or cancer-related consequences.100
The advent of ChatGPT by OpenAI has been recognized for its expansive knowledge of varied subjects. It is a Large Language Model that has become the fastest growing consumer application. Researchers found that ChatGPT has the potential to revolutionize the field of colorectal surgery by providing personalized and precise medical information, reducing errors and complications, and improving patient outcomes.101 Besides, the neuro-oncology was taken as an example to study the ChatGPT responses.102 A quick analysis of radiographic imaging and predictive outcome based on tumor genomics are some of the possible aspects where ChatGPT may help the physicians in diagnostic processing.
Conclusion
AI-ML has flourished in this era due to the technical advances of the time. Previously, these novel innovations were exclusively used for non-medical purposes, but they are now starting to be implemented for the improvement of healthcare around the world. AI and ML have a substantial impact on healthcare and will continue to reshape this field. The potential in the field of oncology is tremendous and has applications in almost every aspect of cancer research including, diagnosis, prognosis, and treatment.
Cancer, one of the life-threatening diseases, may soon have a treatment, but as prevention is preferable to treatment, early and rapid detection, cancer prediction, and disease prognosis prediction are vital. In this article, the most recent deep learning, ML, and AI developments are discussed. Incorporating DL, ML, and AI into many types of cancer prognosis, diagnosis and prediction may one day result in a more effective treatment for cancer. It has the potential to improve the quality of hospital environments for all diseases, not only cancer. The obstacles associated with this debilitating disease will undoubtedly be overcome one day with the help of these increasing algorithms. In light of these aims, additional studies are required to continue to ensure clinical value and analytical and clinical validity.
Funding
Jinling Institute of Science Technology Research Initiation Project (jit-b-202030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi:10.1016/S0140-6736(16)31891-8
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Afolabi LO, Afolabi MO, Sani MM, et al. Exploiting the CRISPR‐Cas9 gene‐editing system for human cancers and immunotherapy. Clinical Translat Immunol. 2021;10(6):e1286. doi:10.1002/cti2.1286
4. World Health Organization. © International Agency for Research on Cancer, 2020. Cancer Today. Available from:https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0. Accessed June 20, 2023.
5. Ahmad M, Khan Z, Rahman ZU, Khattak SI, Khan ZU. Can innovation shocks determine CO2 emissions (CO2e) in the OECD economies? A new perspective. Econ Innov New Technol. 2021;30(1):89–109. doi:10.1080/10438599.2019.1684643
6. Gaur K, Jagtap MM. Role of artificial intelligence and machine learning in prediction, diagnosis, and prognosis of cancer. Cureus. 2022;14(11). doi:10.7759/cureus.31008
7. Dananjayan S, Raj GM. Artificial Intelligence during a pandemic: the COVID −19 example. Int J Health Plann Manage. 2020;35:1260–1262. doi:10.1002/hpm.2987
8. Iqbal MJ, Javed Z, Sadia H, et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: looking into the future. Cancer Cell Int. 2021;21(1):1–11. doi:10.1186/s12935-021-01981-1
9. Huang S, Yang J, Fong S, Zhao Q. Artificial intelligence in cancer diagnosis and prognosis: opportunities and challenges. Cancer Lett. 2020;471:61–71. doi:10.1016/j.canlet.2019.12.007
10. Hollon TC, Pandian B, Adapa AR, et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat Med. 2020;26(1):52–58. doi:10.1038/s41591-019-0715-9
11. Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn. 2002;46(1):389–422. doi:10.1023/A:1012487302797
12. Mori Y, Kudo SE. Detecting colorectal polyps via machine learning. Nat Biomed Eng. 2018;2(10):713–714. doi:10.1038/s41551-018-0308-9
13. Wang K-W, Dong M. Potential applications of artificial intelligence in colorectal polyps and cancer: recent advances and prospects. World J Gastroenterol. 2020;26(34):5090. doi:10.3748/wjg.v26.i34.5090
14. Jianzhu B, Shuang L, Pengfei M, Yi Z, Yanshu Z. Research on early warning mechanism and model of liver cancer rehabilitation based on CS-SVM. J Healthc Eng. 2021;2021. doi:10.1155/2021/6658776
15. Goldenberg SL, Nir G, Salcudean SE. A new era: artificial intelligence and machine learning in prostate cancer. Nat Rev Urol. 2019;16(7):391–403. doi:10.1038/s41585-019-0193-3
16. Hu F, Shi X, Wang H, et al. Is health contagious?—Based on empirical evidence from China family panel studies’ data. Front Public Health. 2021;9:691746. doi:10.3389/fpubh.2021.691746
17. Musa IH, Afolabi LO, Zamit I, et al. Artificial intelligence and machine learning in cancer research: a systematic and thematic analysis of the top 100 cited articles indexed in Scopus database. Cancer Control. 2022;29:10732748221095946. doi:10.1177/10732748221095946
18. Kubat M. An Introduction to Machine Learning. Springer; 2017.
19. Alpaydin E. Introduction to Machine Learning. MIT press; 2020.
20. Rebala G, Ravi A, Churiwala S. An Introduction to Machine Learning. Springer; 2019.
21. Jovel J, Greiner R. An introduction to machine learning approaches for biomedical research. Front Med. 2021;2021:8.
22. Sidey-Gibbons JAM, Sidey-Gibbons CJ. Machine learning in medicine: a practical introduction. BMC Med Res Methodol. 2019;19(1):64. doi:10.1186/s12874-019-0681-4
23. Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. 2015;349(6245):255–260. doi:10.1126/science.aaa8415
24. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. doi:10.1038/nature21056
25. Anderson JP, Parikh JR, Shenfeld DK, et al. Willke RJ: reverse engineering and evaluation of prediction models for progression to type 2 diabetes: an application of machine learning using electronic health records. J Diabetes Sci Technol. 2016;10(1):6–18. doi:10.1177/1932296815620200
26. Ghassemi M, Naumann T, Schulam P, Beam AL, Chen IY, Ranganath R. Practical guidance on artificial intelligence for health-care data. Lancet Digital Health. 2019;1(4):e157–e159. doi:10.1016/S2589-7500(19)30084-6
27. Ertel W. Introduction to Artificial Intelligence. Springer; 2018.
28. Kalis B, Collier M, Fu R. 10 promising AI applications in health care. Harv Bus Rev. 2018;2018:1.
29. Wang H, Zu Q, Chen J, Yang Z, Ahmed MA. Application of artificial intelligence in acute coronary syndrome: a brief literature review. Adv Ther. 2021;38(10):5078–5086. doi:10.1007/s12325-021-01908-2
30. Cao J-S, Z-Y L, Chen M-Y, et al. Artificial intelligence in gastroenterology and hepatology: status and challenges. World J Gastroenterol. 2021;27(16):1664. doi:10.3748/wjg.v27.i16.1664
31. Tran NK, Albahra S, May L, et al. Evolving applications of artificial intelligence and machine learning in infectious diseases testing. Clin Chem. 2022;68(1):125–133. doi:10.1093/clinchem/hvab239
32. Vaishya R, Javaid M, Khan IH, Haleem A. Artificial Intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab Syndr. 2020;14(4):337–339. doi:10.1016/j.dsx.2020.04.012
33. Bhinder B, Gilvary C, Madhukar NS, Elemento O. Artificial intelligence in cancer research and precision medicine. Cancer Discov. 2021;11(4):900–915. doi:10.1158/2159-8290.CD-21-0090
34. Yu C, Helwig EJ. The role of AI technology in prediction, diagnosis and treatment of colorectal cancer. Artif Intell Rev. 2022;55(1):323–343. doi:10.1007/s10462-021-10034-y
35. Kumar Y, Gupta S, Singla R. Hu Y-C: a systematic review of artificial intelligence techniques in cancer prediction and diagnosis. Arch Comput Methods Eng. 2021;2021:1–28.
36. McKinney SM, Sieniek M, Godbole V, et al. International evaluation of an AI system for breast cancer screening. Nature. 2020;577(7788):89–94. doi:10.1038/s41586-019-1799-6
37. Majumder A, Sen D. Artificial intelligence in cancer diagnostics and therapy: current perspectives. Indian J Cancer. 2021;58(4):481. doi:10.4103/ijc.IJC_399_20
38. Pantanowitz L, Quiroga-Garza GM, Bien L, et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: a blinded clinical validation and deployment study. Lancet Digital Health. 2020;2(8):e407–e416. doi:10.1016/S2589-7500(20)30159-X
39. Feng H, Yang B, Wang J, et al. Identifying Malignant Breast Ultrasound Images Using ViT-Patch. Appl Sci. 2023;13(6):3489. doi:10.3390/app13063489
40. Ray A, Chen M, Gelogo Y. Performance Comparison of Different Machine Learning Algorithms for Risk Prediction and Diagnosis of Breast Cancer. In: Smart Technologies in Data Science and Communication. Springer; 2020:71–76.
41. Rana M, Chandorkar P, Dsouza A, Kazi N. Breast cancer diagnosis and recurrence prediction using machine learning techniques. Int J Eng Res Technol. 2015;4(4):372–376. doi:10.15623/ijret.2015.0404066
42. Kharya S, Dubey D, Soni S. Predictive machine learning techniques for breast cancer detection. Int J Comput Sci Inf Technol. 2013;4(6):1023–1028.
43. Liu H, Liu M, Li D, Zheng W, Yin L, Wang R. Recent advances in pulse-coupled neural networks with applications in image processing. Electronics. 2022;11(20):3264. doi:10.3390/electronics11203264
44. Agrawal S, Agrawal J. Neural network techniques for cancer prediction: a survey. Procedia Comput Sci. 2015;60:769–774. doi:10.1016/j.procs.2015.08.234
45. Agarap AFM. On breast cancer detection: an application of machine learning algorithms on the Wisconsin diagnostic dataset. In:
46. Enshaei A, Robson C, Edmondson R. Artificial intelligence systems as prognostic and predictive tools in ovarian cancer. Ann Surg Oncol. 2015;22(12):3970–3975. doi:10.1245/s10434-015-4475-6
47. Miotto R, Li L, Kidd BA, Dudley JT. Deep patient: an unsupervised representation to predict the future of patients from the electronic health records. Sci Rep. 2016;6(1):1–10. doi:10.1038/srep26094
48. Nartowt BJ, Hart GR, Muhammad W, Liang Y, Stark GF, Deng J. Robust machine learning for colorectal cancer risk prediction and stratification. Front Big Data. 2020;3:6. doi:10.3389/fdata.2020.00006
49. Nartowt BJ, Hart GR, Roffman DA, et al. Scoring colorectal cancer risk with an artificial neural network based on self-reportable personal health data. PLoS One. 2019;14(8):e0221421. doi:10.1371/journal.pone.0221421
50. Hart GR, Roffman DA, Decker R, Deng J. A multi-parameterized artificial neural network for lung cancer risk prediction. PLoS One. 2018;13(10):e0205264. doi:10.1371/journal.pone.0205264
51. Stark GF, Hart GR, Nartowt BJ, Deng J. Predicting breast cancer risk using personal health data and machine learning models. PLoS One. 2019;14(12):e0226765. doi:10.1371/journal.pone.0226765
52. Roffman D, Hart G, Girardi M, Ko CJ, Deng J. Predicting non-melanoma skin cancer via a multi-parameterized artificial neural network. Sci Rep. 2018;8(1):1–7. doi:10.1038/s41598-018-19907-9
53. Muhammad W, Hart GR, Nartowt B, et al. Pancreatic cancer prediction through an artificial neural network. Front Artif Intell. 2019;2:2. doi:10.3389/frai.2019.00002
54. Zhao H, Ming T, Tang S, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. 2022;21(1):144. doi:10.1186/s12943-022-01616-7
55. Tian Y, Xiao H, Yang Y, et al. Crosstalk between 5-methylcytosine and N6-methyladenosine machinery defines disease progression, therapeutic response and pharmacogenomic landscape in hepatocellular carcinoma. Mol Cancer. 2023;22(1):1–25. doi:10.1186/s12943-022-01706-6
56. Wrzeszczynski KO, Frank MO, Koyama T, et al. Comparing sequencing assays and human-machine analyses in actionable genomics for glioblastoma. Neurol Genet. 2017;3(4):e164. doi:10.1212/NXG.0000000000000164
57. Dlamini Z, Francies FZ, Hull R, Marima R. Artificial intelligence (AI) and big data in cancer and precision oncology. Comput Struct Biotechnol J. 2020;18:2300–2311. doi:10.1016/j.csbj.2020.08.019
58. Capper D, Stichel D, Sahm F, et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018;136(2):181–210. doi:10.1007/s00401-018-1879-y
59. Lv Z, Yu Z, Xie S, Alamri A. Deep learning-based smart predictive evaluation for interactive multimedia-enabled smart healthcare. ACM Trans Multimedia Comput Commun Appl. 2022;18(1):1–20.
60. Gupta S, Gupta MK, Shabaz M, Sharma A. Deep learning techniques for cancer classification using microarray gene expression data. Front Physiol. 2022;2002:1.
61. Gupta S, Gupta M. Deep learning for brain tumor segmentation using magnetic resonance images. In:
62. Dwivedi AK. Artificial neural network model for effective cancer classification using microarray gene expression data. Neural Comput Appl. 2018;29(12):1545–1554. doi:10.1007/s00521-016-2701-1
63. Gupta S, Gupta MK. Computational prediction of cervical cancer diagnosis using ensemble-based classification algorithm. Comput J. 2022;65(6):1527–1539. doi:10.1093/comjnl/bxaa198
64. Tumuluru P, Ravi B. GOA-based DBN: grasshopper optimization algorithm-based deep belief neural networks for cancer classification. Int J Appl Eng Res. 2017;12(24):14218–14231.
65. Danaee P, Ghaeini R, Hendrix DA: A deep learning approach for cancer detection and relevant gene identification. In:
66. Bębas E, Borowska M, Derlatka M, et al. Machine-learning-based classification of the histological subtype of non-small-cell lung cancer using MRI texture analysis. Biomed Signal Process Control. 2021;66:102446. doi:10.1016/j.bspc.2021.102446
67. Yang S, Li Q, Li W, Li X, Liu -A-A. Dual-level representation enhancement on characteristic and context for image-text retrieval. IEEE Trans Circuits Syst Video Technol. 2022;32(11):8037–8050. doi:10.1109/TCSVT.2022.3182426
68. Avanzo M, Stancanello J, Pirrone G, Sartor G. Radiomics and deep learning in lung cancer. Strahlenther Onkol. 2020;196(10):879–887. doi:10.1007/s00066-020-01625-9
69. Dreher C, Linde P, Boda-Heggemann J, Baessler B. Radiomics for liver tumours. Strahlenther Onkol. 2020;196(10):888–899. doi:10.1007/s00066-020-01615-x
70. Kocher M, Ruge MI, Galldiks N, Lohmann P. Applications of radiomics and machine learning for radiotherapy of malignant brain tumors. Strahlenther Onkol. 2020;196(10):856–867. doi:10.1007/s00066-020-01626-8
71. Bae S, An C, Ahn SS, et al. Robust performance of deep learning for distinguishing glioblastoma from single brain metastasis using radiomic features: model development and validation. Sci Rep. 2020;10(1):1–10. doi:10.1038/s41598-020-68980-6
72. Bibault J-E, Chang DT, Xing L. Development and validation of a model to predict survival in colorectal cancer using a gradient-boosted machine. Gut. 2021;70(5):884–889. doi:10.1136/gutjnl-2020-321799
73. Senders JT, Staples P, Mehrtash A, et al. An online calculator for the prediction of survival in glioblastoma patients using classical statistics and machine learning. Neurosurgery. 2020;86(2):E184–E192. doi:10.1093/neuros/nyz403
74. Kim DW, Lee S, Kwon S, Nam W, Cha I-H, Kim HJ. Deep learning-based survival prediction of oral cancer patients. Sci Rep. 2019;9(1):1–10. doi:10.1038/s41598-018-37186-2
75. Matsuo K, Machida H, Shoupe D, et al. Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer. Obstet Gynecol. 2016;128(4):761. doi:10.1097/AOG.0000000000001647
76. Liu B, He H, Luo H, Zhang T, Jiang J. Artificial intelligence and big data facilitated targeted drug discovery. Stroke Vasc Neurol. 2019;4(4):206–213. doi:10.1136/svn-2019-000290
77. Liu -A-A, Zhai Y, Xu N, Nie W, Li W, Zhang Y. Region-aware image captioning via interaction learning. IEEE Trans Circuits Syst Video Technol. 2021;32(6):3685–3696. doi:10.1109/TCSVT.2021.3107035
78. Basu K, Sinha R, Ong A, Basu T. Artificial intelligence: how is it changing medical sciences and its future? Indian J Dermatol. 2020;65(5):365–370. doi:10.4103/ijd.IJD_421_20
79. Sivashanker K, Duong T, Resnick A, Eappen S. Health care equity: from fragmentation to transformation. NEJM Catal Innov Care Deliv. 2020;15.
80. Zhang P, Schmidt DC, White J, Lenz G. Chapter One - Blockchain Technology Use Cases in Healthcare. In: Raj P, Deka GC, editors. Advances in Computers. Vol. 111. Elsevier; 2018:1–41.
81. Gay V, Leijdekkers P. Bringing health and fitness data together for connected health care: mobile apps as enablers of interoperability. J Med Internet Res. 2015;17(11):e260. doi:10.2196/jmir.5094
82. Ranchal R, Bastide P, Wang X, et al. Disrupting healthcare silos: addressing data volume, velocity and variety with a cloud-native healthcare data ingestion service. IEEE J Biomed Health Inform. 2020;24(11):3182–3188. doi:10.1109/JBHI.2020.3001518
83. Alvarez-Romero C, Martínez-García A, Sinaci AA, et al. FAIR4Health: findable, accessible, interoperable and reusable data to foster health research. Open Res Eur. 2022;2:34. doi:10.12688/openreseurope.14349.1
84. Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. 2016;3(1):160018. doi:10.1038/sdata.2016.18
85. Parra-Calderón CL, Sanz F, McIntosh LD. The challenge of the effective implementation of FAIR principles in biomedical research. Methods Inf Med. 2020;59(04/05):117–118. doi:10.1055/s-0040-1721726
86. Folorunso S, Ogundepo E, Basajja M, et al. FAIR machine learning model pipeline implementation of COVID-19 data. Data Intelli. 2022;4(4):971–990. doi:10.1162/dint_a_00182
87. Zamini M, Reza H, Rabiei M. A review of knowledge graph completion. Information. 2022;13(8):396. doi:10.3390/info13080396
88. Stokman FN, de Vries PH. Structuring Knowledge in a Graph. Rijksuniversiteit Groningen; 1986.
89. Wang Z, Zhang J, Feng J, Chen Z. Knowledge graph embedding by translating on hyperplanes. In:
90. Nie W, Bao Y, Zhao Y, Liu A. Long dialogue emotion detection based on commonsense knowledge graph guidance. IEEE Trans Multimedia. 2023;1–15. doi:10.1109/TMM.2023.3267295
91. Ji S, Pan S, Cambria E, Marttinen P, Philip SY. A survey on knowledge graphs: representation, acquisition, and applications. IEEE Tran Neural Net Learn Sys. 2021;33(2):494–514. doi:10.1109/TNNLS.2021.3070843
92. Mohamed SK, Nounu A, Nováček V. Biological applications of knowledge graph embedding models. Brief Bioinform. 2021;22(2):1679–1693. doi:10.1093/bib/bbaa012
93. Zeng X, Tu X, Liu Y, Fu X, Su Y. Toward better drug discovery with knowledge graph. Curr Opin Struct Biol. 2022;72:114–126. doi:10.1016/j.sbi.2021.09.003
94. Hasan SMS, Rivera D, Wu XC, Durbin EB, Christian JB, Tourassi G. Knowledge graph-enabled cancer data analytics. IEEE J Biomed Health Inform. 2020;24(7):1952–1967. doi:10.1109/JBHI.2020.2990797
95. Gogleva A, Polychronopoulos D, Pfeifer M, et al. Knowledge graph-based recommendation framework identifies drivers of resistance in EGFR mutant non-small cell lung cancer. Nat Commun. 2022;13(1):1667. doi:10.1038/s41467-022-29292-7
96. Alawad M, Gao S, Shekar MC, et al. Integration of domain knowledge using medical knowledge graph deep learning for cancer phenotyping. arXiv preprint arXiv. 2021;2021:210101337.
97. Rowe M. An introduction to machine learning for clinicians. Acad Med. 2019;94(10):1433–1436. doi:10.1097/ACM.0000000000002792
98. Gao Q, Zhu H, Dong L, et al. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 2019;179(2):561–577. e522. doi:10.1016/j.cell.2019.08.052
99. Lucas GM, Gratch J, King A, Morency L-P. It’s only a computer: virtual humans increase willingness to disclose. Comput Human Behav. 2014;37:94–100. doi:10.1016/j.chb.2014.04.043
100. Shaw J, Rudzicz F, Jamieson T, Goldfarb A. Artificial intelligence and the implementation challenge. J Med Internet Res. 2019;21(7):e13659. doi:10.2196/13659
101. Li W, Zhang Y, Chen F. ChatGPT in colorectal surgery: a promising tool or a passing fad? Ann Biomed Eng. 2023. doi:10.1007/s10439-023-03232-y
102. Cifarelli CP, Sheehan JP. Large language model artificial intelligence: the current state and future of ChatGPT in neuro-oncology publishing. J Neurooncol. 2023. doi:10.1007/s11060-023-04336-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
